Abstract
Primary cardiac sarcomas are rare and histologically diverse, with single tumors able to demonstrate histological heterogeneity. Consequently, they are often misdiagnosed as other common cardiac tumors, such as cardiac myxoma or other metastatic malignancies. Incorrect diagnoses can result in incomplete surgeries, inadequate treatment regimens, and early recurrence. In our experience, based on the diverse histological and cytological characteristics of these tumors, immunostaining panels should be used early on to differentiate the exact tumor type. SMA, CD31, myogenin, ERG, and SOX10 panels are used to identify spindle-shaped or polymorphic cell patterns, while CK, LCA, S100, and desmin panels are used for round and monomorphic cell patterns. Using these panels can help identify the histological type of primary cardiac sarcomas, which is normally a challenge for pathologists.
Introduction
Primary cardiac tumors are rare, with incidence ranging from 0.002% to 0.03% during autopsies1. Primary cardiac sarcomas are the most common histological type of cardiac tumor, accounting for 75 — 95% of cardiac tumors2, 3. Angiosarcoma (AS), undifferentiated polymorphic sarcoma (UPS), and leiomyosarcoma (LMS) of the heart are the most common histological subtypes, accounting for approximately 75% of all cardiac sarcomas2, 3, 4. As primary cardiac sarcomas are rare in Viet Nam, to our knowledge, there are currently no statistical studies on their incidence as only isolated cases are reported5, 6, 7.
Primary cardiac sarcomas are histologically diverse; single tumors can display histological heterogeneity. Therefore, they are often misdiagnosed as other common cardiac tumors, such as cardiac myxoma, or other metastatic malignancies. Incorrect diagnoses can lead to incomplete surgeries, inadequate treatment regimens, and early recurrence. Determining the primary malignancy of a cardiac tumor and its histological type is essential for the treatment selection and determining the prognosis. The combination of multiple histological subtypes within the same tumor, such as spindle-shaped and epithelioid cell patterns, myxoid areas mimicking myxofibrosarcoma with thin curved vessels, or myxoma with myxoid stroma, complicates their histological identification8.
Given the rarity of this disease, before 2021, there was almost no consensus on the histopathological criteria of cardiac sarcomas, particularly for cardiac UPS8. In 2021, the World Health Organization (WHO) published a set of histological features for the diagnosis of some primary cardiac sarcomas; however, WHO noted that the immunohistochemical markers were not specific to cardiac UPS8. Therefore, investigating the histopathological and immunohistochemical features of primary cardiac sarcomas will contribute to the evidence supporting the WHO-recommended histological features. Therefore, the current study focuses on the histopathological and immunohistochemical features of several primary cardiac sarcomas to distinguish them from common benign cardiac tumors and other cardiac metastases.
Cases series report
A six-case series of primary cardiac sarcomas that were operated on at E Hospital, Hanoi, Viet Nam, over five years (2018 – 2020) was retrospectively evaluated. The hospital’s Centre of Cardiovascular and Thoracic Surgery is a leading facility in Vietnam for thoracic disease surgery. The patients were admitted with non-specific clinical presentations, with symptoms such as coughs, dyspnea, chest pain, and tiredness8, 9. The patients were a male with AS (case 1), a male with AS (case 2), a male with AS (case 3), a female with LMS (case 4), a female with pleomorphic rhabdomyosarcoma (RMS) (case 5), and a male with primary diffuse large B-cell lymphoma (DLBCL) (case 6).
Clinicopathological features of three angiosarcoma cases (cases 1–3)
The age range of the patients was 34 – 79 years. In cases 1 and 2, the tumor was located in the right atrium, while in case 3, it was in the left atrium. The tumors were large in all three of the cases, occupying most of the right atrium (case 1 and case 2) and left atrium (case 3). Commonly associated lesions included bleeding, necrosis, and thrombosis. The tumors were all sessile. All three cases had pericardial and pleural effusion and pulmonary metastases that consisted of some small nodules with ground-glass opacity in both lungs. Left atrial thrombosis was reported in case 3, where post-operative echocardiography revealed a slightly open mitral valve with no pleural or pericardial effusion. However, the left ventricle’s systolic function was within normal limits.
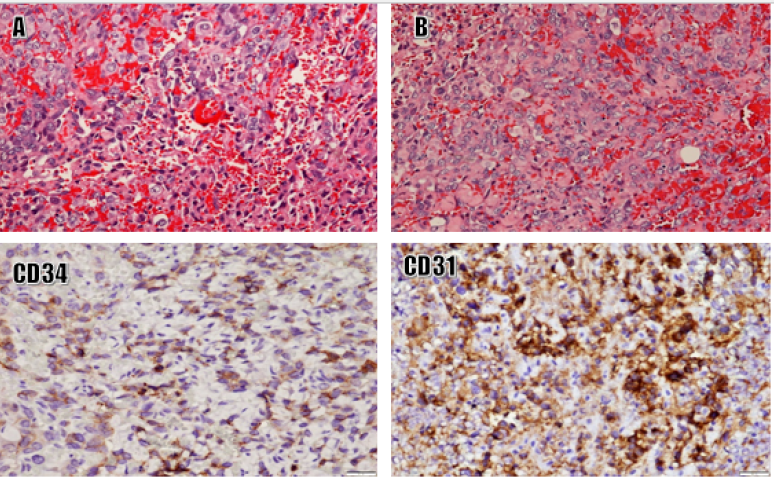
Histologically, the tumoral compositions and growth structures were similar in all three AS cases. Tumor tissues demonstrated two distinct structural growth patterns: solid and narrow slit-shaped lumen. Tumor cells were epithelioid or oval with large, polymorphic nuclei and high mitotic activity. Tumor necrosis was common and extensive. Based on the guidelines proposed by Coindre et al.,10 the necrosis was grade 3. Immunoexpression tests were positive for CD31 and ERG and negative for myogenin, SMA, and S100 (Figure 1).
Clinicopathological features of three cases: leiomyosarcoma (case 4), rhabdomyosarcoma (case 5), and diffuse large B-cell lymphoma (case 6)
The age range of the patients was 29 – 69 years. The tumors were located in the right atrium (case 6), left atrium (case 5), and the auricle of the left atrium and mitral valve (case 4). In case 4, the tumor was 50 × 50 mm. In case 5, it was 87 × 27 mm and pedunculated, and in case 6, it was 56 × 37 mm. In case 4, the tumor almost completely covered the mitral foramen. Commonly associated lesions included bleeding, necrosis, and thrombosis. Pericardial and pleural effusion was present in case 6, as were metastases, represented by small, nodular, ground-glass opacities in both lungs. Thrombosis in the auricle of the left atrium was reported in case 4 and in the left atrium in case 5. Post-operative echocardiography revealed no effusion in the pleura or pericardium for cases 4 and 5, and the left ventricle’s systolic function was within normal limits in both cases. The tumor was not completely resected in case 5.
Leiomyosarcoma (case 4)
Histologically, the tumor tissue had two structural patterns: spindle-shaped and round cells. Aggregations of spindle cells formed bundle or plexus structures, whereas round cells were often dispersed. The mitotic activity was very high in both structures. Tumor necrosis was often extensive and at grade 3 according to the guidelines of Coindre et al.10. Tumor cells were positive for SMA and desmin and negative for myogenin, CD31, and LCA (Figure 1).
Pleomorphic rhabdomyosarcoma (case 5)
The histological structure of the tumor included a typical pattern and a poorly differentiated pattern, including small round cells and a myxoma-like specimen. A high mitotic ratio and extensive necrosis were observed; the necrosis was grade 3 according to the guidelines proposed by Coindre et al. (2006)10. Tumor cells were positive for desmin and myogenin and negative for SMA, S100, LCA, calretinin, and CD31 (Figure 2).
Diffuse large B-cell lymphoma (case 6)
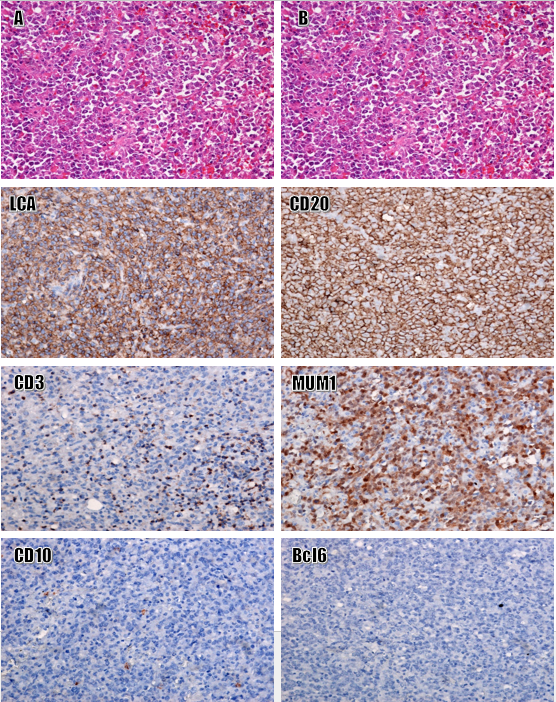
Histologically, the tumor tissue consisted of a large population of anaplastic cells with irregularly shaped nuclei and a high nucleocytoplasmic ratio. Some tumor cells resembled lymphoblasts and prolymphoblasts, with high mitotic activity. Tumor cells were positive for LCA, CD20, and CD79a, and negative for CD3, CD10, desmin, myogenin, S100, CD31, MUM1, and Bcl6 (Figure 3).
Following surgery, five patients dropped out of their treatment regimen due to worsening conditions. Case 5 was transferred to the National Cancer Hospital for medical treatment; this patient’s information was then lost.
Discussion
The characteristic histopathological features and tumor origins for primary malignant cardiac tumors have been controversial topics for decades. In 2021, the WHO classification of primary malignant cardiac tumors formally separated intimal sarcomas and cardiac UPS’s due to the improved understanding of biogenetics and the tendency to bias the anatomical location of the tumour8. The diagnosis of primary cardiac sarcoma is challenging due to its non-specific clinical presentation. The clinical presentation may include symptoms related to obstruction, such as dyspnea, chest pain, or embolism9. Given the challenging and often delayed diagnosis and inadequate surgical treatment due to incomplete tumor resection, cardiac sarcoma management is challenging. The median survival rate for patients is less than one year11.
The primary obstacle for diagnosing primary cardiac malignancies is distinguishing them from cardiac metastases, which occur in 10% of all cancer patients. Compared with primary cardiac cancer, secondary cancer is more common and has a worse prognosis. The most common cancers are metastases from malignant melanomas and carcinomas, such as lung and breast carcinomas, or metastases from non-epithelial tumors, such as AS, mesotheliomas, and lymphomas12, 13.
Several types of primary cardiac sarcomas have been reported. Of these, cardiac AS, UPS, and LMS are the most common, accounting for approximately 75% of all primary cardiac sarcomas. Cardiac AS and UPS each account for approximately 33% of these cases, while LMS accounts for approximately 10%3, 4, 14. These sarcomas are typically found in patients in their fourth or sixth decade of life. AS occurs in the right atrium, with male predominance, while UPS and LMS occur more frequently in the left atrium without gender bias15, 16.
Primary cardiac AS is the most common primary cardiac sarcoma, accounting for 25 – 40% of all cases17. It is a malignancy of the vascular endothelium with a highly proliferative and diffuse capacity, extensive necroses, a high propensity to metastasize, and a poor prognosis18. Expression of CD31 and CD34 markers is typically strong and diffuse1. Examining histological features and CD31/CD34/factor VIII immunoexpression is helpful for the differential diagnosis of AS. Intimal sarcomas are only locally positive for CD31 and CD34, whereas nuclear MDM2 expression is observed in > 70% of cases8. The following characteristics may suggest a cardiac AS diagnosis:
Primary cardiac LMS is a malignant mesenchymal tumor with smooth muscle cell differentiation that accounts for < 20% of all primary cardiac sarcomas8. Histologically, LMS consists of tumor cells often arranged in intersecting fascicles that appear perpendicular on cross-section. Some tumors demonstrate areas of storiform or palisading nuclear patterns. Tumor cells are highly dense and spindle-shaped, with stout, obtuse nuclei and eosinophilic filamentous cytoplasms1. Changes are local and may include myxoid appearance, loose hypocellular areas, and stromal hyalinization. Tumor necrosis and abnormal mitosis are common. Immunoexpression for SMA and desmin is frequently strong and diffuse in these tumor cells1, 8. The following characteristics may suggest a cardiac LMS diagnosis:
RMS is a tumor of skeletal muscle differentiation, representing only 0–5% of all cardiac sarcomas. They mainly occur in adolescents and children8. These tumor cells demonstrate eosinophilic cytoplasms displacing eccentric, round nuclei with prominent central nucleoli and large intracytoplasmic hyaline inclusions8. A rare structure of RMS was observed in the present study, involving diverse growth patterns, such as a high density of perivascular tumor cells, small, poorly differentiated cells, myxoid degeneration of the tumor stroma, and inflammatory infiltration. Interpretation of small biopsies of cardiac RMS should be made carefully due to the complexity of the histological structure, which could be confused with a benign cardiac myxoma. Immunoexpression of myogenin, MyoD1, and desmin is typically strong and diffuse in RMS tumor cells8. The following characteristics may suggest a diagnosis of cardiac RMS:
Primary cardiac lymphomas are extremely rare, accounting for less than 2% of all cardiac tumors and less than 1% of all extra-nodal lymphomas. DLBCL is the most common, accounting for around 80% of cardiac lymphomas. Cardiac DLBCL is usually located in the right atrium and is most common in males in their sixth and seventh decades of life8, 19. Given the difficulty associated with sampling and the lack of clinical symptom specificity, primary cardiac lymphomas are difficult to diagnose. Therefore, detection is often delayed8, 20. Tumor cells are round and poorly adherent, with large and irregular nuclei. Manifestations of cardiac tissue infiltration are obvious. The immunoexpression of CD20 and LCA is typically strong and diffuse in these tumor cells. The following characteristics may suggest a DLBCL diagnosis:
Conclusion
A six-case series of primary cardiac sarcomas, including three ASs, one LMS, one RMS, and one DLBCL, were diagnosed. In our experience, based on the diverse histological and cytological characteristics of primary cardiac sarcomas, immunostaining panels should be used to differentiate the specific type of sarcoma. SMA, CD31, myogenin, ERG, and SOX10 are used to identify spindle-shaped or polymorphic cell patterns, and CK, LCA, S100, and desmin are used to identify round and monomorphic cell patterns. Using these panels enables the identification of the histological type of the primary cardiac sarcoma, which normally presents a problem for pathologists. The addition of other immunomarkers is dependent on the primary immunostaining panels and tumor characteristics.
Abbreviations
Bcl6: B-cell lymphoma 6, CD10: Cluster of differentiation 10, CD20: Cluster of differentiation 20, CD3: Cluster of differentiation 3, CD31: Cluster of differentiation 31, CD34: Cluster of differentiation 34, CD79a: Cluster of differentiation 79a, CK: Cytokeratin, ERG: Erythroblastosis virus E26 oncogene homolog, H&E: Hematoxylin and Eosin, LCA: Leukocyte common antigen, MDM2: Mouse double minute 2, MUM1: Multiple myeloma oncogen-1, S100: Protein S100, SMA: Smooth muscle actin, SOX10: Sry-related HMg-Box 10
Acknowledgments
We would like to thank the University for the study’s approval.
Author’s contributions
NVH, TVC, THN, NTT: Conceptualization, Methodology, Writing-Original draft preparation; NTT, TNM, NCH, DMK: Visualization, Methodology, Software; HTNM, NTN, NHN, TVC : Data curation, Writing-Original draft preparation; DTL, HTNM, NTN, DMK: Validation, investigation, Supervision. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Patel
J.,
Sheppard
M.N.,
Pathological study of primary cardiac and pericardial tumours in a specialist UK Centre: surgical and autopsy series. Cardiovascular Pathology.
2010;
19
(6)
:
343-52
.
View Article PubMed Google Scholar -
Isambert
N.,
Ray-Coquard
I.,
Italiano
A.,
Rios
M.,
Kerbrat
P.,
Gauthier
M.,
Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. European Journal of Cancer (Oxford, England).
2014;
50
(1)
:
128-36
.
View Article PubMed Google Scholar -
Burke
A.P.,
Cowan
D.,
Virmani
R.,
Primary sarcomas of the heart. Cancer.
1992;
69
(2)
:
387-95
.
View Article PubMed Google Scholar -
Simpson
L.,
Kumar
S.K.,
Okuno
S.H.,
Schaff
H.V.,
Porrata
L.F.,
Buckner
J.C.,
Malignant primary cardiac tumors: review of a single institution experience. Cancer.
2008;
112
(11)
:
2440-6
.
View Article PubMed Google Scholar -
Do
T.H.,
Le
X.D.,
Vu
T.T.,
Ngo
T.A.,
Thi
M.H.,
Tran
Q.T.,
Primary cardiac epithelioid angiosarcoma: A case report. Radiology Case Reports.
2022;
17
(9)
:
3349-54
.
View Article PubMed Google Scholar -
Huy
T.,
Manh
P.,
Primary left atrial angiosarcoma mimicking pericarditis: A case report and review of the literature. Journal of Medicine Research.
2021;
148
(E9)
:
115-120
.
View Article Google Scholar -
Ninh
T.P.,
Duc
N.M.,
Trung
N.N.,
Duc
L.A.,
Ngoc
D.V.,
My
T.T.,
Multifocal precursor B-cell lymphoblastic lymphoma in an infant with cardiac involvement: A case report. Radiology Case Reports.
2021;
16
(8)
:
2054
.
View Article PubMed Google Scholar -
Maleszewski
J.J.,
Basso
C.,
Bois
M.C.,
Glass
C.,
Klarich
K.W.,
Leduc
C.,
The 2021 WHO Classification of Tumors of the Heart. Journal of Thoracic Oncology.
2022;
17
(4)
:
510-8
.
View Article PubMed Google Scholar -
Hudzik
B.,
Miszalski-Jamka
K.,
Glowacki
J.,
Lekston
A.,
Gierlotka
M.,
Zembala
M.,
Malignant tumors of the heart. Cancer Epidemiology.
2015;
39
(5)
:
665-72
.
View Article PubMed Google Scholar -
Coindre
J.M.,
Grading of soft tissue sarcomas: review and update. Archives of Pathology & Laboratory Medicine.
2006;
130
(10)
:
1448-53
.
View Article PubMed Google Scholar -
Truong
P.T.,
Jones
S.O.,
Martens
B.,
Alexander
C.,
Paquette
M.,
Joe
H.,
Treatment and outcomes in adult patients with primary cardiac sarcoma: the British Columbia Cancer Agency experience. Annals of Surgical Oncology.
2009;
16
(12)
:
3358-65
.
View Article PubMed Google Scholar -
Bussani
R.,
De-Giorgio
F.,
Abbate
A.,
Silvestri
F.,
Cardiac metastases. Journal of Clinical Pathology.
2007;
60
(1)
:
27-34
.
View Article PubMed Google Scholar -
García-Riego
A.,
Cuiñas
C.,
Vilanova
J. José,
Malignant Pericardial Effusion. Acta cytologica.
;
45
(4)
:
561-566
.
View Article PubMed Google Scholar -
Kim
C.H.,
Dancer
J.Y.,
Coffey
D.,
Zhai
Q.J.,
Reardon
M.,
Ayala
A.G.,
Clinicopathologic study of 24 patients with primary cardiac sarcomas: a 10-year single institution experience. Human Pathology.
2008;
39
(6)
:
933-8
.
View Article PubMed Google Scholar -
Randhawa
J.S.,
Budd
G.T.,
Randhawa
M.,
Ahluwalia
M.,
Jia
X.,
Daw
H.,
Primary Cardiac Sarcoma: 25-Year Cleveland Clinic Experience. American Journal of Clinical Oncology.
2016;
39
(6)
:
593-9
.
View Article PubMed Google Scholar -
Zhang
C.,
Huang
C.,
Zhang
X.,
Zhao
L.,
Pan
D.,
Clinical characteristics associated with primary cardiac angiosarcoma outcomes: a surveillance, epidemiology and end result analysis. European Journal of Medical Research.
2019;
24
(1)
:
29
.
View Article PubMed Google Scholar -
Burke
A.,
Tavora
F.,
The 2015 WHO Classification of tumors of the heart and pericardium. Journal of Thoracic Oncology.
2016;
11
(4)
:
441-52
.
View Article PubMed Google Scholar -
Patel
S.D.,
Peterson
A.,
Bartczak
A.,
Lee
S.,
Chojnowski
S.,
Gajewski
P.,
Primary cardiac angiosarcoma - a review. Medical Science Monitor.
2014;
20
:
103-9
.
View Article PubMed Google Scholar -
Zhao
Y.,
Huang
S.,
Ma
C.,
Zhu
H.,
Bo
J.,
Clinical features of cardiac lymphoma: an analysis of 37 cases. The Journal of International Medical Research.
2021;
49
(3)
:
300060521999558
.
View Article PubMed Google Scholar -
Csizmar
C.M.,
Sachs
Z.,
Cayci
Z.,
Bu
L.,
Linden
M.A.,
Primary Cardiac Lymphoma: Three Case Reports and a Review of the Literature. Open Journal of Blood Diseases.
2021;
11
(4)
:
120-32
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 2 (2023)
Page No.: 5530-5536
Published on: 2023-02-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 4755 times
- PDF downloaded - 1516 times
- XML downloaded - 107 times
 Biomedpress
Biomedpress