Abstract
Background: Pyogenic liver abscess (PLA) is a rare but life-threatening disease, with a frequency ranging from 10.83 to 17.45 per 100,000 persons. The major cause of PLA is bacterial infection of liver parenchyma. The present research study was designed to investigate the common microbes causing PLA in Peshawar (Pakistan) and to evaluate a variety of the most capable and efficient antibiotics for treatment of PLA.
Methods: A 7-year (2012 - 2018) retrospective demographic study of medical records of all PLA patients (n = 379) admitted to the Hayatabad Medical Complex (HMC) and Khyber Teaching Hospital (KTH) was initially performed. The demographic study was followed by biochemical tests and antibiotic resistivity tests of microorganisms, isolated from available samples and selected from literature using web services.
Results & Conclusion: The demographic data revealed that 70% of the PLA patients were under the age of 50, with male predominance (male to female ratio of 3:1). It was concluded that K. pneumonia, poly-microbes (K. pneumonia and Citrobacter), and E. coli are the most common microbes involved in causing PLA in the population of Peshawar. E.coli, Citrobacter and K. pneumonia were sensitive to Cefixime and Ciprofloxacin (100% sensitivity rate), but showed significant resistance against Amoxycillin, Oxacillin and Fusidic Acid. It is, therefore, prudent to practice susceptibility-directed antibiotic therapy.
BACKGROUND
Liver is the most vital metabolic core and the largest organ of the human body. Hepatocytes are the main functional unit of the liver and perform many crucial roles which include bile production, lipid metabolism and protein synthesis1. Pyogenic liver abscess (PLA) is a rare but potentially serious hepatic disease with pathophysiological characteristics that fluctuate over time. The morbidity of PLA patients is significantly amplified in Asia, where the yearly occurrence has gradually increased from 10.83 to 17.45 per 100,000 persons, with a mortality rate of 3 - 19%2, 3. According to recent reports, PLA has become an emerging public health problem worldwide, even affecting the European and American populations4, 5, 6, 7, 8, 9, 10, 11. The diagnosis and therapeutic strategies of PLA are medical challenges due to the wide (non-specific) and numerous symptoms, which include fever, nausea, vomiting, abdominal pain and asthenia12, 13. The pathogenic spectra of hepatic abscess may vary across different regions and areas. For successful treatment of PLA patients, it is necessary to understand the entire etiology. The common microbes responsible for causing hepatic abscess are K. pneumonia, E. coli, Citrobacter, Staphylococcus aureus, Proteus, and anaerobic organisms12, 14, 15, 16, 17. PLA diagnosis has increased due to advances in medical science, including ultrasonography and computed tomography scans18. Since the use of antibiotics, including irrelevant ones, was first initiated in clinical medicine, antibiotic-resistant bacteria strains have gradually developed over time19.
The purpose of the present study was to inspect the pathogenic and clinical features of PLA in Peshawar. A retrospective demographic study was conducted on medical records for all PLA patients (n = 379) admitted to the Hayatabad Medical Complex (HMC) and Khyber Teaching Hospital (KTH) during 2012 - 2018 (7-year review). The aim was to investigate the common pathogens and discern the clinical features and microbiological characteristics of PLA more broadly, as well as to identify the most suitable antibiotics for the prevention and treatment of PLA.
METHODS
Initially, a retrospective study was conducted on PLA patients admitted to the Hayatabad Medical Complex (HMC) and Khyber Teaching Hospital (KTH) in Peshawar, Pakistan, during a 7-year period (January 2012 – December 2018). The study also evaluated the microbiological and clinical attributions of hepatic abscess. The medical and microbiological records of all the PLA patients who were hospitalized and treated at the KTH and HMC were retrospectively reviewed. Demographic data and medical information were also retrospectively collected. Patients with the typical clinical symptoms of liver abscess, imaging evidence, laboratory examination samples (blood or pus), and/or surgical findings were included in the present study. Patients with incomplete medical treatment data were not included in the present study.
All the samples, including blood and pus, were processed for bacterial culture in the Department of Microbiology at Khyber Medical University (KMU); our research collaborators were from the Department of Microbiology, College of Life Sciences, Wuhan University, China. To identify the bacterial isolates, VITEK 2 Compact (bioMérieux, Marcy l’Etoile, France) was used. The pathogens causing PLA, as discussed in previous literature, were also exposed to antibiotic sensitivity testing. The antibiotic susceptibility testing was performed by agar disk diffusion method using antibiotic disks. The antibiotics used for susceptibility test included: Amoxycillin, Cefotaxime, Cefixime, Oxacillin, Marinum, Norfloxacin, Ciprofloxacin, Enoxacin, Gentamicin, Amikacin, Clindamycin, Erythrocin and Fusidic Acid.
RESULTS
Demographic data
| Age in years | No of cases | Males | Females | Male (%) | Female (%) |
| 1 to 10 | 13 | 10 | 3 | 76.92 | 20.08 |
| 11 to 20 | 37 | 21 | 16 | 56.75 | 43.25 |
| 21 to 30 | 67 | 46 | 21 | 68.65 | 31.35 |
| 31 to 40 | 74 | 56 | 18 | 75.67 | 24.33 |
| 41 to 50 | 89 | 68 | 21 | 76.40 | 23.60 |
| 51 to 60 | 32 | 20 | 12 | 62.50 | 37.50 |
| 60 to 70 | 37 | 22 | 15 | 59.46 | 40.54 |
| 70 to 80 | 27 | 19 | 8 | 70.37 | 29.63 |
| 85 to 90 | 3 | 2 | 1 | 66.67 | 33.33 |
| Total | 379 | 264 | 115 | 69.66 | 30.34 |
For demographic data of PLA patients, the database of the KTH and HMC was accessed. Initially, the database showed 431 PLA patients, among which 52 patients were eliminated due to lack of data, and results of pus and blood culture. The demographic data of patients is shown in Table 1. An early recognition and appropriate treatment strategy of PLA patients is required due to its severe complications12, 20. Underlying diseases, such as diabetes and other biliary system complications, can play a key role in the development and progression of PLA. To diagnose PLA, ultrasonography and other imaging assessments are useful, while microbiological techniques are essential to identify the key pathogens and understand the etiology of the disease prior to any therapeutic procedure17, 20, 21.
The present findings are in correlation with previous studies which reported on the male predominance in PLA3, 15, 22, 23, 24. Indeed, in our review, it was noted that 70% of the PLA patients were under the age of 50 years. The predominant sex was males (n = 264, 69.66%), with the mean age of 47.7 ± 10.4 years. The most effective treatment of hepatic abscess is likely percutaneous drainage and supplement of a broad spectrum of antibiotics7, 12.
| Name of micro-organism | Arrangement | Shape | Gram reaction | Motility | Aerobic/anaerobic |
| E. Coli | Scattered | Rod | Gram Negative | Motile | Anaerobic |
| K. pneumoniae | Scattered | Rod | Gram Negative | Non-Motile | Facultative anaerobic |
| Staphylococcus aureus | Clusters | Cocci | Gram Positive | Non-Motile | Facultative anaerobic |
| E. aerogenus | Single | Bacillus | Gram Negative | Motile | Facultative anaerobes |
| Marginella | Single | Rod | Gram Negative | Motile | Facultative anaerobes |
| Citrobacter | Clusters | Bacillus | Gram Negative | Motile | Aerobic |
| Pseudomonas | Single | Bacillus | Gram Negative | Motile | Aerobic |
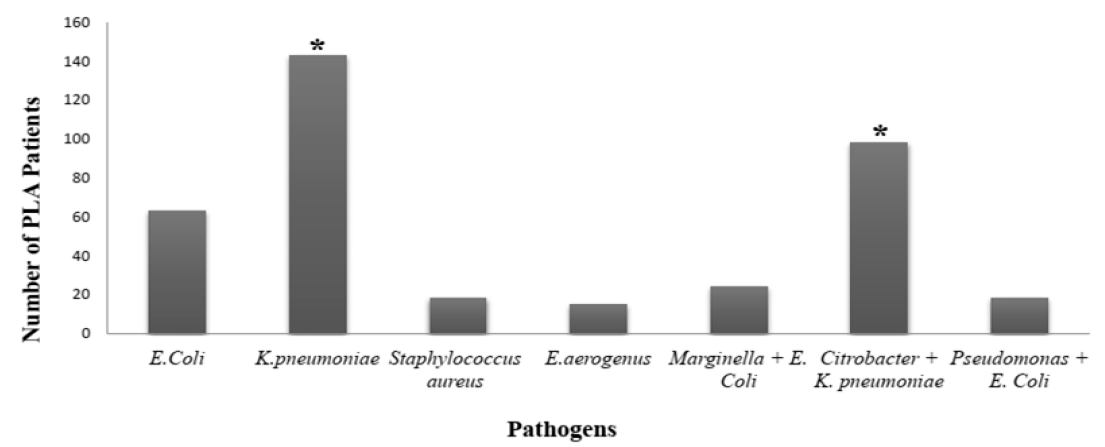
The common microbes causing PLA are Bacteroides, E. coli, Citrobacter, K. pneumoniae, Enterococci, Streptococci, and Staphylococci12, 14, 15, 25. It can be observed from previous reports that during the past few decades, K. pneumonia has surpassed E coli in becoming the leading cause of PLA 12, 26, 27, 28, 29. Similar results were also observed in our current study; K. pneumoniae and E. coli were the most ubiquitous pathogens involve in causing PLA, affecting 143 and 63 patients, respectively. However, Citrobacter, in the presence of K. pneumoniae (i.e. Citrobacter + K. pneumonia) also infected many patients with PLA (n = 98). In a few patients, other pathogens were also detected, such as Marginella + E. Coli (n = 24), and Pseudomonas + E. Coli (n = 18) (Figure 1). Among the microbes detected in PLA patients, Staphylococcus aureus was the only gram-positive bacteria, as seen by the arrangement of cocci in cluster form (Table 2).
Biochemical tests and antibiotic resistivity
The biochemical tests on microbes revealed that the E. coli, E. aerogenes, Staphylococcus aureus, K. pneumoniae and Citrobacter are lactose fermenters by chemical nature. The indole test is usually used to determine the aptitude of an organism to split amino acid tryptophan to form indole compound. Results are shown by either a red or yellow color appearing on the surface layer of the broth. E. coli, Marginella and Citrobacter were found to be positive by indole test, while the rest of the microbes were found to be negative. The methyl red test showed that Pseudomonas, Enterobacter, Staphylococcus aureus and K. pneumoniae were negative for methyl red, as shown in the color change from orange to yellow, while Marginella and Citrobacter were positive for methyl red, as shown by the red color (Table 3).
| Name of microbes | Chemical nature | Motility test | Catalase test | Indole test | Methyl red test | Oxidase test | Vogas-Prosakuer test |
| E. coli | Lactose fermenter | Motile | Positive | Positive | Not done | Negative | Positive |
| Pseudomonas | Doesnot ferment lactose | non-Motile | Positive | Negative | Negative | Positive | Negative |
| E. aerogenus | Lactose fermenter | non-Motile | Positive | Negative | Negative | Negative | Positive |
| Staphlococcus aureus | Lactose fermenter | Motile | Positive | Negative | Negative | Negative | Negative |
| Marginella | Doesnot ferment lactose | Motile | Positive | Positive | Positive | Negative | Negative |
| K. pneumoniae | Lactose fermenter | Motile | Positive | Negative | Negative | Positive | Positive |
| Citrobacter | Lactose fermenter | Motile | Not done | Positive | Positive | Negative | Negative |
The oxidase test is used to verify the potential of the bacteria to produce cytochrome c oxidases. The fluctuation in color from violet to purple indicates a positive test, while production of a light-pink or no color indicates a negative test. Among the microbes subjected to the oxidase test, only Pseudomonas and K. pneumoniae were oxidase-positive. The Voges- Proskauer (VP) test was conducted to investigate the bacterial broth culture for acetoin production. Among the microbes, only E. coli, E. aerogenes and K. pneumoniae have the ability to produce acetoin (Table 3).
K. pneumonia can easily penetrate into the liver through portal circulation, direct spreading or cross the intestinal barrier to cause K. pneumonia-induced hepatic abscess12, 24, 30, 31. Patients should be regularly monitored in order to identify and treat K. pneumonia-induced PLA in a timely manner. Pathogens isolated from PLA patients are highly sensitive to almost every kind of antibiotic, such as β-lactamases, third generation cephalosporins and carbapenems7, 28, 32. The use of amoxicillin or ampicillin may predispose patients to develop K. pneumonia-induced PLA. It has been clinically confirmed that ampicillin or amoxicillin therapy is correlated with K. pneumonia-induced PLA risk22, 33, 34. In a recent animal study, when K. pneumonia-colonized mice were treated with oral ampicillin, K. pneumonia was found to be resistant to ampicillin and induced KP-PLA in the mice; moreover, ampicillin disrupted the intestinal microflora of the mice33. According to the antibiotic susceptibility tests in the present study, the microbes showed a significant rate of resistance against Amoxycillin, Oxacillin and Fusidic Acid. Since the microbes can build resistance to the antibiotics, it is essential before any sort treatment is initiated, to examine the patient’s blood or pus samples in order to accurately identify the key pathogen(s) involved in causing PLA.
β-lactams are broad spectrum antibiotics which include penicillin derivatives, carbapenems and monobactams. These antibiotics inhibit bacterial growth and progression by inhibiting cell wall synthesis of the target bacteria. Among the β-lactam antibiotics applied, Cefixime was found to be the most efficient antibiotic. E. coli, K. pneumoniae, Citrobacter and E. aerogenes were 100% sensitive to Cefixime, followed by Cefotaxime (70%, 58.3%, 67.8% and 52% susceptibility rate, respectively) (Table 4).
| Name of microbes | Amoxycillin | Cofoxitin | Cefotaxime | Cefixime | Oxacillin | Maronum |
| E. coli | 0 | 0 | 70 | 100 | 0 | 17.1 |
| Pseudomonas | 10 | 0 | 27.4 | 80 | 22.1 | 0 |
| E. aerogenus | 0 | 0 | 52 | 100 | 0 | 0 |
| Staphylococcus aureus | 8.33 | 3.84 | 11 | 58.3 | 3.84 | 0 |
| Marginella | 20 | 0 | 19.4 | 60 | 2.31 | 0 |
| K. pneumoniae | 0 | 47.4 | 58.3 | 100 | 3.84 | 11.3 |
| Citrobacter | 9.63 | 0 | 67.8 | 100 | 0 | 10.1 |
Quinolones are a vital group of antibiotics that are responsible for inhibiting DNA synthesis by limiting DNA gyrase activity. Among the quinolones antibiotics used, Ciprofloxacin exhibited the greatest harmful effects on the various bacteria. Indeed, E. coli, E. aerogenes, K. pneumoniae and Citrobacter were 100% sensitive when exposed to Ciprofloxacin (Table 5).
| Name of microbes | Norfloxacin | Ciprofloxacin | Enoxacin |
| E. coli | 16.66 | 100 | 0 |
| Pseudomonas | 0 | 40 | 0 |
| E. aerogenus | 100 | 100 | 0 |
| Staphylococcus aureus | 16.6 | 50 | 0 |
| Marginella | 0 | 40 | 60 |
| K. pneumoniae | 41.6 | 100 | 19.3 |
| Citrobacter | 34 | 100 | 0 |
Besides β-lactam and quinolone antibiotics, there are many different antibiotics that are responsible for inhibiting protein synthesis by interacting with the bacterial 70S ribosome. Among the protein-inhibiting antibiotics used, Erythrocin and Gentamicin significantly limited bacterial progression by inhibiting protein synthesis. E. coli, K. pneumoniae and Citrobacter were noted as being susceptible to Erythrocin (78%, 82.7% and 100% susceptibility rate, respectively) (Table 6).
| Name of microbes | Gentamycin | Amikicin | Clindamycin | Erythrocin | Fusidic acid |
| E. coli | 51.2 | 50 | 33.2 | 78 | 0 |
| Pseudomonas | 40 | 10 | 17.2 | 30 | 0 |
| E. aerogenus | 100 | 0 | 20 | 55.3 | 12 |
| Staphylococcus aureus | 33.3 | 0 | 76.9 | 58 | 0 |
| Marginella | 20 | 53.7 | 20.4 | 43 | 0 |
| K. pneumoniae | 71.1 | 33.8 | 35.6 | 82.7 | 19.4 |
| Citrobacter | 50 | 0 | 25 | 100 | 0 |
CONCLUSION & FUTURE PERSPECTIVES
In conclusion, hepatic abscess is a hazardous health problem common in Asia and rapidly spreading around the world, and one that requires immediate hospitalization. K. pneumonia and E. coli are the leading microbes causing PLA with male predominance. In the present study, a clear profile of the antibiotics with potential against the pathogenic microbes is indicated. Analysis of the microbes demonstrate that they are significantly resistant against Amoxycillin, Oxacillin and Fusidic Acid. As the microbes advance and develop greater resistance to drugs, it is crucial to perform prompt identification of the disease-inducing pathogens, followed by empiric antimicrobial and other effective therapeutic strategies.
A population-based or large-scale study is required to clarify the association between genotypes, resistance spectrum, phenotypes and clones of microbes isolated from patients with PLA. Further studies should be conducted to better understand K. pneumonia-induced PLA and increase awareness, and to develop and deliver more effective treatments for PLA patients.
Acknowledgment
The authors are thankful to the administration and IT team of Hayatabad medical complex (HMC) and Khyber teaching hospital (KTH) for their cooperation and Microbiology laboratory of Khyber medical university (KMU) and Department of microbiology, college of life sciences, Wuhan University, China for providing research facilities.
Funding
No funding was received for the current research work.
Author’s contribution
All the authors equally contributed in the study concept and design, laboratory procedures, data and statistical analysis, initial draft preparation and the revision of the manuscript.
Data availability
The manuscript contains all the analyzed data and make material.
Ethical approval and consent
The Bio-ethics committee of Khyber medical university approved all the study and procedure, while the data of patients accessed was unspecified therefore the ethics committee of both the hospitals approved the retrospective analysis.
Conflict of interest
All the authors declared that they have no conflict of interest.
References
-
Kazmi A, Kazmi A, Shams S, Sajid A, Khan K. Therapeutic role of bone marrow-derived stem cells and zinc sulfate for reduction of liver fibrosis. Progress in Stem Cell. 2019;6(2):269-78.
.
View Article Google Scholar -
Cerwenka H. Pyogenic liver abscess: differences in etiology and treatment in Southeast Asia and Central Europe. World journal of gastroenterology: WJG. 2010;16(20):2458.
.
View Article PubMed Google Scholar -
Chen Y-C, Lin C-H, Chang S-N, Shi Z-Y. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000-2011. Journal of Microbiology, Immunology and Infection. 2016;49(5):646-53.
.
View Article PubMed Google Scholar -
Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. The American journal of gastroenterology. 2005;100(2):322.
.
View Article PubMed Google Scholar -
Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel medicine and infectious disease. 2008;6(4):228-33.
.
View Article PubMed Google Scholar -
Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clinical infectious diseases. 2004;39(11):1654-9.
.
View Article PubMed Google Scholar -
Moore R, O'shea D, Geoghegan T, Mallon P, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection. 2013;41(3):681-6.
.
View Article PubMed Google Scholar -
Melot B, Colot J, Guerrier G. Bacteremic community-acquired infections due to Klebsiella pneumoniae: clinical and microbiological presentation in New Caledonia, 2008-2013. International Journal of Infectious Diseases. 2015;41:29-31.
.
View Article PubMed Google Scholar -
Chavada R, Ng J, Maley M, Descallar J. Emergence of Klebsiella pneumoniae liver abscesses in South-western Sydney. Infection. 2014;42(3):595-6.
.
View Article PubMed Google Scholar -
Fazili T, Sharngoe C, Endy T, Kiska D, Javaid W, Polhemus M. Klebsiella pneumoniae liver abscess: an emerging disease. The American journal of the medical sciences. 2016;351(3):297-304.
.
View Article PubMed Google Scholar -
Chang EK, Kao K-L, Tsai M-S, Yang C-J, Huang Y-T, Liu C-Y, et al. Occult Klebsiella pneumoniae bacteremia at emergency department: A single center experience. Journal of Microbiology, Immunology and Infection. 2015;48(6):684-91.
.
View Article PubMed Google Scholar -
Shi S-h, Zhai Z-l, Zheng S-s, editors. Pyogenic liver abscess of biliary origin: the existing problems and their strategies. Seminars in liver disease; 2018: Thieme Medical Publishers.
.
View Article PubMed Google Scholar -
Liu Y, Wang J-y, Jiang W. An increasing prominent disease of Klebsiella pneumoniae liver abscess: etiology, diagnosis, and treatment. Gastroenterology research and practice. 2013;2013.
.
View Article PubMed Google Scholar -
Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clinical microbiology reviews. 2019;32(3):e00001-19.
.
View Article PubMed Google Scholar -
Jun J-B. Klebsiella pneumoniae liver abscess. Infection & chemotherapy. 2018;50(3):210-8.
.
View Article PubMed Google Scholar -
Ye M, Tu J, Jiang J, Bi Y, You W, Zhang Y, et al. Clinical and genomic analysis of liver abscess-causing Klebsiella pneumoniae identifies new liver abscess-associated virulence genes. Frontiers in cellular and infection microbiology. 2016;6:165.
.
View Article PubMed Google Scholar -
Tulachan SS, Wilkins CE, Cutrona AF, Gemmel D, Marnejon TP. Hepatic abscess associated with Salmonella serotype B in a chronic alcoholic patient. Hepatobiliary & Pancreatic Diseases International. 2013;12(4):440-2.
.
View Article Google Scholar -
Adnan Baig ,Muhammad Ishaq ,Ajeet Kumar ,M. Ishaq Sheikh , Pyogenic liver abscess: a five year retrospective study in slums of Karachi, J. Liaquat Univ. Med. Health Sci. 2012; 11 (1): 19-23.
.
-
Lu P-L, Liu Y-C, Toh H-S, Lee Y-L, Liu Y-M, Ho C-M, et al. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009-2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). International journal of antimicrobial agents. 2012;40:S37-S43.
.
View Article Google Scholar -
Petri A, Höhn J, Hodi Z, Wolfárd A, Balogh A. Pyogenic liver abscess-20 years' experience. Langenbeck's archives of surgery. 2002;387(1):27-31.
.
View Article PubMed Google Scholar -
Zhu X, Wang S, Jacob R, Fan Z, Zhang F, Ji G. A 10-year retrospective analysis of clinical profiles, laboratory characteristics and management of pyogenic liver abscesses in a Chinese hospital. Gut and liver. 2011;5(2):221.
.
View Article PubMed Google Scholar -
Kong H, Yu F, Zhang W, Li X. Clinical and microbiological characteristics of pyogenic liver abscess in a tertiary hospital in East China. Medicine. 2017;96(37).
.
View Article PubMed Google Scholar -
Chen J, Zhang M, Chen J, Ning Y, Cai X, Zhang L, et al. Cryptogenic and non-cryptogenic liver abscess: A retrospective analysis of 178 cases revealed distinct characteristics. Journal of International Medical Research. 2018;46(9):3824-36.
.
View Article PubMed Google Scholar -
Zhang S, Zhang X, Wu Q, Zheng X, Dong G, Fang R, et al. Clinical, microbiological, and molecular epidemiological characteristics of Klebsiella pneumoniae-induced pyogenic liver abscess in southeastern China. Antimicrobial Resistance & Infection Control. 2019;8(1):166.
.
View Article PubMed Google Scholar -
Meddings L, Myers RP, Hubbard J, Shaheen AA, Laupland KB, Dixon E, et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. The American journal of gastroenterology. 2010;105(1):117.
.
View Article PubMed Google Scholar -
Lok K-H, Li K-F, Li K-K, Szeto M-L. Pyogenic liver abscess: clinical profile, microbiological characteristics, and management in a Hong Kong hospital. J Microbiol Immunol Infect. 2008;41(6):483-90.
.
-
Shelat VG, Wang Q, Chia CL, Wang Z, Low JK, Woon WW. Patients with culture negative pyogenic liver abscess have the same outcomes compared to those with Klebsiella pneumoniae pyogenic liver abscess. Hepatobiliary & Pancreatic Diseases International. 2016;15(5):504-11.
.
View Article Google Scholar -
Siu LK, Yeh K-M, Lin J-C, Fung C-P, Chang F-Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. The Lancet infectious diseases. 2012;12(11):881-7.
.
View Article Google Scholar -
Choi HY, Cheon GJ, Kim YD, Han KH, Kim KS, Nah BK. Comparison of clinical characteristics between cryptogenic and biliary pyogenic liver abscess. The Korean journal of gastroenterology Taehan Sohwagi Hakhoe chi. 2007;49(4):238-44.
.
-
Zhang J, Du Z, Bi J, Wu Z, Lv Y, Zhang X, et al. The impact of previous abdominal surgery on clinical characteristics and prognosis of pyogenic liver abscess: a 10-year retrospective study of 392 patients. Medicine. 2018;97(39).
.
View Article PubMed Google Scholar -
Tu Y-C, Lu M-C, Chiang M-K, Huang S-P, Peng H-L, Chang H-Y, et al. Genetic requirements for Klebsiella pneumoniae-induced liver abscess in an oral infection model. Infection and immunity. 2009;77(7):2657-71.
.
View Article PubMed Google Scholar -
Lam MM, Wyres KL, Duchêne S, Wick RR, Judd LM, Gan Y-H, et al. Population genomics of hypervirulent Klebsiella pneumoniae clonal-group 23 reveals early emergence and rapid global dissemination. Nature communications. 2018;9(1):2703.
.
View Article PubMed Google Scholar -
Lin Y-T, Liu C-J, Yeh Y-C, Chen T-J, Fung C-P. Ampicillin and amoxicillin use and the risk of Klebsiella pneumoniae liver abscess in Taiwan. The Journal of infectious diseases. 2013;208(2):211-7.
.
View Article PubMed Google Scholar -
Lee H-L, Lee H-C, Guo H-R, Ko W-C, Chen K-W. Clinical significance and mechanism of gas formation of pyogenic liver abscess due to Klebsiella pneumoniae. Journal of clinical microbiology. 2004;42(6):2783-5.
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 12 (2020)
Page No.: 4190-4196
Published on: 2020-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8792 times
- Download PDF downloaded - 1913 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress



