Is the neutrophil extracellular trap-driven microvascular inflammation essential for diabetes vasculopathy?
Abstract
The neutrophil extracellular traps (NETs) are defined as an extensive web consisting decondensed chromatin, which is released from activated neutrophils, as well as cytotoxic proteins, histones and microbicidal proteases that cause tissue damage. NETs contribute to endothelial damage, inflammation, thrombosis, platelet aggregation, ischemia, that are essential players in the pathobiology of diabetic complications. The objective of the review is to highlight the possible role of NETosis in early diabetes-related vasculopathy beyond cardiovascular complications. Although the clinical significance of NETosis in diabetes beyond early atherosclerosis and cardiovascular complications is not still clear, there is limited data with respect to useful to use biological markers of NETosis aimed early stratification of the diabetics at risk of disease progression. Furthermore, several inductors of NETosis might be a target for novel pharmacological approaches to delay advance in diabetes and prevent diabetes-related vasculopathy.
Introduction
Vasculopathy plays a pivotal role in the development of cardiovascular (CV) complications in diabetes mellitus including the early-onset stage of the disease Schuett et al., 2015. Although the molecular basis responsible for vasculopathy in diabetes mellitus has been widely investigated, the innate pathogenic mechanisms affected various phases at the early stage of the disease have remained yet poorly understood Bhatta et al., 2016. Diabetes-induced vasculopathy may be attributable to hyperglycemia, lipotoxicity, neurohumoral dysregulation of vascular function, increased prolonged exposure to oxidative stress, reduced production and release of nitric oxide, lowgrade microvascular inflammation, pro-thrombotic state, reduced ability to repair endothelial damage by recruitment of endothelial precursors, development of asymptomatic atherosclerosis Bhatta et al., 2016Lehoux and Jones, 2015Leong et al., 2016Vanhoutte et al., 2015. Indeed, hyperglycemia leads to accumulation of advanced glycation end-products (AGEs) that transduces inflammatory and proliferative response through reactive oxygen species generation, or through activation of Receptor for AGEs (RAGE)- mediated pathways Yamagishi et al., 2009. The interaction of AGEs with their receptor RAGE directly elicits proliferative, inflammatory, thrombotic and fibrotic reactions in variety of cells (i.e. mononuclear / macrophages) via inducing vascular endothelial growth factor (VEGF) production and RAGE-NF-κB pathway activation Heier et al., 2015Yamagishi et al., 2009. Therefore, pro-inflammatory cytokines and chemokines may recruit neutrophil subsets in the vascular wall and promote microvascular inflammation through forming neutrophil extracellular traps (NETs) (a process termed NETosis) Xu et al., 2012. However, the exact molecular mechanisms contributed in diabetes- induced vasculopathy via NETs are still not clear. The aim of the review is the summary of knowledge about the role of neutrophil extracellular traps in pathophysiology of diabetes-induced vasculopathy.
Definition of neutrophil extracellular traps
The neutrophil extracellular traps are defined as an extensive web consisting decondensed chromatin, which is released from activated neutrophils, as well as cytotoxic proteins / histones and microbicidal proteases that cause killing of microorganisms and tissue damage Wang et al., 2009. It is deemed that NETosis is initiated with an expression of peptidylarginine deiminase 4 (PAD4), specific enzyme that is essential for modification of arginine residues of histones to citrulline leading to massive chromatin decondensation Wang, 2004. Because of the overexpression of PAD4 might be appeared beyond infection under several conditions (i.e. diabetes) Greene et al., 2015, NETosis could be differentiated into respectively spontaneous or inducible Leavy, 2015, while similar breaking out is not fulfill acceptable.
Biological role and mechanisms of netosis
For the last decade NETs have been known as a component of innate immune system and as part of antimicrobial defense that binds microorganisms, prevents them from spreading, and ensures a high local concentration of antimicrobial agents to degrade virulence factors and kill microorganisms Brinkmann, 2004. Describing a biological role of NETosis Brinkmann and Zychlinsky (2007) Medina, 2009 have called process of producing extracellular structures “beneficial suicide of stimulated neutrophils”. Indeed, neutrophils actively contribute to host defense by killing pathogens via NETosis and they die to make NETs Kobayashi, 2015.
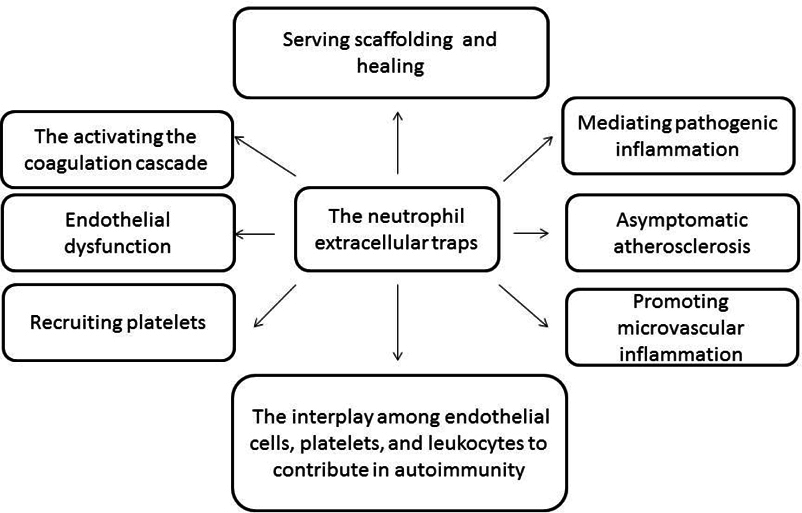
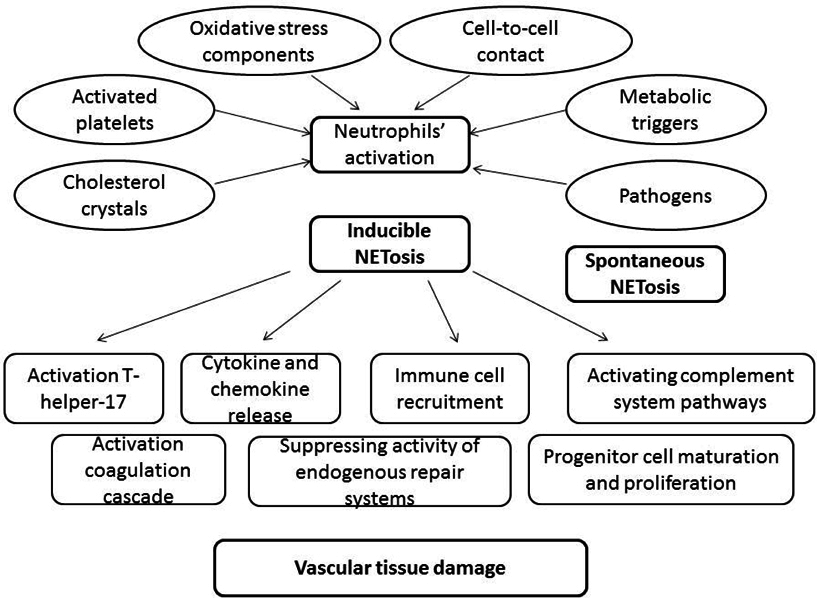
NETs have been implicated in an increasingly large number of human diseases including infectious diseases, sepsis, eclampsia, acute lung injury, thrombosis, autoimmunity, diabetes, and malignancy ( Figure 1 ). The NETosis is the post-translational regulated process forming deiminated chromatin, which is not only able to capture bacterial pathogens and bacterial cell adjuvants, but it may regulate inflammation and tissue damage via indirect mechanisms Dwivedi et al., 2014Dwivedi and Radic, 2013Leshner et al., 2012 including free radical production, forming of immune complexes, autoantibodies, inducing inflammatory cytokines release from competent cells ( Figure 2 ).
It has been suggested that interleukin-8 (IL-8), phorbol myristate acetate (PMA), lipopolysaccharide (LPS), thromboxane A2, β-defensin-1, P-selectin/ P-selectin glycoprotein ligand-1, and even simple contact of various cells with NET-releasing cells might induce NTEosis. Arecent study has shown that several phospholipid mediators, i.e. PAD4, are essential for NET formation Nakashima, 2002. PAD4 is the most investigated inducer of NETosis and it is an enzyme that converts arginine or monomethyl-arginine to citrulline in histones in a calcium-dependent reaction Yang et al., 2016. There are four types of PAD that are distributed in nucleus and cytoplasm of granulocytes. PAD4 is essential for transduction of positive signals in the nucleus via calcium-stimulated ionophores. However, the implication of PAD in histone modifications is widely discussed.
Next main mediator of histone modification is PAF complex (Paf1C) Zhu, 2005. Additionally, Paf1C has been shown to directly regulate gene transcription and RNA polymerase II elongation, coordinate several processes of adhesion and cell trafficking Chen et al., 2015Wang et al., 2015. In this contest, deiminated chromatin may be internalized by host phagocytes on surface of the endothelium, which is able delimiting the NETosis via secretion of apoptotic bodies, but the innate mechanisms responsible for this response remains mysterious.
As well known, post-translational modifications of histones within the externalized NETs are essential component of immune complexes / deposits and may directly damage tissue. There are evidence regarding that the nuclear DNA-binding protein HMGB1 (the high-mobility group box 1), which is released from necrotic cells / late apoptotic cells and is an essential component of DNA-containing immune complexes, may stimulate cytokine production through a Toll-like receptor 9 – myeloid differentiation primary response gene-88 pathway and induce NETosis. Indeed, HMGB1 is tightly attached to the chromatin in apoptotic cells. Moreover, HMGB1-containing nucleosomes from apoptotic cells induced secretion of interleukin 1β, IL-6, IL-10, and tumor necrosis factor (TNF) α and expression of costimulatory molecules in macrophages and dendritic cells (DC), respectively Joshi et al., 2007. Overall, HMGB1 exerts effects on NETs formation through an interaction with TLR2, TLR4 and RAGE, and this process is NADPH oxidase-dependent Grayson and Kaplan, 2015. Activated antigenpresenting cells may crucially contribute to the NETosis development and mediate the tissue damage.
Following activation by several factors mentioned above, neutrophils undergo distinct morphological alterations leading to ultimate NET formation. NETs promote the externalization of modified autoantigens, inducing synthesis of type I interferon, stimulating the formation of various inflammasomes, and activating both the classic and alternative pathways of the complement system Ma et al., 2016Wang et al., 2015. Furthermore, NETs are considered toxic substances for the endothelium that expose immunostimulatory molecules, activate plasmacytoid dendritic cells, and may participate in target-organ damage through incompletely characterized pathways. There are at least two important targets to counter NETosis in the setting of diabetes, such as protein kinase C and NADPH oxidase, which modulate an abnormal response to tissue damage and immune reactions. Consequently, NETs may contribute directly to immunogenicity, endothelial cell dysfunction, vasculopathy, atherosclerotic plaque burden, and thrombosisBerezin, 2015Grayson and Kaplan, 2015.
There is evidence regarding NETs might directly induce epithelial and endothelial damage, because structure of web is composed of DNA, histones and granular proteins, i.e. neutrophil elastase and myeloperoxidase Saffarzadeh et al., 2012. In has suggested that histones and myeloperoxidase are responsible for NET-mediated cytotoxicity in a dose-dependent manner and that this process is regulated by activated protein C Saffarzadeh et al., 2012. Contrary, a high local concentration of neutrophil elastase can cause degradation of the wound matrix and delay healing, and thereby could regulate NET-induced cytotoxicity protecting against activated protein C degradation Saffarzadeh and Preissner, 2013.
Spontaneous / inducible netosis in diabetes-related vasculopathy
The ability of NETosis to induce tissue damage in infections and inflammatory disease is widely known. However, the role of spontaneous NETosis in dysmetabolic states including diabetes mellitus is not fully established. In fact, in diabetes, neutrophils are primed to release NETs and die by NETosis Berezin, 2015. Neutrophils that are isolated from the blood of diabetics have been shown an increased spontaneous NETosis, but an impaired inducible exaggerated NETosis response might be a leading cause of tissue damage in diabetics with healing, such as diabetic food. Moreover, NET components (elastase, histones, NGAL, and proteinase-3) were enriched in nonhealing human with diabetes Fadini et al., 2016a. Wong et al., 2015 have been suggested that diabetes may not only activate neutrophils to overproduce PAD4 and NETs, but NETs are as a key factor delaying wound healing. Nevertheless, it was found that the finely tuned balance of NETosis required protecting the human body from microorganisms yet avoiding self-damage seems to be lost in diabetes Lewis et al., 2015. Cumulatively, the ability of activated antigen-presenting cells to exhibit a spontaneous NETosis is essential for diabetes, whereas the role of inducible exaggerated NETosis remains still unclear.
There are several systemic and local stimuli affected exaggerated level of spontaneous NETosis in diabetes. Freigang et al., 2011 have reported that the oxidative stress-responsive transcription factor NF-E2-related 2 (Nrf2) as an essential positive regulator of inflammasome activation and IL-1- mediated vascular inflammation. Recently studies have revealed that several stimuli, i.e. local ischemia, hypoxia, cholesterol crystals accumulated in vascular wall, necrotic cells, and histones, might act both as priming signals for IL-1β production by and trigger for neutrophils to release NETs via represent an endogenous danger signal that activates Nrf2 and the NLRP3 inflammasome Freigang et al., 2011Nahrendorf and Swirski, 2015Warnatsch et al., 2015. All these could prime macrophages for cytokine release, activating Th17 cells that amplify immune cell recruitment in atherosclerotic plaques and induce microvascular inflammation that leads to vasculopathy Allam et al., 2013Rajamäki et al., 2010Rajamaki et al., 2013. Overall, spontaneous NET-forming neutrophils may assist not only in the innate immune defense against different pathogens, but in early multicellular inflammatory reaction attenuating vascular damage and early stage of atherosclerosis.
Clinical relevance of spontaneous / inducible netosis in diabetes mellitus
Unfortunately, there is no consensus about the clinical significance of NETosis in diabetes beyond early atherosclerosis and CV complications Freigang et al., 2013Mangold et al., 2014. Although chronic lowgrading microvascular inflammation is considered a clue in the pathogenesis of atherosclerosis, the factors that trigger and sustain the inflammation remain elusive. It has been presumed that NETs, which are functionally important players in microvascular inflammation Michel and Ho-Tin-Noé, 2015. Indeed, NETosis contributes in tissue damage and kills endothelial / mononuclear cells and promote inflammation in atherosclerotic plaques, which may contribute to accelerated atherosclerosis. Whether classical NETosis mediates early atherosclerosis beyond plaque formation is still not clear. However, the concept of increased spontaneous NETosis that is suitable for diabetes mellitus might explain the role of low-grading microvascular inflammation as an initial stimulus for development of endothelial dysfunction beyond classical cardiovascular risk factors (A, 2016). Qin et al., 2016 reported that elevated level of circulating components of NETs (neutrophil elastase NE and proteinase 3) in diabetics exhibited closely positive correlation with absolute neutrophil counts. Authors suggest that NET-associated proteins might be novel circulating biomarkers in diabetes reflecting disease activity and target organ damage development. Cumulatively, all these data suggest that an exaggerated NETosis is an integral factor, which might delay healing in target organ damage, i.e. diabetic foot ulcers, diabetic retinopathy, diabetic vasculopathy.
The inflammasomes containing Nrf2 and the NLRP3 are essential regulator for inflammatory response that directly relays oxidative stress to vascular inflammation. Moreover, NETs might be target for further therapy in diabetics and that risk stratification score based on NETs’ identification would be validated for prediction at early stage of diabetes irrespectively CV complications. Taking into consideration that oxidative stress and low-grade inflammation are considered the main modulator of cardiovascular complications in diabetes mellitus, spontaneous / inducible NETosis might discuss as a clue translated regulatory signal from several metabolic triggers to target cells by potentiating the inflammasome-interleukin (IL)-1 family axis and Casp1 activation. Notably, processing and secretion of bioactive IL-1a and IL-1β be activated macrophages, are required Nrf2-regulated forming of the NLRP3 inflammasome. Interestingly, that secretion of NLRP3 inflammasome is under control of several modulators including galectin-3, which is considered as an independent predictor for CV events and disease in general population. Probably, spontaneous / inducible NETosis in diabetes mellitus might relate genetic predisposition and risk of CV complications, i.e. vasculopathy Fadini et al., 2016b. Although the assumptions appear to be attractive, lack of clinical evidence sufficiently limits productive scientific discussion around the topic and it is required more investigations.
Conclusion
NETosis is a novel player in the pathobiology of diabetes contributing to endothelial damage, thrombosis, ischemia, and microvascular inflammation. Diabetes- related vasculopathy might be triggered by metabolic signals that presumably activating inflammasomes and spontaneous / inducible NETosis for neutrophils. Probably, NETs link the various cellular response to metabolic triggers, oxidative stress with innate microvascular inflammation mediating early cardiovascular complications in diabetes. Further investigations are required to explain the role of NETosis in pathophysiology of diabetes and possibility to use triggers of inflammasomes as targets for medical care.
Abbreviations
DC: dendritic cells
DNA: deoxyribonucleic acid
IL: interleukin
LPS: lipopolysaccharide
HMGB1: the high-mobility group box 1
NET: neutrophil extracellular traps
PAD: peptidylarginine deiminase
TNF: tumor necrosis factor
References
-
A.E.
Berezin.
The Neutrophil Extracellular Traps: The Missed Link between Microvascular Inflammation and Diabetes?. Metabolomics.
2016;
06
:
163-166
.
-
R.
Allam,
M.N.
Darisipudi,
J.
Tschopp,
H.-J.
Anders.
Histones trigger sterile inflammation by activating the NLRP3 inflammasome. European Journal of Immunology.
2013;
43
:
3336-3342
.
-
A.E.
Berezin.
Circulating Vascular Endothelial Growth Factor-1 in Cardiovascular Disease. In Biomarkers in Cardiovascular Disease (Springer Science + Business Media).
2015;
:
1-18
.
-
A.
Bhatta,
R.
Sangani,
R.
Kolhe,
H.A.
Toque,
M.
Cain,
A.
Wong,
N.
Howie,
R.
Shinde,
M.
Elsalanty,
L.
Yao.
Deregulation of arginase induces bone complications in high-fat/high-sucrose diet diabetic mouse model. Molecular and Cellular Endocrinology.
2016;
422
:
211-220
.
-
V.
Brinkmann.
Neutrophil Extracellular Traps Kill Bacteria. Science.
2004;
303
:
1532-1535
.
-
Fei X.
Chen,
Ashley R.
Woodfin,
A.
Gardini,
Ryan A.
Rickels,
Stacy A.
Marshall,
Edwin R.
Smith,
R. Shilatifard
Shiekhattar.
PAF1, a Molecular Regulator of Promoter- Proximal Pausing by RNA Polymerase II. Cell.
2015;
162
:
1003-1015
.
-
N.
Dwivedi,
I.
Neeli,
N.
Schall,
H.
Wan,
D.M.
Desiderio,
E.
Csernok,
P.R.
Thompson,
H.
Dali,
J.P.
Briand,
S.
Muller.
Deimination of linker histones links neutrophil extracellular trap release with autoantibodies in systemic autoimmunity. The FASEB Journal.
2014;
28
:
2840-2851
.
-
N.
Dwivedi,
M.
Radic.
Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Annals of the Rheumatic Diseases.
2013;
73
:
483-491
.
-
G.P.
Fadini,
L.
Menegazzo,
M.
Rigato,
V.
Scattolini,
N.
Poncina,
A.
Bruttocao,
S.
Ciciliot,
F.
Mammano,
C.D.
Ciubotaru,
E.
Brocco.
NETosis Delays Diabetic Wound Healing in Mice and Humans. Diabetes.
2016a;
65
:
1061-1071
.
-
G.P.
Fadini,
L.
Menegazzo,
V.
Scattolini,
M.
Gintoli,
M.
Albiero,
A.
Avogaro.
A perspective on NETosis in diabetes and cardiometabolic disorders. Nutrition Metabolism and Cardiovascular Diseases.
2016b;
26
:
1-8
.
-
S.
Freigang,
F.
Ampenberger,
G.
Spohn,
S.
Heer,
A.T.
Shamshiev,
J.
Kisielow,
M.
Hersberger,
M.
Yamamoto,
M.F.
Bachmann,
M.
Kopf.
Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. European Journal of Immunology.
2011;
41
:
2040-2051
.
-
S.
Freigang,
F.
Ampenberger,
A.
Weiss,
T.-D.
Kanneganti,
Y.
Iwakura,
M.
Hersberger,
M.
Kopf.
Fatty acid- induced mitochondrial uncoupling elicits inflammasomeindependent IL-1α and sterile vascular inflammation in atherosclerosis. Nature Immunology.
2013;
14
:
1045-1053
.
-
P.C.
Grayson,
M.J.
Kaplan.
At the Bench: Neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. Journal of Leukocyte Biology.
2015;
99
:
253-264
.
-
C.S.
Greene,
A.
Krishnan,
A.K.
Wong,
E.
Ricciotti,
R.A.
Zelaya,
D.S.
Himmelstein,
R.
Zhang,
B.M.
Hartmann,
E.
Zaslavsky,
S.C.
Sealfon.
Understanding multicellular function and disease with human tissue-specific networks. Nature Genetics.
2015;
47
:
569-576
.
-
M.
Heier,
H.D.
Margeirsdottir,
M.
Gaarder,
K.H.
Stensæth,
C.
Brunborg,
P.A.
Torjesen,
I.
Seljeflot,
K.F.
Hanssen,
K.
Dahl-Jørgensen.
Soluble RAGE and atherosclerosis in youth with type 1 diabetes: a 5-year follow-up study. Cardiovasc Diabetol.
2015;
14
.
-
C.S.
Joshi,
E.S.
Priya,
S.
Venkataraman.
Acute and subacute toxicity studies on the polyherbal antidiabetic formulation Diakyur in experimental animal models. Journal of health science.
2007;
53
:
245-249
.
-
Y.
Kobayashi.
Neutrophil biology: an update. EXCLI journal.
2015;
14
:
220
.
-
O.
Leavy.
Inflammation: NETting a one-two punch. Nat Rev Immunol.
2015;
15
:
526-527
.
-
S.
Lehoux,
E.A.
Jones.
Shear stress, arterial identity and atherosclerosis. Thromb Haemost.
2015;
115
:
467-473
.
-
A.
Leong,
S.A.
Berkowitz,
V.A.
Triant,
B.
Porneala,
W.
He,
S.J.
Atlas,
D.J.
Wexler,
J.B.
Meigs.
Hypoglycemia in Diabetes Mellitus as a Coronary Artery Disease Risk Factor in Patients at Elevated Vascular Risk. The Journal of Clinical Endocrinology & Metabolism.
2016;
101
:
659-668
.
-
M.
Leshner,
S.
Wang,
C.
Lewis,
H.
Zheng,
X.A.
Chen,
L.
Santy,
Y.
Wang.
PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immun.
2012;
3
.
-
H.D.
Lewis,
J.
Liddle,
J.E.
Coote,
S.J.
Atkinson,
M.D.
Barker,
B.D.
Bax,
K.L.
Bicker,
R.P.
Bingham,
M.
Campbell,
Y.H.
Chen.
Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nature Chemical Biology.
2015;
11
:
189-191
.
-
Y.-H.
Ma,
T.-t.
Ma,
C.
Wang,
H.
Wang,
D.-Y.
Chang,
M.
Chen,
M.-H.
Zhao.
High-mobility group box 1 potentiates antineutrophil cytoplasmic antibody-inducing neutrophil extracellular traps formation. Arthritis Res Ther.
2016;
18
.
-
A.
Mangold,
S.
Alias,
T.
Scherz,
T.
Hofbauer,
J.
Jakowitsch,
A.
Panzenböck,
D.
Simon,
D.
Laimer,
C.
Bangert,
A.
Kammerlander.
Coronary Neutrophil Extracellular Trap Burden and Deoxyribonuclease Activity in STElevation Acute Coronary Syndrome Are Predictors of STSegment Resolution and Infarct SizeNovelty and Significance. Circulation Research.
2014;
116
:
1182-1192
.
-
E.
Medina.
Neutrophil Extracellular Traps: A Strategic Tactic to Defeat Pathogens with Potential Consequences for the Host. J Innate Immun.
2009;
1
:
176-180
.
-
J.-B.
Michel,
B.
Ho-Tin-Noé.
Thrombi and Neutrophils. Circulation Research.
2015;
116
:
1107-1108
.
-
M.
Nahrendorf,
F.K.
Swirski.
Neutrophilmacrophage communication in inflammation and atherosclerosis. Science.
2015;
349
:
237-238
.
-
K.
Nakashima.
Nuclear Localization of Peptidylarginine Deiminase V and Histone Deimination in Granulocytes. Journal of Biological Chemistry.
2002;
277
:
49562-49568
.
-
J.
Qin,
S.
Fu,
C.
Speake,
C.J.
Greenbaum,
J.M.
Odegard.
NETosis-associated serum biomarkers are reduced in type 1 diabetes in association with neutrophil count. Clin Exp Immunol.
2016
.
-
K.
Rajamäki,
J.
Lappalainen,
K.
Öörni,
E.
Välimäki,
S.
Matikainen,
P.T.
Kovanen,
K.K.
Eklund.
Cholesterol Crystals Activate the NLRP3 Inflammasome in Human Macrophages: A Novel Link between Cholesterol Metabolism and Inflammation. PLoS ONE.
2010;
5
:
e11765
.
-
K.
Rajamaki,
T.
Nordstrom,
K.
Nurmi,
K.E.O.
Akerman,
P.T.
Kovanen,
K.
Oorni,
K.K.
Eklund.
Extracellular Acidosis Is a Novel Danger Signal Alerting Innate Immunity via the NLRP3 Inflammasome. Journal of Biological Chemistry.
2013;
288
:
13410-13419
.
-
M.
Saffarzadeh,
C.
Juenemann,
M.A.
Queisser,
G.
Lochnit,
G.
Barreto,
S.P.
Galuska,
J.
Lohmeyer,
K.T.
Preissner.
Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE.
2012;
7
:
e32366
.
-
M.
Saffarzadeh,
K.T.
Preissner.
Fighting against the dark side of neutrophil extracellular traps in disease. Current Opinion in Hematology.
2013;
20
:
3-9
.
-
K.A.
Schuett,
M.
Lehrke,
N.
Marx,
M.
Burgmaier.
High-Risk Cardiovascular Patients: Clinical Features, Comorbidities, and Interconnecting Mechanisms. Front Immunol.
2015;
6
.
-
P.M.
Vanhoutte,
H.
Shimokawa,
M.
Feletou,
E.H.C.
Tang.
Endothelial Dysfunction and Vascular Disease - A Thirthieth Anniversary Update. Acta Physiologica.
2015
.
-
H.
Wang,
C.
Wang,
M.H.
Zhao,
M.
Chen.
Neutrophil extracellular traps can activate alternative complement pathways. Clin Exp Immunol.
2015;
181
:
518-527
.
-
Y.
Wang.
Human PAD4 Regulates Histone Arginine Methylation Levels via Demethylimination. Science.
2004;
306
:
279-283
.
-
Y.
Wang,
M.
Li,
S.
Stadler,
S.
Correll,
P.
Li,
D.
Wang,
R.
Hayama,
L.
Leonelli,
H.
Han,
S.A.
Grigoryev.
Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol.
2009;
184
:
205-213
.
-
A.
Warnatsch,
M.
Ioannou,
Q.
Wang,
V.
Papayannopoulos.
Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science.
2015;
349
:
316-320
.
-
S.L.
Wong,
M.
Demers,
K.
Martinod,
M.
Gallant,
Y.
Wang,
A.B.
Goldfine,
C.R.
Kahn,
D.D.
Wagner.
Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nature Medicine.
2015;
21
:
815-819
.
-
S.
Xu,
L.
Lind,
L.
Zhao,
B.
Lindahl,
P.
Venge.
Plasma Prolylcarboxypeptidase (Angiotensinase C) Is Increased in Obesity and Diabetes Mellitus and Related to Cardiovascular Dysfunction. Clinical Chemistry.
2012;
58
:
1110-1115
.
-
S.-i.
Yamagishi,
T. Nakamura K.
Matsui.
Role of Advanced Glycation End Products, Oxidative Stress, and Inflammation in Diabetic Vascular Complications. In Neurovascular Medicine (Oxford University Press (OUP)).
2009;
:
521-539
.
-
Y.
Yang,
W.
Li,
M.
Hoque,
L.
Hou,
S.
Shen,
B.
Tian,
B.D.
Dynlacht.
PAF Complex Plays Novel Subunit- Specific Roles in Alternative Cleavage and Polyadenylation. PLoS Genet.
2016;
12
:
e1005794
.
-
B.
Zhu.
The human PAF complex coordinates transcription with events downstream of RNA synthesis. enes & Development.
2005;
19
:
1668-1673
.
Comments
Downloads
Article Details
Volume & Issue : Vol 3 No 05 (2016)
Page No.: 618-624
Published on: 2016-05-27
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 6207 times
- Download PDF downloaded - 1728 times
- View Article downloaded - 7 times
 Biomedpress
Biomedpress