Abstract
Microbes release important metabolites that regulate various physiological activities inside and outside of organisms. The human gastrointestinal tract is a reservoir of microbes that play important regulatory roles in modulating the immune system and numerous other physiological functions. Thus, there is substantial interest in these microbial products and their clinical significance. These microbial metabolites have shown promise as therapies for cancer, inflammation, neurological disorders, and many other diseases. Here, we discuss microbial metabolites with substantial therapeutic potential, including proteasome inhibitors, therapeutic enzymes, bacteriocins, polyamines, and flavonoids.
Introduction
Therapies involving metabolites produced and released by microbes have received substantial attention from the scientific and medicinal community in recent decades. Microbial metabolites are microorganism-produced compounds with potential therapeutic applications. Microbial metabolites are being assessed as novel therapeutic tools for various diseases, including cancer and immune disorders1. The first therapeutic use of microbes to treat infection occurred during World War II after Alexander Fleming isolated penicillin in 19282. Currently, researchers are using different biological and chemical methods to study the biological effects of microbial metabolites on humans3. There is a growing demand for using substances generated from microorganisms in medicine, agriculture, the food industry, and scientific research4. Developing anticancer drugs with reduced side effects from the microbiome has been a research priority for many years. Researchers have discovered that natural products can help treat cancer, illness, infection, allergy, and many other diseases5, demonstrating that microorganisms are viable sources of therapeutics. Microbes associated with the human body impact pathophysiological processes, including metabolic disorders, mental disorders, and even cancer6. Actinomycetes produce 0.1% of known microbial secondary metabolites, Bacillus produce 7.0% and other bacteria produce 1–2%7. Other diseases, such as tuberculosis (TB), have long been treated using natural remedies derived from microbial secondary metabolites8. Currently, four drugs, isoniazid, rifampin, pyrazinamide, and streptomycin, are used to treat TB. The World Health Organization (WHO) plan for TB helps patients limit its spread worldwide. Microbial natural products benefit patients, avoiding injections and acting as alternatives to synthetic drugs and other regular therapies9. Microbial amino acids have been utilized in nutritional supplements and food for humans and animals. It is promising and economically advantageous to produce essential amino acids on an industrial scale using microbial metabolites10. Piericidins are a large class of microbial metabolites commonly formed by species of the genus Streptomyces and comprise a 4-pyridinol core skeleton with a methylated polyketide side chain11. Piericidin application has been developed over time. Streptomyces broth culture was screened in 1993 to assess the antitumor effects of piericidin as a novel phosphatidylinositol turnover inhibitor. Piericidins have been isolated from soil, water, and insect samples12. Here we summarize the main types of microbial metabolites that exhibit different therapeutic properties and can be potentially of clinical and therapeutic use. These metabolites are either secreted by microbes outside of the body or associated with the microbiome.
Biomimicry of Microbial Metabolites
The word biomimicry is derived from the Greek bios, meaning life, and mimesis, meaning imitation. Biomimicry approaches are frequently used in drug discovery. The novel approach of microbial metabolite imitation can increase the chemical repertoire of future pharmaceuticals. In medicine, biomimicry involves the development of homologs of host-endogenous molecules that target specific receptors and provide a desirable result13. The future development of pharmaceuticals will likely be broadened by the use of microbial metabolites that imitate promiscuous ligand–receptor interactions. Xenobiotic nuclear receptors, such as PXR and AhR, are prototypical host receptors with weak ligand interactions. Microbial metabolite mimicry using PXR and AhR as model xenobiotic receptors has been found to result in powerful and non-toxic treatments, mediating pathophysiological disorders involving these receptors. It is also plausible that these weaker receptor-ligand interactions have evolved to the host's benefit to avoid receptor overstimulation, which may have certain negative effects. Additionally, not all microbial metabolites are advantageous; some promote inflammation and cancer development13. Microbial metabolite mimics with distinct antibacterial effect can be tested against intestinal bacteria in vitro or in consortia-inoculated germ-free animals to explore diversity control as a method for host disease control. Thus, mimicry enables the diversification of the microbiome and the upkeep of host health homeostasis by increasing the metabolite repertoire14. Kaempferol, a natural flavanol, has anti-arthritis properties, among other pharmacological effects. Intraperitoneal (20 mg kg-1 d-1) and intragastric (200 mg kg-1 d-1) kaempferol delivery has been assessed for efficacy and mechanistic action in collagen-induced arthritis (CIA) mice. Kaempferol retained in the gastrointestinal tract diversified the microbiota. These findings support the idea that microbiome diversity contributes to the therapeutic effect. Kaempferol mimics with strong microbial remodeling capabilities can be used for arthritis therapy15. Intestinal microorganisms may produce indole/indole-3-propionic acid, which, when activated by PXR, downregulates the TLR4-NF-B inflammatory pathway in mice; an indole/indole-3-propionic acid small-molecule mimic of PXR enhance receptor activation. Unlike other PXR xenobiotics, the small-molecules FKK5 and FKK6 mimic the natural indole metabolites, avoiding toxic effects16.
The path toward using microbial metabolites as potential therapeutics
Humans have used numerous conventional drugs formulated from herbs, fungi, and synthetic chemistry as pharmaceuticals for centuries16. These products have effectively cured ailments such as cancer17. However, due to the limitations of past pharmaceuticals in curing and treating novel medical problems, drug and pharmaceutical development has grown exponentially in the past five decades, replacing older drugs to eliminate severe side effects and improve efficacy. Scientists and researchers have become more interested in microbes and their products, and the positive findings in clinical studies evaluating microbial products have led companies and agencies to opt for these strategies in novel drug development18. Many strategies, such as metal-based compounds from microbes, enzymes, and proteasomes, have been targeted for their clinical and therapeutic significance (Table 1).
Microbial proteasome inhibitors
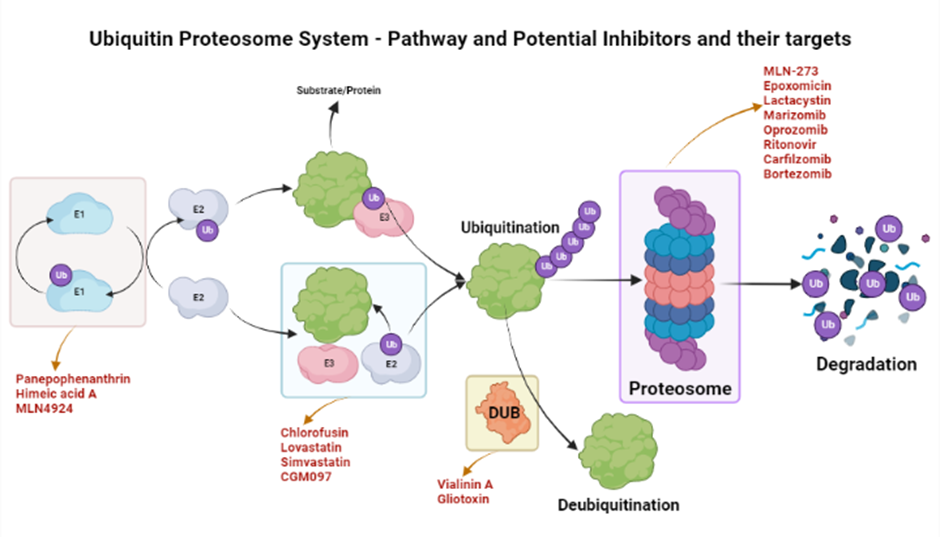
Proteosomes are the regulatory machinery that regulates the homeostatic conditions of the body by removing and degrading regulatory proteins. Many of these proteins play a substantial role in developing immunity against pathogens19, 20. A proteosome of the TB-causing bacteria Mycobacterium tuberculosis was targeted by two small drugs, namely, MMV019838 and MMV687146. These drugs showed promise for curing TB and achieved better outcomes in in silico tests21. Proteosomes are Ntn-hydrolases (N-terminal nucleophiles) that require ATP to cleave amide bonds22, 23. Regulatory proteins such as CDK inhibitors, cyclins, and tumor suppressors are extremely vulnerable to this machinery if demonstrating abnormalities in structure or function. Proteosome Inhibitors (PIs) target proteosomes and affect their functionality19. Biophysical parameters, such as the local concentration of proteins and their binding affinities, are critical for the PI efficacy24. PIs enhance immune responses and control the growth of cancerous cells by preventing proteosomes from removing regulatory proteins19. PI development was initiated approximately fifteen years ago25; due to their immunosuppressive effects, PIs have demonstrated substantial potential in the development of drugs for inflammation, carcinoma, immune disorders, and muscular dystrophies25, 26. PIs have demonstrated particular efficacy for hematological malignancies, multiple myeloma, and multiple cell lymphoma27.
The ubiquinone–proteosome system (UPS) is a major protein turnover regulator in mammalian cells and can be effectively targeted by PIs to cure malignancies (Figure 1)28. Fellutamides are UPS inhibitors produced by Penicillium spp. and Aspergillus spp. in the gastrointestinal (GI) tract of Apogon endekataenia, a marine fish. Fellutamides are potently cytotoxic against in vitro a wide range of cancer cells, including sarcoma cells, fibroblasts, solid tumor cells, and human epidermoid carcinoma KB cells29. The mechanisms by which UPS functions within protein systems are shown in Figure 1.
Lactacystin, synthesized by Streptomyces actinobacterium 30, was the first-in-class PI discovered and incorporated into clinical research as a therapeutic31. Lactacystin effectively treats many diseases in animal models and is being evaluated in clinical trials. A rat model study used lactacystin to target UPS to address synaptic plasticity in Alzheimer’s disease (AD), successfully restoring synaptic tagging and capture (STC) and impairing activity-dependent synaptic plasticity in vitro and associative long-term memory in vivo. Thus, lactacystin is a potential therapy for AD32.
Belactosin (β-Lactone), mainly extracted from Streptococcus spp., is a PI with antitumor properties and potential bioactivity against viruses and bacteria. Marizomib, a naturally occurring β-Lactone extracted from the Salinispora tropica, has shown clinical and preclinical improvements in multiple myeloma33, 34.
| Sr. | Metabolite | Microbial Specie | Molecular targets | Clinical Significance | References |
|---|---|---|---|---|---|
| 1 | Prodigiosin | Serratia marcescens | Herpes simplex virus | (PG) is a natural red pigment secondary metabolite. That exhibit cellular targets altering apoptosis and proapoptotic anticancer effects | 35 |
| 2 | Serrawettin | Serratia marcescens | Methicillin-resistant Staphylococcus aureus (MRSA) | Serrawettin, which was isolated from the green potato rhizosphere, has potent antifungal properties. According to reports, serratia produce antibacterial chemicals and secondary metabolites such as the red pigment prodigiosin. | 36 |
| 3 | Ethyl acetate extract | Pseudoalteromonas rubra, Virgibacillus salaries | Nocardiopsis dassonvillei | The ethyl acetate was used to extract the active compounds that were tested for bacterial growth inhibitory activity against human clinical pathogens. The EA extract was prepared as described and analysed for its content of triptolide and tripdiolide, which are responsible for up to 90% of the bioactiviA extract. | 37 |
| 4 | Aminoglycosides (S-137-R) | Bacillus velezensis | Plasmid-mediated quinolone resistance | Aminoglycosides (S-137-R) used for the treatment of severe Gram-negative bacterial infections. Streptococcal and enterococcal endocarditis can be treated with some treatments for severe Pseudomonas aeruginosa infections, brucellosis, and in low dosages as a synergistic approach. | 38 |
| 5 | Methanolic pigment extract | Micrococcus sp. | Canthaxanthin (4′,4′-diketo-13-carotene) | It is still being done to screen bioactive substances to uncover new chemical structures for the methanolic pigment extract utilized in pharmaceuticals. Some substances have been developed as antibiotics, and they are essential for the survival and growth of microorganisms in bacterial populations or the ability to withstand nutritional stressors. | 39 |
| 6 | Germicidins, c-Actinorhodin | Streptomyces lanatus | Pyricularia oryzae | Some Streptomyces strains produce germicidins, which serve as autoregulators of spore germination. | 40 |
| 7 | Juglomycin A | Streptomyces achromogenes E91CS4 | Streptozotocin | Juglomycins have bactericidal action against both Gram-positive and Gram-negative bacteria, as well as anticancer activity. There have been a few reports of racemic juglomycin syntheses | 41 |
| 8 | Acyl depsipeptide (ADEP) | Streptomyces hawaiiensis | ClpP serine protease | The caseinolytic protease (ClpP protease), the proteolytic centre of bacterial ATP-dependent proteases, was discovered to be the target of acyldepsipeptides (ADEPs), a new class of antibacterial chemical and its derivative. Treatment with ADEP lengthened Leptospira and slowed its growth kinetics. | 42 |
| 9 | Lipopeptide lipid 430 | Algibacter sp. M09B557 and M09B04 | Chloropid, Bacteroidetes | Human TLR2 transfected human embryonic kidney cells were triggered by Lipopeptide lipid 430, which also caused wild-type mice's blood CCL2 (MCP-1) levels to rise. | 43 |
Microbial therapeutic enzymes
Enzymes have long been used in industry and were initially explored in the context of medical therapy in the 1950s, altering our perception of medicinal drugs and therapeutics. Due to their anti-inflammatory and anticancer effects, enzymes have been developed for several ailments, including cancer, AD, and hyperuricemia44.
Inflammation is an immune response mostly characterized by swelling at the site of homeostatic disturbance due to environmental agents such as pathogens, chemicals, or abrasions45. Conventional drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs), have been used to overcome inflammation; however, such drugs are associated with several side effects, such as GI ulcers. Trends have shifted toward the use of microbial enzymes to overcome these problems, for example, serratiopeptidase, which has high efficacy in inflammation and almost no harmful effects46. In a study on bowel disease in mice, the gut microbiota schistosome-derived enzyme P28GST (28 kDa glutathione S-transferase) showed promising anti-inflammatory results in the colon, restoring the regulatory responses between T-helper 1 and T-helper 2 cells47.
Serratiopeptidase is produced by the gram-negative bacterium Serratia marcescens. Serratiopeptidase is a protease in the trypsin family and demonstrates highly anti-inflammatory proteolytic activity48, 49. Serratiopeptidase restores wound sites through an unusual mode of action, recruiting immune cells from the lymph nodes to the affected area to promote healing50. The efficacy of serratiopeptidase increases exponentially when used in combination with other drugs, such as NSAIDs48.
Another protease enzyme, collagenase, hydrolyzes collagen fibers50. Collagenase was used in enzymatic wound debridement prior to the discovery of its anti-inflammatory properties. Das et al. assessed the anti-inflammatory application of collagenase in mice in 2018. Wound-healing macrophages loaded with collagenase santyl ointment (CSO) were implanted into the mice, and anti-inflammatory cytokine production was increased in the CSO-treated groups, improving inflammatory wound healing51. Similarly, the suppression of pro-inflammatory cytokines was observed when simvastatin-loaded porous microspheres were injected into the tendons of the collagenase-induced Achilles tendinitis rats. The production of anti-inflammatory cytokines also increased52. Numerous other studies on the collagenase produced by Clostridium histolyticum have revealed that it is healthy, noninvasive, and safe in treating several pathological conditions53.
Superoxide dismutase is another clinically significant anti-inflammatory agent that can be derived from Cyanobacteria, such as Anabaena cylindrica, Plectonema borynarum, Nostoc commune, and Microcystis aerunginosa50.
L-asparaginase is a microbe-derived enzyme that can play an important role in the cure of cancer. Numerous molecules influence the proliferation of cancer cells and downregulate their growth50. L-asparaginase can be derived from Escherichia coli, Leucosporidium muscorum, Aspergillus terreus, Yersinia pseudotuberculosis, and Pseudomonas otitidis54, 55. L-asparaginase generates ammonia and aspartic acid by breaking down L-asparagine, which is the driver of its anti-cancerous ability. L-asparaginase is effective in treating lymphoblastic leukemia55.
Bacteriocins are the chemicals bacteria produce to eliminate competitors from ecological niches56 and are generally used in intra-specific interactions, for example, in competition for food and shelter57. Some bacteriocidins demonstrate anticancer activity with selective toxicity toward cancer cells58. Many bacteriocin molecules, such as Nisin A derived from Lactococcus lactis, are effective in head and neck squamous cell carcinoma (HNSCC) through inducing cell cycle arrest and blocking cell division59. Bacteriocins have also been found effective against breast cancer60. Bivocin H5C derived from Streptococcus bovis, Laterosporulin 10 derived from Brevibacillus sp, and Colicins A and E1 extracted from Escherichia coli also have antitumor properties61.
Arginine deiminase is an enzyme produced and secreted by Mycoplasma spp., such as M. hominis, M. arginine, and Pseudomonas furukawaii 61. Arginine has shown encouraging antitumor and anticancer properties and can play a key role in the cure and treatment of numerous auxotrophic carcinomas through arginine depletion62. The absence of arginosuccinate synthetase 1 (ASS1) in most cancers is the key aspect of the functionality of ADI (arginine deiminase). ASSI is critical for L-arginine production; cancer cells take up L-arginine for growth. This dependence of the cells on external sources can be pivotal in the anti-cancerous activity of arginine deiminases63.
Microbial antibiotic resistance is rising; thus, there the development of novel therapies is urgent64, 65. Antibacterial drugs and therapeutics are a prominent focus for handling this increasing challenge and controlling human and animal pathogens57. Several strategies and therapeutics have been developed, such as bacteriocins and lysin enzymes, referred to as enzybiotics. Enzybiotics are microbial enzymes and products that hinder the growth of pathogenic bacteria. Since their discovery in 2001, enzybiotics have played a significant role in the development of microbial based antibacterial drugs to combat antibiotic resistance44.
Bacteriocins are the secondary metabolites bacteria produce to target competitors, hindering the growth and reproduction of other microbes66. Bacteriocins are antimicrobial peptides (AMPs) 12–100 amino acids in length67. AMPs have recently been found effective against human microbial pathogens derived from lactic acid bacteria (LAB), Lactobacillus 68. Bacteriocins have shown great efficacy towards several microbe-borne diseases, including methicillin-resistant Streptococcus aureus (MRSA)69. LABs have been successfully employed in managing MRSA in clinical trials70. Another bacterial-produced TFnt, lysostaphin, is a bacteriocin that has demonstrated encouraging bactericidal activity against mutant Staphylococcus aureus strains71.
Endolysins are hydrolases that prevent the formation of the cell wall, cell membrane, envelope, and biofilm around antibiotic resistant strains of bacteria by lysing the peptidoglycan layer44, 72. Different endolysin enzymes have different mechanisms and target sites in Gram-positive and Gram-negative bacteria44. Many endolysins have shown efficacy in clinical trials. LysK-like endolysin derived from Staphylococcus spp. and LysSAP33 encoded by bacteriophage SAP33 have substantial lytic activity against the antibiotic-resistant Staphylococcus aureus, targeting biofilm formation73. Similarly, many endolysins, namely, Abtn-4, derived from a bacteriophage D2 (vB_AbaP_D2), have shown substantial therapeutic potential, preventing biofilm formation by multiple phage-resistant Gram-positive and Gram-negative bacteria (Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterococcus, and Salmonella). The aforementioned findings highlight the therapeutic potential of this endolysin74. Another important endolysin, LysAB54, was extracted from the phage p54 of the multi-drug resistant Acinetobacter baumannii and showed promising antibacterial activity this and other gram-negative bacteria, such as Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli75.
Other metabolites
Numerous other gut microbiome-derived metabolites also have immense therapeutic potential76. These metabolites play a substantial role in living organisms' biological machinery, influencing physiological, pathological, and metabolic activities and signaling pathways77. Gut microbial metabolites, such as short-chain fatty acids (SCFA) and many other organic compounds, have shown encouraging anticancer, brain modulation, anti-obesity, anti-bacterial, neurological, and mineral absorption properties78. These metabolites are very effective, especially for neurological diseases, including ASD, PD, AD, and neuroinflammation79, 80. Some other metabolites, such as TMA and TMAO, are also effective for cardiovascular diseases, including heart failure (72), hypertension81, and atherosclerosis82. Understanding the mechanisms of action of these metabolites will open doors for new therapeutic approaches81. Other such metabolites include TMAO, tryptophan, indoles, 4-ethylphenylesulfate, fatty acids, and protein-derived metabolites.
Protein-derived Metabolites
Protein-derived metabolites are functional, covalent protein modifications produced during or after protein digestion through endogenous, intrinsically reactive metabolites without the aid of enzymes83. Many protein-derived metabolites have been shown to be therapeutic. Taurine is a sulfur-containing β-amino acid and can be generated from cystine metabolism84, 85. Taurine has many important functions in animals' nervous system, muscles, kidneys, cardiovascular system, and immune system because of its anti-inflammatory and anti-oxidative effects85, 86. Taurine plays a vital role in maintaining homeostasis and many other physiological activities, such as gene expression and energy metabolism87, 88. Traumatic brain injury (TBI) is lethal, and inflammatory responses can result in death in other traumatic conditions. Taurine was administered to 32 patients with TBI conditions along with Standard Entera Meal. The levels of the inflammation biomarkers IL-6, IL-10, and TNF-α were recorded before and after the treatment, and serum IL-6 levels decreased significantly, showing the encouraging anti-inflammatory properties of taurine and its potential clinical use89. Taurolidine (TRD) is a taurine N-methylol derivative that exerts bactericidal and anti-inflammatory effects by inhibiting proinflammatory cytokines, such as IL-6, IL-8, and IL-1β90.
Imidazole propionate is another protein-derived microbial metabolite produced in the gut microbiome through histidine metabolism and is closely linked with glucose metabolism91, 92. Imidazole propionate disrupts glucose metabolism and causes several diseases, including type 2 diabetes and hypertension93, 94. T2D has been found to be closely associated with elevated imidazole propionate, which alters histidine metabolism and impairs glucose metabolism and insulin signaling by activating Mtor1, p38γ, and S6K1 signaling92. Imidazole propionate is a potential activator of p38γ map kinase, inhibiting metformin activity95, and is associated with increased pro-inflammatory cytokines that cause gastrointestinal inflammation and inflammatory bowel disease93.
Microbiota-associated polyamines are organic cations produced by the gut microbiota organisms, such as Clostridia96. These polyamines are derived from arginine-containing proteins97 and are involved in numerous metabolic and cellular activities, including cell growth and differentiation and the production of DNA, RNA, and several proteins96. Many polyamines are also uremic toxins that hasten the progression of renal fibrosis and uremia98. However, polyamines such as spermine can suppress inflammatory cytokine production and regulate NF-κB activation96. Spermine is also associated with prostate cancer (PCa); significantly decreased levels of spermine have been observed in PCa patients. Spermine levels are a marker of PCa, and understanding the mechanism of action of spermine may lead to its use as a therapeutic target99. Another polyamine, putrescine, is naturally produced in peri-ovulating women and decreased in women with age-related infertility, demonstrating the role of putrescine in ova production and its potential for improving ova quality as a treatment for infertile women of advanced maternal age100.
Flavonoids
Flavonoids are plant-based nutritional constituents with substantial health benefits101. Certain flavonoids may have hepatoprotective characteristics that reduce the risk of coronary heart disease102. Moreover, these flavonoids have anti-inflammatory and anticancer properties103. Flavonoids demonstrate structural and functional effects through enzyme inhibition, acting as antioxidants, damaging cells, triggering host defense mechanisms, and blocking virus cell attachment and penetration103, 104. As secondary plant metabolites, flavonoids constitute the majority of the non-energetic aspect of human nutrition. Most dietary flavonoids are O-glycosides and are primarily consumed as D-glucose; fermented foodstuffs such as wine, tempeh, and certain teas also contain flavonoids. The polarity of the flavonoid molecule increases with glycosylation, which is necessary for preservation in plant cell vacuoles105. Similarly, many GI tract microbial metabolites may be responsible for or contribute to quercetin's effect when taken orally since the flavonoid metabolites DHPA and 4-methylcatechol reduce arterial blood pressure106. The absorption, distribution, digestion, and elimination of flavonoids by the GI tract are critical processes affecting human health107. These features are influenced by how flavonoids interact with other dietary components, the host, the environment, and GI flora108. Flavonoids can target the microbiome, various GI tract cell types, and compounds present in luminal material107, 108, 109.
Bile acids
Bile acids are microbiological byproducts with potential therapeutic applications. The gut microbiota produces bile acids, which are crucial for dietary lipid solubilization110. The gut microbiota facilitates digestion and has been linked to diseases and health risks. Despite the considerable variation in gut microbiota composition, it is challenging to link intestinal microbiota patterns to health and nutrition because the gut microbiota has a high level of functional redundancy. Metabolomics has successfully identified gut-produced microbial metabolites that may be significant mediators of diet-induced host–microbial interaction in several studies. Among the metabolites derived from nutrition are short-chain fatty acids, secondary bile acids inferred from primary bile acids, microbial tryptophan catabolites that originate from proteolysis, imidazole propionate originating from histidine, and trimethylamine N-oxide111, 112. Bile acids can potentially be used to treat non-alcoholic fatty liver disease (NAFLD), cholestatic and metabolic liver diseases, infant jaundice, and other liver problems113, 114, 115, 116. NAFLD is the most frequent chronic liver disease worldwide115. The link between the gut microbiota & NAFLD has been widely researched. The gut microbiota controls NAFLD by fermenting undigested foodstuff, interacting with intestinal mucosal immune system, and altering intestinal barrier function, leading to signaling changes117. Microbial metabolites, including SCFAs, TMAO, BAs, endogenous ethanol, and indole, play important roles in NAFLD regulation. However, changes in microbial metabolites in NAFLD are undeniable. Microbial metabolites impact the signaling pathway in the stomach and the liver, which is distant from the gut117, 118. Microbes, in part, control the host's immune system by generating metabolites. An increasing body of research suggests that some microbial metabolites affect the immune system significantly through host receptors and other target molecules. P2X7, GPR41, GPR43, GPR109A, aryl hydrocarbon receptor precursor (AhR), farnesoid X receptor (FXR), PXR, and TGR5 are some of the metabolite-specific receptors expressed by immune cells119, 120, 121. Changes in food, bodily functions, and the immune system produce a range of signals from microbial metabolites and their receptors. Gut bacteria produce many lipid-modifying and metabolizing enzymes. For instance, polyunsaturated fatty acids are transformed into hydroxy fatty acids by gut bacteria such as Lactobacillus plantarum, which also encodes the enzymes that saturate polyunsaturated fatty acids122.
Polyamines
Polyamines are polycationic compounds with over two amino groups that are biosynthesized from ornithine and arginine. Polyamines, primarily putrescine, spermidine, and spermine, are abundant in the digestive system and are derived from food or biosynthesized by the host and bacteria. Polyamines are chiefly produced within the host by arginase 1 (which converts l-arginine to l-ornithine), ornithine decarboxylase (ODC), which metabolizes ornithine to putrescine, and enzymes that catalysis spermidine, putrescine, and spermine interconversion. In contrary to host polyamine metabolism, bacteria create polyamines using constitutive or inducible amino acid decarboxylases. Polyamines play an important role in cell proliferation, immune system activation, and cell differentiation. Polyamines are crucial during the cell development; a low content of polyamines inside the cell has been associated with cell growth abnormalities. Increased amounts of polyamines are needed by tumor cells relative to normal cells to sustain fast development; increased polyamine concentrations are observed in blood/urine samples from cancer patients relative to those in samples from healthy and normal individuals. Dysregulation of polyamine metabolism in the host or gut bacteria potentially contributes to colorectal cancer (CRC)123. Polyamines in the gut includes cadaverine, putrescine, and spermidine, although bacteria can produce other polyamines. Polyamine pathway enzymes have been found in various organisms. However, only a few species have been functionally characterized in terms of polyamine production. Polyamines are produced, accumulated, or required/used by Staphylococcus aureus, Haemophilus influenzae, E. coli, Enterococcus faecalis, Neisseria flava, and Vibrio cholera. Cadaverine is a lysine decarboxylation product produced by the bacterium enzymes LdcC and CadA. Cadaverine can be produced by humans and microorganisms. Cadaverine biosynthetic enzymes are also found in Streptococcus and Escherichia coli. Polyamine metabolism is dysregulated in pancreatic adenocarcinoma. The fact that modification of the polyamine cycle can amplify or alleviate the effects of traditional cytostatic therapy emphasizes the functional importance of polyamine production in (human) pancreatic cancer124.
Tri-methylamine N-Oxide
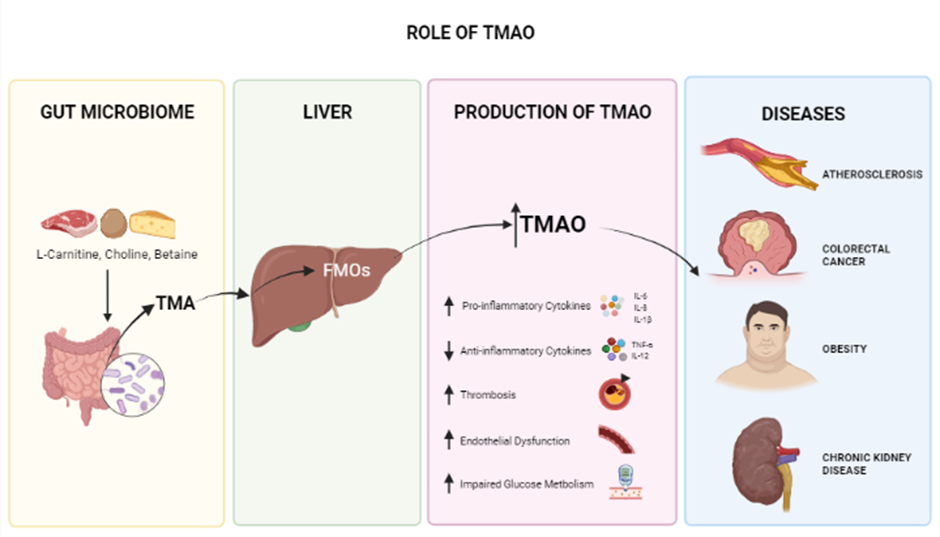
Trimethylamine N-Oxide is an osmolyte and a metabolite produced in the gut microbiome and is generated from dietary choline, betaine, and L-carnitine125, 126 crucial for stabilizing protein structures in the presence of urea126. Several studies have shown that high circulating blood levels of TMAO are associated with the development of many chronic diseases, including cardiovascular diseases (CVD), atherosclerosis127, obesity (potentially)128, diabetes, colorectal cancer129, acute kidney disease (AKD) (Figure 2)130, and neurological disorders involving synaptic plasticity131, 132. Since TMAO plays a significant role in heart failure (HF) pathogenesis, one of several cardiovascular diseases (CVDs) caused by vascular inflammation, it can serve as an early and timely prognostic biomarker before HF becomes critical81, 133, 134. This inflammatory response associated with TMAO is also seen in conditions such as psoriatic arthritis (PsA). In a study conducted on 38 PsA patients, TMAO and other chemicals, such as Trimethylamine (TMA), choline, and carnitine, were found to be involved in inflammation. Thus targeting TMAO is a potential treatment for PsA135. In another in vitro study of vascular smooth muscle cells (VSMC) from rats and humans, TMAO was found to play a substantial role in the development of inflammatory responses; the Nox4-PRMT5-VCAM-1 pathway was revealed as a potential target for resolving this condition136. Recently, a study of 48 patients with preeclampsia (PE) revealed, by analyzing fecal matter plasma lipopolysaccharide (LPS) and Trimethylamine N-Oxide (TMAO) and in comparison with healthy controls, that elevated LPS and TMAO are present in PE patients137.
Fatty Acids
Short-chain fatty acids are the fermentation products of some gut-microbiome bacteria produced from non-digestible carbohydrates (NDCs)138. The most common SCFA-producing bacteria species are Anaerostipes caccae, Bacteroides eggerthii, and Clostridial spp.139. Therapeutically, SCFAs are important because have substantial anti-obesity, anti-inflammatory, and anti-diabetic potential140. Numerous SCFAs, such as valproic acid, acetate, butyrate, and propionate, hold immense therapeutic potential. Valproic acid (VPA) is an anti-convulsant and anti-epileptic SCFA with applications in many neurological disorders, including autism, epilepsy, migraine, and bipolar disorder141, 142. VPA also has anticancer activities143. VPA (3 μM) in combination with arsenic trioxide (ATO) (3 μM) showed synergistic anticancer effects in NCI-H460 and NCI-H1299 lung cancer cells in vivo and in vitro141. VPA has also shown antioxidant, anti-inflammatory, and antilipidemic properties in a female mouse model with type 1 diabetes. Wistar Rats with type 2 diabetes have also been treated with VPA (100, 300, and 600 mg/kg body weight) and metformin (100 mg/kg body weight), revealing anti-diabetic and pro-antioxidant effects144. Butyrate, another very important SCFA metabolite, is synthesized by gut microbiota and recognized as a very critical mediator in the regulation of whole-body energy metabolism145, particularly functioning as an important nutrient source for colonocytes, influencing their differentiation and growth146. Decreased butyrate levels have been observed in patients with ulcerative colitis (UC), leading to inflammation in the intestinal mucosa. Later it was discovered that butyrate downregulates the expression of genes involved in the inflammatory pathways147.
Long chain fatty acids (LCFA) are also therapeutically important. Omega-3 fatty acid (O3FA) is an important long-chain fatty acid not synthesized by the body. It is of great therapeutic interest due to its anti-inflammatory148, antineoplastic149, antithrombotic, insulin resistance, antiarrhythmic150, neuroprotective151, and immunomodulatory152 effects. Eicosatetraenoic acid (EPA) is one of the two main types of O3FA. It has the potential to control the signs and symptoms of depression. Previously, the brain was thought to contain very little or no EPA; however, EPA was recently found to penetrate the blood-brain barrier, where it is immediately esterified into phospholipids; thus, EPA does not build up in the brain and is instead found in at low levels in microglia. Randomized clinical trials are required to demonstrate the therapeutic potential of EPA in combatting major depression153. Recent studies have also demonstrated that EPA is essential for normal brain function. In controlled trials conducted on 92 children (aged 6–12) with attention deficit hyperactivity disorder (ADHD), in comparison with the placebo, high doses of EPA (1.2 g) improved overall focused attention and vigilance. Thus, EPA treatment may improve cognitive symptoms in ADHD-affected youth154. EPA has also shown anti-inflammatory effects155.
Conjugated linoleic acid (CLA) is another naturally occurring LCFA with substantial therapeutic potential due to its anti-carcinogenic, anti-atherosclerosis, and anti-obesogenic properties156. The therapeutic potential of CLA in cancer has been demonstrated in several animal and cellular models studies; clinical trials of CLA for breast cancer and prostate cancer have also been performed157.
4-Ethylephenylesulfate (4-EPS)
4-Ethylephenylesulfate (4-EPS) is a gut-microbiome-derived metabolite considered a uremic toxin158, 159. Elevated levels of 4-EPS have been observed during the atypical neurodevelopment of neuronal tissues in mice. Similarly, 4-EPS entered the brain in another mouse study and induced unusual brain activity and behaviors. In ex vivo culture, this disturbance of brain activity and behavior patterns was due to the decreased interaction between neuronal cells and oligodendrocytes due to impaired maturation. This impaired activity in mice could be cured by the pharmacological differentiation of oligodendrocytes, demonstrating the toxic effects of 4-EPS on behavior. These gut–brain interactions due to metabolites can be a potential target in treating complex behavior diseases including, autism spectrum disorder (ASD)76, 159. Similarly, higher levels of 4-EPS have been reported in the maternal immune activation (MIA) ASD mouse model, and this increased level of 4-EPS was controlled by the administration of Bacteroides fragilis. Axial Biotherapeutics also observed similar elevated concentrations in children with ASD160.
Conclusion
Numerous studies demonstrate the broad potential of microbial metabolites in the treatment of multiple diseases, especially those for which conventional therapies have failed; thus, these metabolites may represent the future of the drug and pharmaceutical industries. The broader efficacy of microbial metabolite therapies remains debatable, and more studies and clinical trials are required. However, previous small-scale studies indicate these therapies are potentially highly effective. Further investigation into microbial metabolites will allow their use to become more common beyond clinical trials. The resulting treatments will likely be comfortably incorporated into approaches for various diseases.
Abbreviations
AD: Alzheimer's Disease, ADHD: Attention Deficit Hyperactivity Disorder, ADI: Arginine Deiminase I, AKD: Acute Kidney Disease, AMPs: Antimicrobial Peptides, ASD: Autism Spectrum Disorder, ASSI: Arginosuccinate Synthetase, ATO: Arsenic Trioxide, CIA: Collagen-Induced Arthritis, CRC: Colorectal Cancer, CSO: Collagenase Santyl Ointment, CVD: Cardiovascular Diseases, EPA: Eicosatetraenoic Acid, EPS: Ethylephenylesulfate, FXR: Farnesoid X Receptor, GIT: Gastro-Intestinal Tract, GST: Glutathione S-Transferase, HF: Heart Failure, HNSCC: Head And Neck Squamous Cell Carcinoma, LAB: Lactic Acid Bacteria, LCFA: Long Chain Fatty Acids, LPS: Lipopolysaccharide, MIA: Maternal Immune Activation, MRSA: Methicillin Resistant Streptococcus Aureus, NAFLD: Non-Alcoholic Fatty Liver Disease, NDC: Non-Digestible Carbohydrates, NSAID: Non-Steroidal Anti-Inflammatory Drugs, ODC: Ornithine Decarboxylase, Pca: Prostate Cancer, PE: Preeclampsia, Pis: Proteosome Inhibitors, PXR: Pregnane X Receptors, SCFA: Short-Chain Fatty Acids, STC: Synaptic Tagging and Capture, TB: TuberculosisTMAO: Trimethylamine N-Oxide, TRD: Taurolidine, UC: Ulcerative Colitis, UPS: Ubiquinone-Proteosome System, VPA: Valproic Acid, VSMC: Vascular Smooth Muscle Cells, WHO: World Health Organization
Acknowledgments
The authors are thankful to the Vice Chancellor, University of Narowal, Narowal, Pakistan for providing the support for the accomplishment of this manuscript.
Author’s contributions
Muhammad Babar Khawar, Muddasir Hassan Abbasi, And Nadeem Sheikh contributed to the study conception and design. Literature Search, data collection and visualization were performed by Ali Afzal, Muhammad Abu Talha Safdar Hashmi, Rimsha Naseem, Nayab Shahid, Adil Farooq, Maryam Mukhtar, Muhammad Ahsan Ashraf, and Syeda Eisha Hamid. The first draft of the manuscript was written by Muhammad Abu Talha Safdar Hashmi, Rimsha Naseem, Nayab Shahid, Rabia Mehmood, and Ali Afzal. Sara Shahzaman, Syeda Eisha Hamid, Muhammad Idnan and Ume Habiba revised the final version of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Luu
M.,
Visekruna
A.,
Microbial metabolites: novel therapeutic tools for boosting cancer therapies. Trends in Cell Biology.
2021;
31
(11)
:
873-5
.
View Article PubMed Google Scholar -
Tan
S.Y.,
Tatsumura
Y.,
Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Medical Journal.
2015;
56
(7)
:
366-7
.
View Article PubMed Google Scholar -
Abdelghani
Z.,
Hourani
N.,
Zaidan
Z.,
Dbaibo
G.,
Mrad
M.,
Hage-Sleiman
R.,
Therapeutic applications and biological activities of bacterial bioactive extracts. Archives of Microbiology.
2021;
203
(8)
:
4755-76
.
View Article PubMed Google Scholar -
Danovaro
R.,
Corinaldesi
C.,
Dell'Anno
A.,
Rastelli
E.,
Potential impact of global climate change on benthic deep-sea microbes. FEMS Microbiology Letters.
2017;
364
(23)
.
View Article PubMed Google Scholar -
Kovács
T.,
Mikó
E.,
Vida
A.,
Seb\Ho
É.,
Toth
J.,
Csonka
T.,
Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Scientific Reports.
2019;
9
(1)
:
1300
.
View Article PubMed Google Scholar -
Maruvada
P.,
Leone
V.,
Kaplan
L.M.,
Chang
E.B.,
The human microbiome and obesity: moving beyond associations. Cell Host & Microbe.
2017;
22
(5)
:
589-99
.
View Article PubMed Google Scholar -
Zitvogel
L.,
Ayyoub
M.,
Routy
B.,
Kroemer
G.,
Microbiome and anticancer immunosurveillance. Cell.
2016;
165
(2)
:
276-87
.
View Article PubMed Google Scholar -
Hussain
A.,
Rather
M.A.,
Bhat
Z.S.,
Majeed
A.,
Maqbool
M.,
Shah
A.M.,
In vitro evaluation of dinactin, a potent microbial metabolite against Mycobacterium tuberculosis. International Journal of Antimicrobial Agents.
2019;
53
(1)
:
49-53
.
View Article PubMed Google Scholar -
Dirlikov
E.,
Raviglione
M.,
Scano
F.,
Global tuberculosis control: toward the 2015 targets and beyond. Annals of Internal Medicine.
2015;
163
(1)
:
52-8
.
View Article PubMed Google Scholar -
Zhou
X.,
Liang
Z.,
Li
K.,
Fang
W.,
Tian
Y.,
Luo
X.,
Exploring the natural piericidins as anti-renal cell carcinoma agents targeting peroxiredoxin 1. Journal of Medicinal Chemistry.
2019;
62
(15)
:
7058-69
.
View Article PubMed Google Scholar -
Azad
S.M.,
Jin
Y.,
Ser
H.L.,
Goh
B.H.,
Lee
L.H.,
Thawai
C.,
Biological insights into the piericidin family of microbial metabolites. Journal of Applied Microbiology.
2022;
132
(2)
:
772-84
.
View Article PubMed Google Scholar -
Engl
T.,
Kroiss
J.,
Kai
M.,
Nechitaylo
T.Y.,
Svatoš
A.,
Kaltenpoth
M.,
Evolutionary stability of antibiotic protection in a defensive symbiosis. Proceedings of the National Academy of Sciences of the United States of America.
2018;
115
(9)
:
2020-9
.
View Article PubMed Google Scholar -
Dvořák
Z.,
Sokol
H.,
Mani
S.,
Drug mimicry: promiscuous receptors PXR and AhR, and microbial metabolite interactions in the intestine. Trends in Pharmacological Sciences.
2020;
41
(12)
:
900-8
.
View Article PubMed Google Scholar -
Haag
L.M.,
Siegmund
B.,
Exploring & exploiting our `other self' - does the microbiota hold the key to the future therapy in Crohn's?. Best Practice & Research. Clinical Gastroenterology.
2014;
28
(3)
:
399-409
.
View Article PubMed Google Scholar -
Aa
L.X.,
Fei
F.,
Qi
Q.,
Sun
R.B.,
Gu
S.H.,
Di
Z.Z.,
Rebalancing of the gut flora and microbial metabolism is responsible for the anti-arthritis effect of kaempferol. Acta Pharmacologica Sinica.
2020;
41
(1)
:
73-81
.
View Article PubMed Google Scholar -
Dvorak
Z.,
Klapholz
M.,
Burris
T.P.,
Willing
B.P.,
Gioiello
A.,
Pellicciari
R.,
Weak microbial metabolites: A treasure trove for using biomimicry to discover and optimize drugs. Molecular Pharmacology.
2020;
98
(4)
:
343-9
.
View Article PubMed Google Scholar -
Huang
M.,
Lu
J.J.,
Ding
J.,
Natural products in cancer therapy: past, present and future. Natural Products and Bioprospecting.
2021;
11
(1)
:
5-13
.
View Article PubMed Google Scholar -
Puebla-Barragan
S.,
Reid
G.,
Forty-five-year evolution of probiotic therapy. Microbial Cell.
2019;
6
(4)
:
184-96
.
View Article PubMed Google Scholar -
Momose
I.,
Kawada
M.,
The therapeutic potential of microbial proteasome inhibitors. International Immunopharmacology.
2016;
37
:
23-30
.
View Article PubMed Google Scholar -
Kisselev
A.F.,
Site-specific proteasome inhibitors. Biomolecules.
2021;
12
(1)
:
54
.
View Article PubMed Google Scholar -
Tyagi
R.,
Srivastava
M.,
Jain
P.,
Pandey
R.P.,
Asthana
S.,
Kumar
D.,
Development of potential proteasome inhibitors against Mycobacterium tuberculosis. Journal of Biomolecular Structure & Dynamics.
2022;
40
(5)
:
2189-203
.
View Article PubMed Google Scholar -
Zhang
H.,
Lin
G.,
Microbial proteasomes as drug targets. PLoS Pathogens.
2021;
17
(12)
:
e1010058
.
View Article PubMed Google Scholar -
Artymiuk
P.J.,
A sting in the (N-terminal) tail. Nature Structural Biology.
1995;
2
(12)
:
1035-7
.
View Article PubMed Google Scholar -
Pohl
C.,
Dikic
I.,
Cellular quality control by the ubiquitin-proteasome system and autophagy. Science.
2019;
366
(6467)
:
818-22
.
View Article PubMed Google Scholar -
Génin
E.,
Reboud-Ravaux
M.,
Vidal
J.,
Proteasome inhibitors: recent advances and new perspectives in medicinal chemistry. Current Topics in Medicinal Chemistry.
2010;
10
(3)
:
232-56
.
View Article PubMed Google Scholar -
Lee
H.S.,
Jeong
G.S.,
Salinosporamide A, a marine-derived proteasome inhibitor, inhibits T cell activation through regulating proliferation and the cell cycle. Molecules (Basel, Switzerland).
2020;
25
(21)
:
5031
.
View Article PubMed Google Scholar -
Leonardo-Sousa
C.,
Carvalho
A.N.,
Guedes
R.A.,
Fernandes
P.M.,
Aniceto
N.,
Salvador
J.A.,
Revisiting Proteasome Inhibitors: Molecular Underpinnings of Their Development, Mechanisms of Resistance and Strategies to Overcome Anti-Cancer Drug Resistance. Molecules (Basel, Switzerland).
2022;
27
(7)
:
2201
.
View Article PubMed Google Scholar -
Zhang
X.,
Linder
S.,
Bazzaro
M.,
Drug development targeting the ubiquitin - proteasome system (UPS) for the treatment of human cancers. Cancers (Basel).
2020;
12
(4)
:
902
.
View Article PubMed Google Scholar -
Staszczak
M.,
Fungal Secondary Metabolites as Inhibitors of the Ubiquitin-Proteasome System. International Journal of Molecular Sciences.
2021;
22
(24)
:
13309
.
View Article PubMed Google Scholar -
de Bettignies
G.,
Coux
O.,
Proteasome inhibitors: dozens of molecules and still counting. Biochimie.
2010;
92
(11)
:
1530-45
.
View Article PubMed Google Scholar -
Ōmura
S.,
Crump
A.,
Lactacystin: first-in-class proteasome inhibitor still excelling and an exemplar for future antibiotic research. The Journal of Antibiotics.
2019;
72
(4)
:
189-201
.
View Article PubMed Google Scholar -
Krishna-K
K.,
Baby
N.,
Raghuraman
R.,
Navakkode
S.,
Behnisch
T.,
Sajikumar
S.,
Regulation of aberrant proteasome activity re-establishes plasticity and long-term memory in an animal model of Alzheimer's disease. The FASEB Journal.
2020;
34
(7)
:
9466-79
.
View Article PubMed Google Scholar -
Bazou
D.,
Le
G.,
Boyle
A.,
Blum
A.,
O'Gorman
P.,
Marizomib: A novel therapeutic approach for the treatment of central nervous system myeloma. eJHaem.
2020;
1
(1)
:
315-7
.
View Article PubMed Google Scholar -
Wang
J.,
Shi
Y.,
Jiang
D.,
β-Lactone Derivatives and Their Anticancer Activities: A Short Review. Current Topics in Medicinal Chemistry.
2021;
21
(18)
:
1645-56
.
View Article PubMed Google Scholar -
Karbalaei-Heidari
H.R.,
Partovifar
M.,
Memarpoor-Yazdi
M.,
Evaluation of the bioactive potential of secondary metabolites produced by a new marine micrococcus species isolated from the Persian Gulf. Avicenna Journal of Medical Biotechnology.
2020;
12
(1)
:
61-5
.
PubMed Google Scholar -
Ahmad
T.,
Arora
P.,
Nalli
Y.,
Ali
A.,
Riyaz-Ul-Hassan
S.,
Antibacterial potential of Juglomycin A isolated from Streptomyces achromogenes, an endophyte of Crocus sativus Linn. Journal of Applied Microbiology.
2020;
128
(5)
:
1366-77
.
View Article PubMed Google Scholar -
Dhara
A.,
Hussain
M.S.,
Kanaujia
S.P.,
Kumar
M.,
Acyldepsipeptide activated ClpP1P2 macromolecule of Leptospira, an ideal Achilles' heel to hamper the cell survival and deregulate ClpP proteolytic activity. Research in Microbiology.
2021;
172
(2)
:
103797
.
View Article PubMed Google Scholar -
Olsen
I.,
Nichols
F.C.,
Are sphingolipids and serine dipeptide lipids underestimated virulence factors of Porphyromonas gingivalis?. Infection and Immunity.
2018;
86
(7)
:
e00035-18
.
View Article PubMed Google Scholar -
Pournejati
R.,
Gust
R.,
Karbalaei-Heidari
H.R.,
An aminoglycoside antibacterial substance, S-137-R, produced by newly isolated Bacillus velezensis strain RP137 from the Persian Gulf. Current Microbiology.
2019;
76
(9)
:
1028-37
.
View Article PubMed Google Scholar -
Ramalingam
V.,
Rajaram
R.,
Archunan
G.,
Padmanabhan
P.,
Gulyás
B.,
Structural Characterization, Antimicrobial, Antibiofilm, Antioxidant, Anticancer and Acute Toxicity Properties of N-(2-hydroxyphenyl)-2-phenazinamine From Nocardiopsis exhalans (KP149558). Frontiers in Cellular and Infection Microbiology.
2022;
12
:
794338
.
View Article PubMed Google Scholar -
Hennessy
R.C.,
Dichmann
S.I.,
Martens
H.J.,
Zervas
A.,
Stougaard
P.,
Serratia inhibens sp. nov., a new antifungal species isolated from potato (Solanum tuberosum). International Journal of Systematic and Evolutionary Microbiology.
2020;
70
(7)
:
4204-11
.
View Article PubMed Google Scholar -
Motoyama
T.,
Secondary metabolites of the rice blast fungus Pyricularia oryzae: biosynthesis and biological function. International Journal of Molecular Sciences.
2020;
21
(22)
:
8698
.
View Article PubMed Google Scholar -
Suryawanshi
R.K.,
Koujah
L.,
Patil
C.D.,
Ames
J.M.,
Agelidis
A.,
Yadavalli
T.,
Bacterial pigment prodigiosin demonstrates a unique antiherpesvirus activity that is mediated through inhibition of prosurvival signal transducers. Journal of Virology.
2020;
94
(13)
:
e00251-20
.
View Article PubMed Google Scholar -
Datta
S.,
Rajnish
K.N.,
George Priya Doss
C.,
Melvin Samuel
S.,
Selvarajan
E.,
Zayed
H.,
Enzyme therapy: a forerunner in catalyzing a healthy society?. Expert Opinion on Biological Therapy.
2020;
20
(10)
:
1151-74
.
View Article PubMed Google Scholar -
Germolec
D.R.,
Shipkowski
K.A.,
Frawley
R.P.,
Evans
E.,
Markers of inflammation. Immunotoxicity Testing: Methods and Protocols.
2018;
2018
:
57-79
.
View Article Google Scholar -
El-Abd
M.A.,
Ibrahim
E.A.,
Production and one-step purification of serratiopeptidase enzyme from Serratia marcescens with potent anti-inflammatory and antioxidant power. Egyptian Pharmaceutical Journal..
2020;
19
(3)
:
238
.
View Article Google Scholar -
Foligné
B.,
Plé
C.,
Titécat
M.,
Dendooven
A.,
Pagny
A.,
Daniel
C.,
Contribution of the gut microbiota in P28GST-mediated anti-inflammatory effects: experimental and clinical insights. Cells.
2019;
8
(6)
:
577
.
View Article PubMed Google Scholar -
Jadhav
S.B.,
Shah
N.,
Rathi
A.,
Rathi
V.,
Rathi
A.,
Serratiopeptidase: insights into the therapeutic applications. Biotechnology Reports (Amsterdam, Netherlands).
2020;
28
:
e00544
.
View Article PubMed Google Scholar -
Metkar
S.K.,
Girigoswami
A.,
Vijayashree
R.,
Girigoswami
K.,
Attenuation of subcutaneous insulin induced amyloid mass in vivo using Lumbrokinase and Serratiopeptidase. International Journal of Biological Macromolecules.
2020;
163
:
128-34
.
View Article PubMed Google Scholar -
Vachher
M.,
Sen
A.,
Kapila
R.,
Nigam
A.,
Microbial therapeutic enzymes: A promising area of biopharmaceuticals. Current Research in Biotechnology.
2021;
3
:
195-208
.
View Article Google Scholar -
Das
A.,
Datta
S.,
Roche
E.,
Chaffee
S.,
Jose
E.,
Shi
L.,
Novel mechanisms of Collagenase Santyl Ointment (CSO) in wound macrophage polarization and resolution of wound inflammation. Scientific Reports.
2018;
8
(1)
:
1696
.
View Article PubMed Google Scholar -
Jeong
C.,
Kim
S.E.,
Shim
K.S.,
Kim
H.J.,
Song
M.H.,
Park
K.,
Exploring the in vivo anti-inflammatory actions of simvastatin-loaded porous microspheres on inflamed tenocytes in a collagenase-induced animal model of achilles tendinitis. International Journal of Molecular Sciences.
2018;
19
(3)
:
820
.
View Article PubMed Google Scholar -
Hoy
S.M.,
Collagenase clostridium histolyticum: a review in Peyronie's disease. Clinical Drug Investigation.
2020;
40
(1)
:
83-92
.
View Article PubMed Google Scholar -
Costa-Silva
T.A.,
Flores-Santos
J.C.,
Freire
R.K.,
Vitolo
M.,
Pessoa-Jr
A.,
Microbial cell disruption methods for efficient release of enzyme L-asparaginase. Preparative Biochemistry & Biotechnology.
2018;
48
(8)
:
707-17
.
View Article PubMed Google Scholar -
Muneer
F.,
Siddique
M.H.,
Azeem
F.,
Rasul
I.,
Muzammil
S.,
Zubair
M.,
Microbial L-asparaginase: purification, characterization and applications. Archives of Microbiology.
2020;
202
(5)
:
967-81
.
View Article PubMed Google Scholar -
Juturu
V.,
Wu
J.C.,
Microbial production of bacteriocins: latest research development and applications. Biotechnology Advances.
2018;
36
(8)
:
2187-200
.
View Article PubMed Google Scholar -
Drider
D.,
Bendali
F.,
Naghmouchi
K.,
Chikindas
M.L.,
Bacteriocins: not only antibacterial agents. Probiotics and Antimicrobial Proteins.
2016;
8
(4)
:
177-82
.
View Article PubMed Google Scholar -
Kaur
S.,
Kaur
S.,
Bacteriocins as potential anticancer agents. Frontiers in Pharmacology.
2015;
6
:
272
.
View Article PubMed Google Scholar -
Joo
N.E.,
Ritchie
K.,
Kamarajan
P.,
Miao
D.,
Kapila
Y.L.,
Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Medicine.
2012;
1
(3)
:
295-305
.
View Article PubMed Google Scholar -
Paiva
A.D.,
de Oliveira
M.D.,
de Paula
S.O.,
Baracat-Pereira
M.C.,
Breukink
E.,
Mantovani
H.C.,
Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology (Reading, England).
2012;
158
(Pt 11)
:
2851-8
.
View Article PubMed Google Scholar -
Karpiński
T.M.,
Adamczak
A.,
Anticancer activity of bacterial proteins and peptides. Pharmaceutics.
2018;
10
(2)
:
54
.
View Article PubMed Google Scholar -
Huang
Z.,
Hu
H.,
Arginine Deiminase Induces Immunogenic Cell Death and Is Enhanced by N-acetylcysteine in Murine MC38 Colorectal Cancer Cells and MDA-MB-231 Human Breast Cancer Cells In Vitro. Molecules (Basel, Switzerland).
2021;
26
(2)
:
511
.
View Article PubMed Google Scholar -
Rogers
L.C.,
Van Tine
B.A.,
Innate and adaptive resistance mechanisms to arginine deprivation therapies in sarcoma and other cancers. Cancer Drug Resistance (Alhambra, Calif.).
2019;
2
(3)
:
516-26
.
View Article PubMed Google Scholar -
Angelopoulou
A.,
Warda
A.K.,
Hill
C.,
Ross
R.P.,
Non-antibiotic microbial solutions for bovine mastitis - live biotherapeutics, bacteriophage, and phage lysins. Critical Reviews in Microbiology.
2019;
45
(5-6)
:
564-80
.
View Article PubMed Google Scholar -
Zaslavskaya
M.,
Makhrova
T.,
Aleksandrova
N.,
Ignatova
N.,
Belova
I.,
Tochilina
A.,
Prospects for using bacteriocins of normal microbiota in antibacterial therapy. Современные технологии в медицине.
2019;
11
(3)
:
136-44
.
View Article Google Scholar -
Aslam
R.S.,
Ashraf
M.,
Mohsin
M.,
Iqbal
Z.,
Production and therapeutic potential of bacteriocin produced by indigenous isolates of Bacillus subtilis. Pakistan Journal of Agricultural Sciences.
2020;
57
(5)
:
1403-1411
.
-
Radaic
A.,
de Jesus
M.B.,
Kapila
Y.L.,
Bacterial anti-microbial peptides and nano-sized drug delivery systems: the state of the art toward improved bacteriocins. Journal of Controlled Release.
2020;
321
:
100-18
.
View Article PubMed Google Scholar -
Mokoena
M.P.,
Omatola
C.A.,
Olaniran
A.O.,
Applications of lactic acid bacteria and their bacteriocins against food spoilage microorganisms and foodborne pathogens. Molecules (Basel, Switzerland).
2021;
26
(22)
:
7055
.
View Article PubMed Google Scholar -
Walsh
L.,
Johnson
C.N.,
Hill
C.,
Ross
R.P.,
Efficacy of phage-and bacteriocin-based therapies in combatting nosocomial MRSA infections. Frontiers in Molecular Biosciences.
2021;
8
:
654038
.
View Article PubMed Google Scholar -
Yaacob
S.N.,
Wahab
R.A.,
Misson
M.,
Sabullah
M.K.,
Huyop
F.,
Zin
N.M.,
Lactic acid bacteria and their bacteriocins: new potential weapons in the fight against methicillin-resistant Staphylococcus aureus. Future Microbiology.
2022;
17
(9)
:
683-99
.
View Article PubMed Google Scholar -
Kovalskaya
N.Y.,
Herndon
E.E.,
Foster-Frey
J.A.,
Donovan
D.M.,
Hammond
R.W.,
Antimicrobial activity of bacteriophage derived triple fusion protein against Staphylococcus aureus. AIMS Microbiology.
2019;
5
(2)
:
158-75
.
View Article PubMed Google Scholar -
Rahman
M.U.,
Wang
W.,
Sun
Q.,
Shah
J.A.,
Li
C.,
Sun
Y.,
Endolysin, a promising solution against antimicrobial resistance. Antibiotics (Basel, Switzerland).
2021;
10
(11)
:
1277
.
View Article PubMed Google Scholar -
Yuan
Y.,
Li
X.,
Wang
L.,
Li
G.,
Cong
C.,
Li
R.,
The endolysin of the Acinetobacter baumannii phage vB_AbaP_D2 shows broad antibacterial activity. Microbial Biotechnology.
2021;
14
(2)
:
403-18
.
View Article PubMed Google Scholar -
Yuan
Y.,
Li
X.,
Wang
L.,
Li
G.,
Cong
C.,
Li
R.,
The endolysin of the Acinetobacter baumannii phage vB_AbaP_D2 shows broad antibacterial activity. Microbial Biotechnology.
2021;
14
(2)
:
403-18
.
-
Khan
F.M.,
Gondil
V.S.,
Li
C.,
Jiang
M.,
Li
J.,
Yu
J.,
A novel Acinetobacter baumannii bacteriophage endolysin LysAB54 with high antibacterial activity against multiple Gram-negative microbes. Frontiers in Cellular and Infection Microbiology.
2021;
11
:
637313
.
View Article PubMed Google Scholar -
Descamps
H.C.,
Herrmann
B.,
Wiredu
D.,
Thaiss
C.A.,
The path toward using microbial metabolites as therapies. EBioMedicine.
2019;
44
:
747-54
.
View Article PubMed Google Scholar -
Caspani
G.,
Swann
J.,
Small talk: microbial metabolites involved in the signaling from microbiota to brain. Current Opinion in Pharmacology.
2019;
48
:
99-106
.
View Article PubMed Google Scholar -
Peredo-Lovillo
A.,
Romero-Luna
H.E.,
Jiménez-Fernández
M.,
Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Research International.
2020;
136
:
109473
.
View Article PubMed Google Scholar -
Park
J.,
Kim
C.H.,
Regulation of common neurological disorders by gut microbial metabolites. Experimental & Molecular Medicine.
2021;
53
(12)
:
1821-33
.
View Article PubMed Google Scholar -
Haase
S.,
Wilck
N.,
Haghikia
A.,
Gold
R.,
Mueller
D.N.,
Linker
R.A.,
The role of the gut microbiota and microbial metabolites in neuroinflammation. European Journal of Immunology.
2020;
50
(12)
:
1863-70
.
View Article PubMed Google Scholar -
Zhang
W.Q.,
Wang
Y.J.,
Zhang
A.,
Ding
Y.J.,
Zhang
X.N.,
Jia
Q.J.,
TMA/TMAO in hypertension: novel horizons and potential therapies. Journal of Cardiovascular Translational Research.
2021;
14
(6)
:
1117-24
.
View Article PubMed Google Scholar -
Zhu
Y.,
Li
Q.,
Jiang
H.,
Gut microbiota in atherosclerosis: focus on trimethylamine N-oxide. APMIS.
2020;
128
(5)
:
353-66
.
View Article PubMed Google Scholar -
Bollong
M.J.,
Lee
G.,
Coukos
J.S.,
Yun
H.,
Zambaldo
C.,
Chang
J.W.,
A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature.
2018;
562
(7728)
:
600-4
.
View Article PubMed Google Scholar -
Kurtz
J.A.,
VanDusseldorp
T.A.,
Doyle
J.A.,
Otis
J.S.,
Taurine in sports and exercise. Journal of the International Society of Sports Nutrition.
2021;
18
(1)
:
39
.
View Article PubMed Google Scholar -
Wu
G.,
Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids.
2020;
52
(3)
:
329-60
.
View Article PubMed Google Scholar -
Baliou
S.,
Adamaki
M.,
Ioannou
P.,
Pappa
A.,
Panayiotidis
M.I.,
Spandidos
D.A.,
Protective role of taurine against oxidative stress (Review). Molecular Medicine Reports.
2021;
24
(2)
:
1-19
.
View Article PubMed Google Scholar -
Jakaria
M.,
Azam
S.,
Haque
M.E.,
Jo
S.H.,
Uddin
M.S.,
Kim
I.S.,
Taurine and its analogs in neurological disorders: focus on therapeutic potential and molecular mechanisms. Redox Biology.
2019;
24
:
101223
.
View Article PubMed Google Scholar -
Wen
C.,
Li
F.,
Zhang
L.,
Duan
Y.,
Guo
Q.,
Wang
W.,
Taurine is involved in energy metabolism in muscles, adipose tissue, and the liver. Molecular Nutrition & Food Research.
2019;
63
(2)
:
e1800536
.
View Article PubMed Google Scholar -
Vahdat
M.,
Hosseini
S.A.,
Soltani
F.,
Cheraghian
B.,
Namjoonia
M.,
The effects of Taurine supplementation on inflammatory markers and clinical outcomes in patients with traumatic brain injury: a double-blind randomized controlled trial. Nutrition Journal.
2021;
20
(1)
:
53
.
View Article PubMed Google Scholar -
Iwegbulem
O.,
Wang
J.,
Pfirrmann
R.W.,
Redmond
H.P.,
The role of taurine derivatives in the putative therapy of COVID-19-induced inflammation. Irish Journal of Medical Science.
2022;
191
(1)
:
485-6
.
View Article PubMed Google Scholar -
Zhu
L.,
Li
J.,
Li
Y.,
Ivey
K.,
Lee
K.H.,
Eliassen
H.,
Histidine Intake, Human Gut Microbiome, Plasma Levels of Imidazole Propionate, and Coronary Heart Disease Risk in US Adults. Current Developments in Nutrition.
2022;
6
:
1041
.
View Article Google Scholar -
Molinaro
A.,
Bel Lassen
P.,
Henricsson
M.,
Wu
H.,
Adriouch
S.,
Belda
E.,
Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nature Communications.
2020;
11
(1)
:
1-10
.
View Article PubMed Google Scholar -
Wu
B.,
Tan
L.,
Wang
W.,
Feng
X.,
Yan
D.,
Imidazole Propionate is Increased in Diabetes and Associated with Stool Consistency. Diabetes, Metabolic Syndrome and Obesity.
2022;
15
:
1715-24
.
View Article PubMed Google Scholar -
van Son
J.,
Serlie
M.J.,
St\aahlman
M.,
Bäckhed
F.,
Nieuwdorp
M.,
Aron-Wisnewsky
J.,
Plasma Imidazole Propionate Is Positively Correlated with Blood Pressure in Overweight and Obese Humans. Nutrients.
2021;
13
(8)
:
2706
.
View Article PubMed Google Scholar -
Koh
A.,
Manneras-Holm
L.,
Yunn
N.-O.,
Nilsson
P.M.,
Ryu
S.H.,
Molinaro
A.,
Microbial imidazole propionate affects responses to metformin through p38γ-dependent inhibitory AMPK phosphorylation. Cell metabolism.
2020;
32
(4)
:
643-53. e4
.
View Article Google Scholar -
Hertli
S.,
Zimmermann
P.,
Molecular interactions between the intestinal microbiota and the host. Molecular Microbiology.
2022;
117
(6)
:
1297-307
.
View Article PubMed Google Scholar -
Chen
Y.,
Chen
Y.X.,
Microbiota-Associated Metabolites and Related Immunoregulation in Colorectal Cancer. Cancers (Basel).
2021;
13
(16)
:
4054
.
View Article PubMed Google Scholar -
Feng
Y.L.,
Cao
G.,
Chen
D.Q.,
Vaziri
N.D.,
Chen
L.,
Zhang
J.,
Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cellular and Molecular Life Sciences.
2019;
76
(24)
:
4961-78
.
View Article PubMed Google Scholar -
Peng
Q.,
Wong
C.Y.,
Cheuk
I.W.,
Teoh
J.Y.,
Chiu
P.K.,
Ng
C.F.,
The Emerging Clinical Role of Spermine in Prostate Cancer. International Journal of Molecular Sciences.
2021;
22
(9)
:
4382
.
View Article PubMed Google Scholar -
Tao
Y.,
Tartia
A.,
Lawson
M.,
Zelinski
M.B.,
Wu
W.,
Liu
J.Y.,
Can peri-ovulatory putrescine supplementation improve egg quality in older infertile women?. Journal of Assisted Reproduction and Genetics.
2019;
36
(3)
:
395-402
.
View Article PubMed Google Scholar -
Jucá
M.M.,
Cysne Filho
F.M.,
de Almeida
J.C.,
Mesquita
D.D.,
Barriga
J.R.,
Dias
K.C.,
Flavonoids: biological activities and therapeutic potential. Natural Product Research.
2020;
34
(5)
:
692-705
.
View Article PubMed Google Scholar -
Osborn
L.J.,
Claesen
J.,
Brown
J.M.,
Microbial Flavonoid Metabolism: A Cardiometabolic Disease Perspective. Annual Review of Nutrition.
2021;
41
(1)
:
433-54
.
View Article PubMed Google Scholar -
Ullah
A.,
Munir
S.,
Badshah
S.L.,
Khan
N.,
Ghani
L.,
Poulson
B.G.,
Important Flavonoids and Their Role as a Therapeutic Agent. Molecules (Basel, Switzerland).
2020;
25
(22)
:
5243
.
View Article PubMed Google Scholar -
Billowria
K.,
Ali
R.,
Rangra
N.K.,
Kumar
R.,
Chawla
P.A.,
Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Critical Reviews in Analytical Chemistry.
2022;
:
1-15
.
View Article PubMed Google Scholar -
Nemeth
K.,
Piskula
M.K.,
Food content, processing, absorption and metabolism of onion flavonoids. Critical Reviews in Food Science and Nutrition.
2007;
47
(4)
:
397-409
.
View Article PubMed Google Scholar -
Pourová
J.,
Najmanová
I.,
Vopršalová
M.,
Migkos
T.,
Pilařová
V.,
Applová
L.,
Two flavonoid metabolites, 3,4-dihydroxyphenylacetic acid and 4-methylcatechol, relax arteries ex vivo and decrease blood pressure in vivo. Vascular Pharmacology.
2018;
111
:
36-43
.
View Article PubMed Google Scholar -
Oteiza
P.I.,
Fraga
C.G.,
Mills
D.A.,
Taft
D.H.,
Flavonoids and the gastrointestinal tract: local and systemic effects. Molecular Aspects of Medicine.
2018;
61
:
41-9
.
View Article PubMed Google Scholar -
Wang
L.,
Gao
M.,
Kang
G.,
Huang
H.,
The potential role of phytonutrients flavonoids influencing gut microbiota in the prophylaxis and treatment of inflammatory bowel disease. Frontiers in Nutrition.
2021;
8
:
798038
.
View Article PubMed Google Scholar -
Oteiza
P.I.,
Fraga
C.G.,
Mills
D.A.,
Taft
D.H.,
Flavonoids and the gastrointestinal tract: local and systemic effects. Molecular Aspects of Medicine.
2018;
61
:
41-9
.
View Article PubMed Google Scholar -
Perino
A.,
Schoonjans
K.,
Metabolic Messengers: bile acids. Nature Metabolism.
2022;
4
(4)
:
416-23
.
View Article PubMed Google Scholar -
Roager
H.M.,
Dragsted
L.O.,
Diet-derived microbial metabolites in health and disease. Nutrition Bulletin.
2019;
44
(3)
:
216-27
.
View Article Google Scholar -
Collins
S.L.,
Stine
J.G.,
Bisanz
J.E.,
Okafor
C.D.,
Patterson
A.D.,
Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nature Reviews. Microbiology.
2023;
21
(4)
:
236-47
.
View Article PubMed Google Scholar -
van der Schoor
L.W.,
Verkade
H.J.,
Bertolini
A.,
de Wit
S.,
Mennillo
E.,
Rettenmeier
E.,
Potential of therapeutic bile acids in the treatment of neonatal Hyperbilirubinemia. Scientific Reports.
2021;
11
(1)
:
11107
.
View Article PubMed Google Scholar -
Camilleri
M.,
Gores
G.J.,
Therapeutic targeting of bile acids. American Journal of Physiology. Gastrointestinal and Liver Physiology.
2015;
309
(4)
:
209-15
.
View Article PubMed Google Scholar -
Lin
C.H.,
Kohli
R.,
Bile acid metabolism and signaling: potential therapeutic target for nonalcoholic fatty liver disease. Clinical and Translational Gastroenterology.
2018;
9
(6)
:
164
.
View Article PubMed Google Scholar -
Evangelakos
I.,
Heeren
J.,
Verkade
E.,
Kuipers
F.,
Role of bile acids in inflammatory liver diseases. Seminars in Immunopathology.
2021;
43
(4)
:
577-90
.
View Article PubMed Google Scholar -
Arab
J.P.,
Karpen
S.J.,
Dawson
P.A.,
Arrese
M.,
Trauner
M.,
Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology (Baltimore, Md.).
2017;
65
(1)
:
350-62
.
View Article PubMed Google Scholar -
Dai
X.,
Hou
H.,
Zhang
W.,
Liu
T.,
Li
Y.,
Wang
S.,
Microbial metabolites: critical regulators in NAFLD. Frontiers in Microbiology.
2020;
11
:
567654
.
View Article PubMed Google Scholar -
Thomas
C.,
Gioiello
A.,
Noriega
L.,
Strehle
A.,
Oury
J.,
Rizzo
G.,
TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metabolism.
2009;
10
(3)
:
167-77
.
View Article PubMed Google Scholar -
Pircher
P.C.,
Kitto
J.L.,
Petrowski
M.L.,
Tangirala
R.K.,
Bischoff
E.D.,
Schulman
I.G.,
Farnesoid X receptor regulates bile acid-amino acid conjugation. The Journal of Biological Chemistry.
2003;
278
(30)
:
27703-11
.
View Article PubMed Google Scholar -
Staudinger
J.L.,
Goodwin
B.,
Jones
S.A.,
Hawkins-Brown
D.,
MacKenzie
K.I.,
LaTour
A.,
The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proceedings of the National Academy of Sciences of the United States of America.
2001;
98
(6)
:
3369-74
.
View Article PubMed Google Scholar -
Kim
C.H.,
Immune regulation by microbiome metabolites. Immunology.
2018;
154
(2)
:
220-9
.
View Article PubMed Google Scholar -
Peng
Y.,
Nie
Y.,
Yu
J.,
Wong
C.C.,
Microbial metabolites in colorectal cancer: basic and clinical implications. Metabolites.
2021;
11
(3)
:
159
.
View Article PubMed Google Scholar -
Kiss
B.,
Mikó
E.,
Sebo
É.,
Toth
J.,
Ujlaki
G.,
Szabó
J.,
Oncobiosis and microbial metabolite signaling in pancreatic adenocarcinoma. Cancers (Basel).
2020;
12
(5)
:
1068
.
View Article PubMed Google Scholar -
Yang
S.,
Li
X.,
Yang
F.,
Zhao
R.,
Pan
X.,
Liang
J.,
Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Frontiers in Pharmacology.
2019;
10
:
1360
.
View Article PubMed Google Scholar -
Ganguly
P.,
Polák
J.,
Vegt
N.F. van der,
Heyda
J.,
Shea
J.E.,
Protein stability in TMAO and mixed urea-TMAO solutions. The Journal of Physical Chemistry B.
2020;
124
(29)
:
6181-97
.
View Article PubMed Google Scholar -
Cully
M.,
Microbiome therapeutics go small molecule. Nature Reviews. Drug Discovery.
2019;
18
(8)
:
569-72
.
View Article PubMed Google Scholar -
Dehghan
P.,
Farhangi
M.A.,
Nikniaz
L.,
Nikniaz
Z.,
Asghari-Jafarabadi
M.,
Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: an exploratory systematic review and dose-response meta- analysis. Obesity Reviews.
2020;
21
(5)
:
e12993
.
View Article PubMed Google Scholar -
Jalandra
R.,
Dalal
N.,
Yadav
A.K.,
Verma
D.,
Sharma
M.,
Singh
R.,
Emerging role of trimethylamine-N-oxide (TMAO) in colorectal cancer. Applied Microbiology and Biotechnology.
2021;
105
(20)
:
7651-60
.
View Article PubMed Google Scholar -
Zhang
W.,
Miikeda
A.,
Zuckerman
J.,
Jia
X.,
Charugundla
S.,
Zhou
Z.,
Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice. Scientific Reports.
2021;
11
(1)
:
518
.
View Article PubMed Google Scholar -
Govindarajulu
M.,
Pinky
P.D.,
Steinke
I.,
Bloemer
J.,
Ramesh
S.,
Kariharan
T.,
Gut metabolite TMAO induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Frontiers in Molecular Neuroscience.
2020;
13
:
138
.
View Article PubMed Google Scholar -
Janeiro
M.H.,
Ramírez
M.J.,
Milagro
F.I.,
Martínez
J.A.,
Solas
M.,
Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients.
2018;
10
(10)
:
1398
.
View Article PubMed Google Scholar -
Mohan
V.K.,
George
M.,
A Review of The Contribution of Gut-Dependent Microbiota Derived Marker, Trimethylamine N-oxide (TMAO), in Coronary Artery Disease. Current Research in Nutrition and Food Science.
2021;
9
(3)
:
712-21
.
View Article Google Scholar -
Liu
Y.,
Trimethylamine N-oxide generated by the gut microbiota is associated with vascular inflammation: new insights into atherosclerosis. Mediators of inflammation.
2020;
2020
:
4634172
.
View Article Google Scholar -
Coras
R.,
Kavanaugh
A.,
Boyd
T.,
Huynh
D.,
Lagerborg
K.A.,
Xu
Y.J.,
Choline metabolite, trimethylamine N-oxide (TMAO), is associated with inflammation in psoriatic arthritis. Clinical and Experimental Rheumatology.
2019;
37
(3)
:
481-4
.
PubMed Google Scholar -
Liu
H.,
Jia
K.,
Ren
Z.,
Sun
J.,
Pan
L.L.,
PRMT5 critically mediates TMAO-induced inflammatory response in vascular smooth muscle cells. Cell Death & Disease.
2022;
13
(4)
:
299
.
View Article PubMed Google Scholar -
Wang
J.,
Gu
X.,
Yang
J.,
Wei
Y.,
Zhao
Y.,
Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Frontiers in Cellular and Infection Microbiology.
2019;
9
:
409
.
View Article PubMed Google Scholar -
Harris
H.C.,
Morrison
D.J.,
Edwards
C.A.,
Impact of the source of fermentable carbohydrate on SCFA production by human gut microbiota in vitro - a systematic scoping review and secondary analysis. Critical Reviews in Food Science and Nutrition.
2021;
61
(22)
:
3892-903
.
View Article PubMed Google Scholar -
Bloom
P.P.,
Tapper
E.B.,
Young
V.B.,
Lok
A.S.,
Microbiome therapeutics for hepatic encephalopathy. Journal of Hepatology.
2021;
75
(6)
:
1452-64
.
View Article PubMed Google Scholar -
Roy
R.,
Nguyen-Ngo
C.,
Lappas
M.,
Short-chain fatty acids as novel therapeutics for gestational diabetes. Journal of Molecular Endocrinology.
2020;
65
(2)
:
21-34
.
View Article PubMed Google Scholar -
Singh
D.,
Gupta
S.,
Verma
I.,
Morsy
M.A.,
Nair
A.B.,
Ahmed
A.F.,
Hidden pharmacological activities of valproic acid: A new insight. Biomedicine and Pharmacotherapy.
2021;
142
:
112021
.
View Article PubMed Google Scholar -
Lipska
K.,
Gumieniczek
A.,
Filip
A.A.,
Anticonvulsant valproic acid and other short-chain fatty acids as novel anticancer therapeutics: possibilities and challenges. Acta Pharmaceutica (Zagreb, Croatia).
2020;
70
(3)
:
291-301
.
View Article PubMed Google Scholar -
Park
H.K.,
Han
B.R.,
Park
W.H.,
Combination of arsenic trioxide and valproic acid efficiently inhibits growth of lung cancer cells via G2/M-phase arrest and apoptotic cell death. International Journal of Molecular Sciences.
2020;
21
(7)
:
2649
.
View Article PubMed Google Scholar -
Igunnu
A.,
Omotehinse
A.,
David
I.,
Ogunsola
O.,
Oyegoke
R.,
Valproic acid displays anti-diabetic and pro-antioxidant effects in high-fat diet and streptozotocin-induced type 2 diabetic rats. Nigerian Journal of Pure & Applied Science.
2019;
32
:
3324-36
.
-
Zhang
L.,
Liu
C.,
Jiang
Q.,
Yin
Y.,
Butyrate in energy metabolism: there is still more to learn. Trends in Endocrinology and Metabolism.
2021;
32
(3)
:
159-69
.
View Article PubMed Google Scholar -
Fu
X.,
Liu
Z.,
Zhu
C.,
Mou
H.,
Kong
Q.,
Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Critical reviews in food science nutrition.
2019;
59
(sup1)
:
S130-S52
.
View Article Google Scholar -
Magnusson
M.K.,
Isaksson
S.,
Öhman
L.,
The anti-inflammatory immune regulation induced by butyrate is impaired in inflamed intestinal mucosa from patients with ulcerative colitis. Inflammation.
2020;
43
(2)
:
507-17
.
View Article PubMed Google Scholar -
Giacobbe
J.,
Benoiton
B.,
Zunszain
P.,
Pariante
C.M.,
Borsini
A.,
The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Frontiers in Psychiatry.
2020;
11
:
122
.
View Article PubMed Google Scholar -
Fadallah
M.,
Zahran
M.H.,
El-Assmy
A.M.,
Barakat
N.M.,
Khater
S.,
Awadalla
A.,
Omega-3 polyunsaturated fatty acids: a modified approach for chemo-prevention of bladder cancer in a rat model and molecular studies of antineoplastic mechanisms. Molecular Biology Reports.
2022;
49
(7)
:
6357-65
.
View Article PubMed Google Scholar -
Tadic
M.,
Sala
C.,
Grassi
G.,
Mancia
G.,
Taddei
S.,
Rottbauer
W.,
Omega-3 fatty acids and coronary artery disease: more questions than answers. Journal of Clinical Medicine.
2021;
10
(11)
:
2495
.
View Article PubMed Google Scholar -
Chitre
N.M.,
Moniri
N.H.,
Murnane
K.S.,
Omega-3 fatty acids as druggable therapeutics for neurodegenerative disorders. CNS & Neurological Disorders - Drug Targets.
2019;
18
(10)
:
735-49
.
View Article PubMed Google Scholar -
Malekahmadi
M.,
Pahlavani
N.,
Firouzi
S.,
Islam
S.M.,
Zonooz
S.R.,
Moghaddam
O.M.,
The Effect of Immunomodulatory Diet (Omega-3 Fatty Acid, γ-Linolenic Acid and Antioxidants) on clinical outcomes in critically ill patients. Authorea.
2020;
:
preprint
.
View Article Google Scholar -
Bazinet
R.P.,
Metherel
A.H.,
Chen
C.T.,
Shaikh
S.R.,
Nadjar
A.,
Joffre
C.,
Brain eicosapentaenoic acid metabolism as a lead for novel therapeutics in major depression. Brain, Behavior, and Immunity.
2020;
85
:
21-8
.
View Article PubMed Google Scholar -
Chang
J.P.,
Su
K.P.,
Mondelli
V.,
Satyanarayanan
S.K.,
Yang
H.T.,
Chiang
Y.J.,
High-dose eicosapentaenoic acid (EPA) improves attention and vigilance in children and adolescents with attention deficit hyperactivity disorder (ADHD) and low endogenous EPA levels. Translational Psychiatry.
2019;
9
(1)
:
303
.
View Article PubMed Google Scholar -
Nelson
J.R.,
Raskin
S.,
The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgraduate Medicine.
2019;
131
(4)
:
268-77
.
View Article PubMed Google Scholar -
den Hartigh
L.J.,
Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients.
2019;
11
(2)
:
370
.
View Article PubMed Google Scholar -
Dachev
M.,
Bryndová
J.,
Jakubek
M.,
Mou\vcka
Z.,
Urban
M.,
The effects of conjugated linoleic acids on cancer. Processes (Basel, Switzerland).
2021;
9
(3)
:
454
.
View Article Google Scholar -
Ephraim
E.,
Jewell
D.,
The Influence of Fiber Source on Circulating Concentration of Age-Related Metabolites and the Gut Microbial Composition in Senior Cats. Current Developments in Nutrition.
2021;
5
:
13
.
View Article Google Scholar -
Needham
B.D.,
Funabashi
M.,
Adame
M.D.,
Wang
Z.,
Boktor
J.C.,
Haney
J.,
A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature.
2022;
602
(7898)
:
647-53
.
View Article PubMed Google Scholar -
Kang
D.W.,
Adams
J.B.,
Vargason
T.,
Santiago
M.,
Hahn
J.,
Krajmalnik-Brown
R.,
Distinct fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy. MSphere.
2020;
5
(5)
:
e00314-20
.
View Article PubMed Google Scholar
Comments
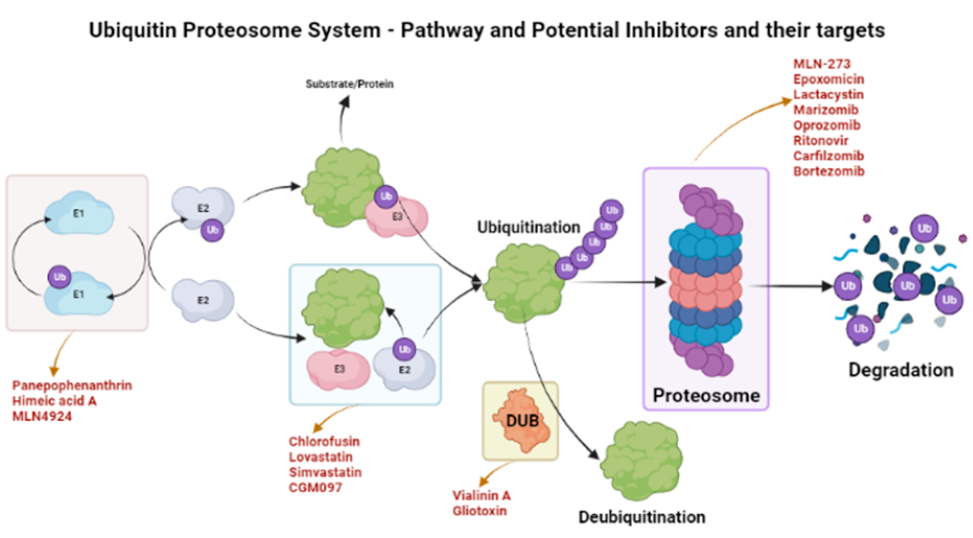
Article Details
Volume & Issue : Vol 10 No 4 (2023)
Page No.: 5638-5653
Published on: 2023-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6361 times
- PDF downloaded - 1606 times
- XML downloaded - 195 times
 Biomedpress
Biomedpress