Mini-invasive treatment for delayed or non-union: the use of percutaneous autologous bone marrow injection
Abstract
Delayed union or nonunion of bone fracture is becoming less frequent, but still remains a challenging clinical problem. Autologous cancellous bone grafting that is the gold standard method, often involves donor site morbidities and complications. Once these fractures have been mechanically stabilized, other local factors should be investigated to promote delayed healing. The purpose of this study was to evaluate the initial outcome of the percutaneous injection of autologous bone marrow after concentration for the treatment of delayed or nonunion. Our subjects included 10 patients of delayed or nonunion fracture (3 female, 7 male) with an average age of 28 years. All fractures were mechanically stabilized after accident. Delayed or nonunion affected the femur in 2 patients, the tibia in 5 patients, the humerus in 2 patients, and the ulna in 1 patient. Bone marrow aspirates were obtained from both the posterior superior iliac crest. Bone marrow aspiration concentrate was produced via density gradient centrifugation. Trocars were inserted in the delayed or nonunion gap under fluoroscopic guidance. The bone marrow aspiration concentrate was injected slowly. As results, all of 10 delayed or nonunion healed after treatment with percutaneous injection of autologous bone marrow. The mean time for new bone formation was 3.3 months, for clinical union was 5.2 months, and for radiological union was 11.8 months. The current study is encouraging in the initial outcome and percutaneous bone marrow implantation could be an effective and safe treatment for delayed or nonunion.
Introduction
Despite recent advances in the biomechanical stabilization of fracture and understanding in the biological processes of healing, delayed union or nonunion is becoming less frequent, but still remains a challenging clinical problem.Autologous cancelous bone grafting is still the gold standard biological method for bone healing by introduction ofvital local components: osteoconduction, osteoinduction, and osteogenic cells Pountos, 2010.This bone graft often involves remarkable donor site morbidities and complications, as well as additional procedures.
The development of alternative techniques of biological stimulation, which must also provide these osteogenic components, is required. Once the stabilization of fracture has been mechanically met, other local factors should be investigated to promote delayed healing Pountos, 2010. More recently, numerous bone graft substitutes have been presented with varying degrees of successful outcome Zimmermann, 2010. Connolly and Shindell first demonstrated that nonunion could be treated successfully in the clinic with the percutaneous injection of bone marrow Connolly, 1989 Connolly, 1991. However, bone marrow after aspiration was directly injected to fracture site. This led to low rate of the implanted bone marrow. The purpose of this study was to evaluate the initial outcome of the percutaneous injection of autologous bone marrow after concentration for the treatment of delayed union or nonunion fractures.
Material and methods
The prospective study was performed in patients who were treated at our Center from January 2013 to December 2014 with delayed union or nonunion fractures following fracture stabilization. The protocol for this clinical study was approved by the Committee of Medical Training and Research Center. Our subjects included 10 patients (3 female, 7 male) from 18 to 48 year old with an average age of 28 years. Delayed union or nonunion affected the femur in 2 patients, the tibia in 5 patients, the humerus in 2 patients, and the ulna in 1 patient. All fractures that were presented with Müller AO fracture classification were stabilized. Two closed fractures at the femur and tibia had been stabilized by inter-locking intramedullary nail. Three cases of open fractures at the tibia, which had a very bad scar, were stabilized by an external device. All the remaining fractures were osteosynthesed by plates. Patient demographic, medical history, initial fracture treatment, and operative and follow-up data were recorded.
A diagnosis of delayed union or nonunion is based mainly on the clinical and radiographic examination. Clinical evidence of delayed union was determined by documented pain, motion at the fracture site, and tenderness on stress at the fracture site. An injury is deemed "delayed union" when an adequate period of time has elapsed without achieving bone union, with a time period of six months or less since the initial injury. "Nonunion" was determined by lack of radiographic evidence of bridging osteophytes at six months or a fracture that has not displayed progression of healing over a 3-month period Hernigou, 2005bTressler,2011. Clinical assessment and preoperative laboratories tests such as white blood cell count, erythrocyte sedimentation rate, and C-active protein were performed to rule out infection. Exclusion criteria included fractures with delayed or nonunion due to a mechanical cause related to the bone fixation necessitating revision of the stabilization, and the presence of a gap at the fracture site more than 10 mm wide which needed to be filled with a structural graft Akram, 2014Kassem, 2013.
No patients were included in analysis if they were actively being treated for an infection at the fracture site or ongoing treatment with immunosuppressant drugs: glucocorticosteroids, chemotherapy or colchicines at the time of nonunion revision Hernigou, 2013Tressler, 2011. Patients were also excluded if they were pregnant and lactating, or had autoimmune deficiency syndrome, hepatitis, or a medical history of alcohol or drug abuse Jager, 2011.
Isolation and concentration of bone marrow
The procedure of bone marrow aspiration was performed under general anaesthesia. Patients were placed in a prone position on an operating table. Bone marrow aspirates (350 ml) were aspirated from both posterior superior iliac crests of the patients by the Jamshidi vacuum. The samples were collected by qualified hematologists. Mesenchymal stem cells (MSCs) processing techniques were established as previously described (Giannotti et al., 2013). Bone marrow aspiration concentrate (BMAC) was made via a density gradient centrifugation using the SorvallTMcentrifuge (Thermo Scientific, MA, USA) at 3,670 rpm for 7 min. Finally, a total volume of 10 ml BMAC was poured into a syringe for injection Jager et al., 2011.
MSCs reimplantation
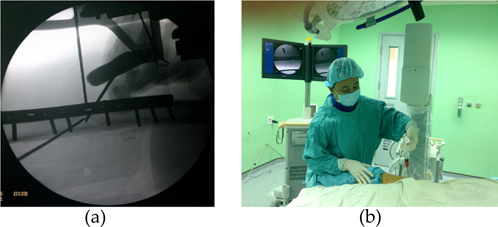
MSCs reimplantation was performed using a technique described by Hernigou et al. Hernigou, 2006. Under general anaesthesia, the patient was placed on the operating table in a supine position. The area of the nonunion site was prepared and draped in a sterile fashion. The tip of the trocars (15 G Barker-Bier Luer Lock, Unimed SA, Lausanne, Switzerland) was navigated into the delayed or nonunion gap and around the bone ends at several directions guided by the images of C-arm fluoroscopy (Philips Medical Systems NL B.V., Best, The Netherlands) ( Figure 1 ). The bone marrow aspiration concentrate (BMAC) was injected slowly at a speed of 20 ml/minute in a controlled fashion. After injection, the trocars, with the stylet in place, was withdrawn slowly to blood clot gradually bit of trocar paths Hernigou, 2006. The patient was asked to keep in the same position for the first hour to allow for cell attachment. There was one case of the humeral fracture stabilizing by plate and screws; one screw that had transversed the fracture line needed removal before injection. Two cases with an intramedullary nail at the femoral and tibial fracture were dynamized by removal of the distal locking screw. In two cases of tibial fracture, external device was removed and the fracture was stabilized by plaster cast Centeno C.J., 2011.
Assessment of fracture healing
These patients were monitored using the same protocol during the postoperative period. Patients were followed up at 1 month, 3 months, 6 months, 9 months, and 12 months, and continuously followed up until full bone union was achieved, as determined by clinical examination and radiographs. Weight bearing was not allowed during the first month after bone marrow transplantation. If callus was observed radiographically after one month, partial weight bearing was allowed. At the third month, if the patient had no more pain and there was cortical callus or disappearance of the fracture lines on radiographs, full weight-bearing was allowed. And if a plaster cast was used it would be removed. The physical examination assessed pain, sensation of stability, and ability to walk with or without crutches. Anteroposterior and lateral x-rays were performed at follow-ups. Assessment of bone union and remodelling was based on the modified Lane and Sandhu radiological scoring system. Three experts blindly assessed the radiological scores, which were the sum of the scores of bone formation and remodelling. The score for bone formation was determined as 0 (no bone formation), 1 (<25% bone formation), 2 (25– 50% bone formation), 3 (50–75% bone formation), and 4 (>75% bone formation). The score of union was 0 (full fracture line), 2 (partial fracture line), or 4 (absent fracture line). The remodelling score was 0 (no evidence of remodelling), 2 (remodelling of the intramedullary channel), and 4 (full remodelling of cortex). The maximum point could be achieved was 12.
Bone Marrow Analysis
The bone marrow cells that achieved after a density gradient centrifugation were analysed. In brief, the total mononuclear cells from bone marrow samples were counted with the use of a SysmexXS 800i Haematology Analyser (Sysmex Europe GmbH, Norderstedt, Germany). Cells then were displayed by immunofluorescence staining with antibodies against CD 34 (Becton, Dickinson and Company, New Jersey, United States). CD34+cells were counted and expressed as % CD34+ cells and CD34+ cells/ml. For cell viability evaluation, the Trypan Blue dye was used to enter cells with compromised membranes, making them appear dark upon bright field imaging. Colonyforming unit (CFU) and fibroblast colony-forming units (CFU-F) were used as an indicator of cell activity. Colony assays using the medium of Methocult™ H4434 Classic (Stemcell Technologies Inc., Vancouver, Canada). Fibroblast colonies were determined using mediums of MesenCult™MSC Basal and MesenCult™ Stimulatory Supplements (Stemcell Technologies Inc., Vancouver, Canada). Finally, BMAC sample were tested for sterility using BD BACTECTM 9050 Blood Culture System (Becton, Dickinson and Company, New Jersey, United States).
Data Management and Statistical Analysis
Data were collected, stored, and managed using paper- based case report forms. All data are shown as the mean and the standard deviation. Clinical characteristics of patients were resumed as counts for categorical variables.
Results
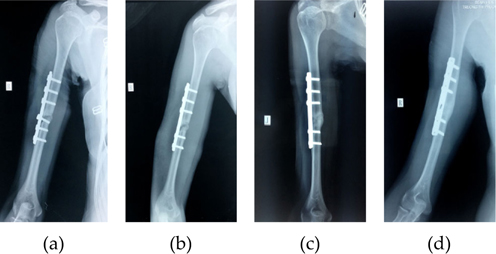
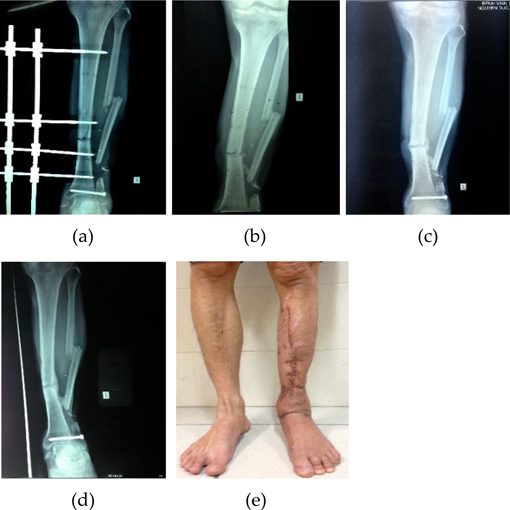
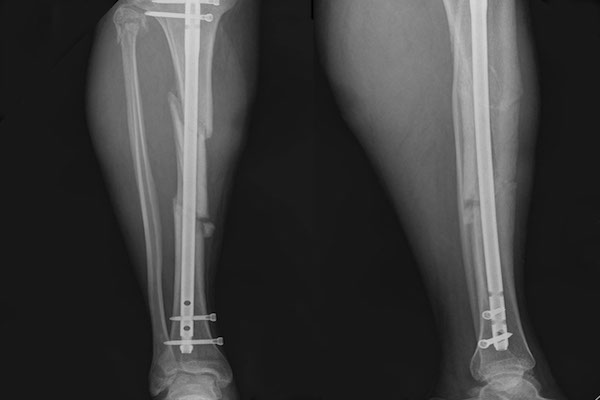
All the fractures were grossly stable. The average time from the initial injury to the MSCs implantation was 6.4 months (range, 6 - 11 months). None of the patients presented peri-operative complications that could be attributed to the percutaneous harvesting and reimplanting technique. The average follow-up period was 13.4 months (range, 6 - 32 months). All of 10 delayed or nonunion healed after treatment with percutaneous injection of autologous BMAC. The mean time for new bone formation was 3.3 months (range, 2 - 4 months), for clinical union was 5.2 months (range, 2 - 7 months), and for radiological union was 11.8 months (range, 11 - 15 months) ( Figure 2 and Figure 3 ).
After concentration of bone marrow aspirates, progenitors preparing injections contained a mean ratio of 1.75 ± 0.85 (%) CD34+ cells, a mean concentration of 2.53 ± 0.75 (x106) CD34+ cells/ml, a mean number of 21.14 ± 5.41 (x106) CD34+ cells, and a mean viability of 98.10 ± 1.29 (%) CD34+cells. Colony-forming unit (CFU) and fibroblast colony-forming units (CFU-F) were detected in all the samples incubated in vitro. The result of all samples of BMAC was negative for bacteria cultures.
Discussion
Our study showed that all 10 patients treated with percutaneous concentrated autologous bone marrow implantation performed significant bone union on their radiographs and good return to normal activities as before the fracture occurred. Healing time after bone marrow injection was significantly shortened. There was no local and systemic complication related to the intervention. Haematoma formation or infection was not found at the position of aspiration. Aspiration of bone marrow from the both iliac crests has not been a cause for chronic pain.
For the treatment of the delayed or nonunion, mechanical stabilization has been the main technique. All our patients presented with stable delayed or nonunion fractures, which there was no excessive movements at the fracture site. Beside mechanical and biochemical aspects, biological processes played a major role in the causes of delayed or nonunion fracture in recent studies Bhargava, 2007Bigham-Sadegh, 2014Hernigou,2005b. The rate of bone union was influenced by the cellular proliferation that results in the formation of the callus, the periosteum, and the status of bone marrowin the generation of osteoblasts Fox, 2014Hernigou,2005bQin, 2014.
Studies of Hernigou et al. showed that deficiency in the population of local connective tissue by progenitors may happen in the cases of previous infection, tissue defects, and injured vascularity, and this could be the cause of delayed or nonunion. Furthermore, the periosteum that has an osteogenic potential had usually been lost in these cases; consolidation could only be achieved by the osteoblasts at the fracture site. The number of osteoblasts was insufficient, so the proliferative capacity could be lower and bony union could be difficultly achieved Hernigou, 2005aReichert, 2007.
Over the past two decades, progress in the understanding of the role of the biology of bone union had led to the development of other forms of bone grafting. The purpose being to facilitate osteoinduction and osteoconduction, and to lead to osteogenesis Liebergall. M., 2013Oryan A., 2014Shah, 2013Shenoy,2014. The harvest of autologous bone marrow has been developed in previous studies Bielecki, 2006. Since bone marrow contains osteogenic progenitors, the injection of bone marrow is considered to have the potential for effective bone regeneration Hernigou, 2006Undale, 2009. The implantation of bone marrow can increase the local biology and optimize the potential of osteoprogenitors Ohgushi, 2014. On the other hand, autologous bone marrow injection enhances cellular numbers and stimulates the cells to fabricate a structural osteoconductive substrate with extremely low donor site morbidity Pountos, 2010. Bone marrow injection is a mini-invasive technique and can contribute to bone union in cases of stable delayed or nonunion Bhutia, 2015Singh, 2013Tseng, 2008. It has reduced soft tissue damage, improved the recovery period, and minimized scaring. It is also consistent with the modern treatment trends in the fields of Orthopaedics, especially in difficult situations Pountos, 2010.
In our studies, biologic scaffolds were not used in any of these procedures and the trocars were not remove the callus or fibrous tissue between bone gaps. Bone marrow was directly injected in the delayed or nonunion gap and around the bones ends. Using trocars to cannulate the fracture site is the mechanism that allows the transformation of fibrous tissue into callus. This may well create enough traumas to make a whole marrow scaffold. Then, the fibrous tissue interposed between the bones ends can be ossified and stop the micro-motion at the delayed or nonunion site in order to allow union of the gap Centeno C.J., 2011. All of 10 patients in our series healed their fracture.
Despite the success rates of fresh bone marrow aspirates for implantation in recent studies Hauzeur, 2010Kassem, 2013Singh, 2013Wilkins, 2003, an apparent correlation was observed between the number of the injected progenitor cells and the bone union rate Pountos, 2010. The success of whole bone marrow implantation is dependent on the sufficient populations of the transferred progenitors. Therefore, techniques that are able to enhance the volume of osteoprogenitor cells will contribute good clinical outcomes Hernigou,2005a. The mean ratio of CD34+ cells in bone marrow grafting prior injection in the study of Gangji et al. achieved 1.0 ± 0.2 (%) of CD34+ cells (Gangji et al). In present study, bone marrow aspirates was concentrated by centrifugation contained a mean ratio of 1.75 ± 0.85 (%) CD34+ cells, a mean number of 21.14 ± 5.41 (x106) CD34+ cells, and a mean viability of 98.10 ± 1.29 (%) CD34+ cells prior to injection. Bacterial cultures of BMAC were completely negative, and all the cultures of the CFU and CFU-F displayed positive staining in vitro. We believe that the clinical application of BMAC is a safe procedure. Hernigou showed that the amount of available oxygen at fracture site might be one of the limiting factors after implantation Hernigou, 2005aet. The implanted progenitor cells have to be a competitor with other cells for oxygen. The concentration of bone marrow aspiration helps to exclude all other cells andoptimizes progenitor cell survival for bone formation Pountos, 2010.
Our study has the limitations because the number of patient was limited and a measurement of the number of CFU-F was not performed. Therefore, it is very difficult to allow conclusive judgments. Since the volume of callus formation in this technique is limited, the bone defect gap should be indicated in a limited way.
Conclusion
The result of current study is encouraging in the initial outcome. Percutaneous autologous bone marrow implantation could be an effective and safe option for the treatment of delayed or nonunion fractures. The efficiency related to the population of progenitor cells in the concentrated bone marrow aspiration.However, more studies are needed for definitive conclusions. Beside of pure bone marrow implantation, composites of the porous implantable material with concentrated bone marrow will be evaluated for their efficacy in delayed union or nonunion in future application.
Abbreviation
Mesenchymal stem cells (MSCs); bone marrow aspiration concentrate (BMAC); Colony-forming unit (CFU); fibroblast colony-forming unit (CFU-F).
References
-
M.
Akram,
M.
Irshad,
F.M.
Farooqi,
R.K.
Sah,
M.L.
Shahzad,
A.H.
Sarfraz,
S.M
Awais.
Role of injecting bone marrow aspiration injection in treating delayed union and nonunion. J Pak Med Assoc.
2014;
64
:
154-157
.
-
R.
Bhargava,
S.
Sankhla,
A.
Gupta,
R.
Changani,
K
Gagal.
Percutaneous autologus bone marrow injection in the treatment of delayed or nonunion. Indian J Orthop.
2007;
41(1)
:
67-71
.
-
K.U.
Bhutia,
A.A.
Bary,
A.K.
Singh,
A.M.
Singh,
R.
Raghuvanshi,
C
Hmar.
Role of percutaneous autologous bone marrow injection in treatment of delayed union and nonunion of long bones. Journal Of Dental And Medical Sciences.
2015;
14
:
07-13
.
-
T.M.
Bielecki,
T.S
Gazdzik.
Percutaneous injection of autogenous growth factors in patient with nonunion of the humerus. A case report. J Orthopaedics.
2006;
3(3)
:
E15
.
-
A.
Bigham-Sadegh,
A
Oryan.
Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. International Wound Journal doi: 10.1111/iwj.12231.
2014
.
-
C.J. S. J.R.
Centeno,
M.
Cheever,
M.
Freeman,
B
Robinson.
A case series of percutaneous treatment of nonunion fractures with autologous, culture expanded, bone marrow derived, mesenchymal stem cells and platelet lysate. J Bioengineer & Biomedical Sci Doi:10.4172/2155-9538.
2011;
S2
:
007
.
-
J.F.
Connolly,
R.
Guse,
J.
Tiedeman,
R
Dehne.
Autologous marrow injection for delayed unions of the tibia. In A pre-liminary report (J Orthop Trauma).
1989;
:
276
.
-
J.F.
Connolly,
R.
Guse,
J.
Tiedman,
R
Dehne.
Autologous marrow injection as a substitute of operative grafting of tibial nonunions. Clin Orthop Relat Res.
1991;
289
:
259-270
.
-
J.M.
Fox,
P.G
Genever.
Use of adult stem cells for orthopedic regenerative medicine applications. Journal Cell & Tissue Transplantation & Therapy.
2014;
6
:
19-25
.
-
V.
Gangji,
J.P.
Hauzeur,
C.
Matos,
V.D.
Maertelaer,
M.
Toungouz,
M.
Lambermont.
Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am..
2004;
86
:
1153-1160
.
-
J.P.
Hauzeur,
V
Gangji.
Phases 1-3 clinical trials using adult stem cells in osteonecrosis and nonunion fractures. Stem Cells International doi:10.4061/2010/410170.
2010
.
-
J.
Hernigou,
F
Schuind.
Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop.
2013;
37
:
883-887
.
-
P.
Hernigou,
G.
Mathieu,
A.
Poignard,
O.
Manicom,
F.
Beaujean,
H
Rouard.
Percutaneous autologous bone marrow grafting for nonunion surgical technique. J Bone Joint Surg 88-A.
2006;
:
322-327
.
-
P.
Hernigou,
A.
Poignard,
F.
Beaujean,
H
Rouard.
Percutaneous autologous bone marrow grafting for nonunion: influence of the number and concentration of progenitor cells. J Bone Joint Surg 87-A.
2005a;
7
:
1430-1447
.
-
P.
Hernigou,
A.
Poignard,
O.
Manicom,
G.
Mathieu,
H
Rouard.
The use of percutaneous autologous bone marrow transplantation in non-union and avascular necrosis of bone. J Bone Joint Surg 87-B.
2005b;
7
:
896-902
.
-
M.
Jager,
M.
Herten,
U.
Fochtmann,
J.
Fischer,
P.
Hernigou,
C.
Zilkens,
C.
Hendrich,
R
Krauspe.
Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res.
2011;
29
:
173-180
.
-
M.S.
Kassem.
Percutaneous autogenous bone marrow injection for delayed union or non-union of fractures after internal fixation. Acta Orthop.
2013;
79
:
711-717
.
-
M. J. S.
Liebergall.,
M.
Rami.
Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. The American Society Of Gene & Cell Therapy Doi:10.1038/Mt.2013.109.
2013
.
-
H.
Ohgushi.
Osteogenically differentiated mesenchymal stem cells and ceramics for bone tissue engineering. Expert Opin Biol Ther.
2014;
14(2)
:
197-208
.
-
A. A. S.
Oryan,
A.
Moshiri,
N
Maffulli.
Bone regenerative medicine: classic options, novel strategies, and future directions. Journal of Orthopaedic Surgery and Research.
2014;
9
:
18
.
-
I.
Pountos,
T.
Georgouli,
G.
Kontakis,
P.V
Giannoudis.
Efficacy of minimally invasive techniques for enhancement of fracture healing: evidence today. International Orthopaedics.
2010;
34
:
3-12
.
-
Y.
Qin,
J.
Guan,
C
Zhang.
Mesenchymal stem cells: mechanisms and role in bone regeneration. Postgrad Med J.
2014;
90
:
643-647
.
-
P.
Reichert,
R.
Rutowski,
J.
Gosk,
K.
Zimmer,
K
Skiba.
Treatment of delayed union of long bones by percutaneous injection of autologous stem cells. Adv Clin Exp Med 16.
2007;
1
:
43-48
.
-
F.A.
Shah,
Z.
Khan,
Z.A.
Durrani,
I.
Kifayatullah,
H
Khan.
Role of percutaneous autologous bone marrow grafting in nonunion tibia. Journal Of Surgery Pakistan 18.
2013;
(2)
:
92-96
.
-
R.M.
Shenoy,
D.
Pinto,
K.V.N.
Dinesh,
G
Chandra.
Osteoinduction using autologous bone marrow in difficult orthopaedic problems - a clinical study. International Journal Of Biomedical And Advance Research 5.
2014;
(06)
:
288-291
.
-
A.K.
Singh,
S.
Shetty,
J.J.
Saraswathy,
A
Sinha.
Percutaneous autologous bone marrow injections for delayed or nonunion of bones. Journal of Orthopaedic Surgery and Research.
2013;
21(1)
:
60-64
.
-
M.A.
Tressler,
J.E.
Richards,
D.
Sofianos,
F.K.
Comrie,
P.J.
Kregor,
W.T
Obremskey.
Bone morphogenetic protein-2 compared to autologous iliac crest bone graft in the treatment of long bone nonunion. Orthopedics 34.
2011;
(12)
:
877-884
.
-
S.S.
Tseng,
M.A.
Lee,
Reddi
MD.
Nonunions and the potential of stem cells in fracture-healing. J Bone Joint Surg Am.
2008;
90
:
92-98
.
-
A.H.
Undale,
J.J.
Westendorf,
M.J.
Yaszemski,
S
Kchosla.
Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin Proc.
2009;
84(10)
:
893-902
.
-
R.M.
Wilkins,
B.T.
Chimenti,
R.M
Rifkin.
Percutaneous treatment of long bone nonunion: the use of autologous bone marrow and allograft bone matrix. Orthopedics 26.
2003;
(5)
:
549-554
.
-
G.
Zimmermann,
A
Moghaddam.
Trauma: nonunion: new trends. European Instructional Lectures.
2010
.
Comments
Downloads
Article Details
Volume & Issue : Vol 2 No 11 (2015)
Page No.: 389-395
Published on: 2015-11-24
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 10289 times
- Download PDF downloaded - 2227 times
- View Article downloaded - 14 times
 Biomedpress
Biomedpress