Abstract
Introduction: Gastric cancer is the most common cancer with significant increasing trends during the last decade in Iran. The aim of this study was to evaluate the epidemiologic profile of gastric cancer along with gastric cancer-specific survival analysis.
Methods: This was an analytical cross-sectional study in which all gastric cancer data were analyzed using the database of the East Azerbaijan Population-Based Cancer Registry (EA-PBCR). The incidents of definitive gastric cancer diagnosis were between the period of March 20th, 2015 to March 19th, 2017 ( = 3 Iranian solar years). The survival analysis was performed using the Kaplan-Meier method and life tables for 1- to 5-year survival data. The Log-rank test and Cox regression were computed to test the equality of survival function and mortality hazard.
Results: Overall, 2,631 newly diagnosed gastric cancer cases were registered for 3 years. Gastric cancer was 2.35 times more common in men than women. The most common age group was the 7th decade- with 531 (31.2%) gastric cancer cases. Most of the gastric cancer cases were non-cardia (n = 2,244, 85.29%) cancer, and the proportion of non-cardia to cardia gastric cancer was 5.8:1. Overall survival was 60.1%, and 1- to 5-year survival proportions were 91.61%, 64.21%, 58.53%, 30.14% and 24.77%, respectively. Cardia cancers had a worse survival rate than non-cardia cancers, and the hazard of mortality was 1.33 times higher in cardia than non-cardia cancers (hazard ratio or HR = 1.33; 95% CI: 1.05 - 1.68; P = 0.017).
Conclusion: Non-cardia gastric cancer is still the most dominant subsite in East Azerbaijan, Iran. There was a higher 1- to 5- year survival proportion in East Azerbaijan, with lower overall mortality rates, compared to other regions of Iran.
Introduction
The incidence of gastric cancer is particularly high in Iran, where it remains a leading cause of cancer-related deaths. Gastric cancer is the most common cancer among Iranian men, with an age-standardized incidence rate (ASR) of 21.6 per 100,000 males, and is third among females (ASR = 9.44/100,000 women)1, 2. The incidence and mortality of gastric cancer, by contrast, rank fifth and third, respectively, in both genders worldwide1.
Studies have shown that there has been an overall decrease of ASRs (between 2007 and 2017) by 6% worldwide and for all socio demographic index (SDI) quintiles. Gastric cancer was the most common cancer in men compared to women, especially in high-middle SDI countries, with 1 in 33 men and 1 in 78 women worldwide developing stomach cancer over a lifetime3.
Although the global incidence of gastric cancer has decreased in recent decades, in Iran as well4, there is still a high incidence of this cancer in the north and northwest regions of Iran compared to other regions of the country. Clustering the incidence of gastric cancer during 2004-2008 for the provincial-level, and in 2010 for the country-level, showed that the north and northwest of Iran are high-risk clusters4, 5, 6. The Ardebil Province in Northwest Iran has been introduced as a high-risk area- with the highest ASRs and age-standardized mortality rates (ASMRs) of gastric cancer7, 8, 9, 10. Moreover, there has been a six-fold difference in gastric cancer mortality rates between the northern and southern parts of Iran11. Recently, in the East Azerbaijan Province (located in the northwest of Iran), gastric cancer has become the most common cancer with significant increasing trends- by the overall annual percentage change (APC) statistical test- which showed 1.8% APC in both genders during the last decade, which was higher in men (APC, 3.2%) than in women (APC, 1.3%)12, 13.
Since the incidence and mortality rates of gastric cancer have varied significantly in recent years in the different regions and provinces of Iran 4, 14, 15, it is necessary to have an epidemiological study of the incidence, gender and age distribution, and morphological type of gastric cancer. The aim of this study was to evaluate the epidemiologic profile of gastric cancer, along with 1- to 5-year gastric cancer specific survival analysis, in cases from East Azerbaijan, Iran.
Methods
Data Sources
All newly diagnosed cancer data were collected in EA-PBCR database, during the period of the 20th of March 2015 to the 19th of March 2017 (= 3 Iranian solar years), and from different data sources. The major sources of data collection related to the cancer cases were reports from pathology laboratories, hospital records, radiotherapy centers, and death registry databases.
The main demographic data, including the first and last name, national identification (NID) number, date of birth, date of diagnosis, gender, place of birth, and contact information were all recorded in the database. The morphology (including histology, behavior and grade of the cancer) and topography (primary site of the tumor) were coded and collected according to the International Classification of Disease for Oncology (ICD-O-3).
Population
This was an analytical cross-sectional study in which all gastric cancer data were analyzed using the database of the East Azerbaijan Population Based Cancer Registry (EA-PBCR), which included the definitive diagnoses of gastric cancer cases in the entire East Azerbaijan Province.
The East Azerbaijan Province is located in the northwest of Iran, and has a population of approximately 4 million people from the 20 counties, 62 cities, and 44 districts which comprise the largest Azeri Ethnic population in Iran.
Quality Control
Statistical data analysis, as well as quality and consistency checks, were performed to ensure clean and non-duplicated data. Most of the cases provided their address and contact information; follow-up and outcome data were obtained by contacting the patients' relatives and/or from the Hospital Information System (HIS).
Statistical Analysis
Gastric cancer-specific 1- to 5-year survival analysis and mortality rates were calculated using STATA MP 14.2 (Stata Corp LP, College Station, TX, USA). The survival status was considered from the date of diagnosis (as new cases were recorded in the EA-PBCR) to the date of gastric cancer-related death, and for censored cases until the last follow-up time. Patients were followed until the end of September 2019. The survival analysis was performed using the Kaplan-Meier method and life tables for 1- to 5-year survival data. The log-rank test and Cox regression analysis were used to test the equality of survival function and mortality hazard (HR, with 95% CI) by age, gender, morphology, subsite, stage and grade.
Results
Study Sample
We registered 2,631 new gastric cancer cases during 3 years: 918 (34.89%) cases during 2015, 782 (29.72%) during 2016, and 931 (35.39%) during 2017. Of the cases, 1,849 (70.19%) were males and 784 (29.81%) were females; the male to female ratio was 2.35:1. The mean age of the patients was 67.52 (SD 12.77) years, with a range of 21-99 years. The most common age group was the 7th decade- with 531 (31.2%) cases having gastric cancer.
The most common morphological type was intestinal type adenocarcinoma (n = 1322, 50.25%), followed by diffuse type AC (n = 677, 25.73%). Of the cases, 387 (14.71%) had cardia gastric cancer and 2,244 (85.29%) had non-cardia gastric cancer; the proportion of non-cardia to cardia was 5.8:1.
The stage of tumor was defined for 257 cases, whereby 53.70% of the cases (n = 138) were in stage II, and 27.63% of the cases were in stage III. The grade of cancer was available in 2,445 cases; of these, 384 (15.71%) were grade I, 150 cases (6.13%) were grade II, 253 cases (10.35%) were grade III, and most of the cases (n = 1,658, 67.81%) were grade IV gastric cancer. The descriptive data are shown in Table 1 .
| Characteristics | Total Cohort (n = 2,631) | Cohort with Follow-up (n = 1,288) | |
| Age | Mean (± SD) | 67 (± 12.77) | 66.70 (± 12.37) |
| Range | 21 - 99 | 26 - 97 | |
| ≤ 65 years of age | 1,068 (40.59%) | 557 | |
| > 65 years of age | 1,563 (59.41%) | 731 (56.75%) | |
| Sex | Male | 1,847 (70.20%) | 929 (72.13%) |
| Female | 784 (29.80%) | 359 (27.87%) | |
| Subsite | Non-Cardia | 2,244 (85.29%) | 1,027 (79.74%) |
| Cardia | 387 (14.71%) | 261 (20.26%) | |
| Morphology | AC, Intestinal Type | 1322 (50.25%) | 665 (51.63%) |
| AC, Diffuse Type | 677 (25.73%) | 380 (29.50%) | |
| Others | 632 (24.02%) | 243 (18.87%) | |
| Grade | I | 384 (15.71%) | 171(13.32%) |
| II | 150 (6.13%) | 112 (8.72%) | |
| III | 253 (10.35%) | 213 (16.59%) | |
| IV | 1,658 (67.81%) | 788 (61.37%) | |
| Total (n = 2,445) | Total (n = 1,284) | ||
| Stage | I | 46 (17.90%) | 27 (13.99%) |
| II | 138 (53.70%) | 96 (49.74%) | |
| III | 47 (18.29%) | 52 (26.94%) | |
| IV | 26 (10.12%) | 18 (9.33%) | |
| Total (n = 257) | Total (n = 193) | ||
| Years of study | Case (percent) | No. of subject* (%) | 5-Years Mortality rate/100,000 people | Overall survival | Mean Overall Survival |
| 2015 | 182 (14.13%) | 188 (36.58%) | 66.69 | 0.00% | 14.99 |
| 2016 | 463 (35.95%) | 144 (28.02%) | 10.58 | 68.9% | 29.37 |
| 2017 | 643 (49.92%) | 182 (35.40%) | 13.58 | 70.8% | 21.11 |
| Total | 1288 | 514 | 17.19 | 60.1% | 23.22 |
| Variable | Uni-variate Cox Regression | Multivariate Cox Regression* | ||||||||
| Freq. (Percent %) | HR† | 95% Confidence Interval | P value | HR* | 95% Confidence Interval | P value | ||||
| Age | ≤ 65 | 557 (43.25%) | Ref. | - | - | - | Ref. | - | - | - |
| > 65 | 731 (56.75%) | 1.01 | 0.85 | 1.21 | 0.14 | 1.05 | 0.60 | 1.82 | 0.867 | |
| Sex | Male | 929 (72.13%) | Ref. | - | - | - | Ref. | - | - | - |
| Female | 359 (27.87%) | 1.00 | 0.83 | 1.22 | 0.961 | 1.04 | 0.60 | 1.79 | 0.893 | |
| Subside | Non-Cardia | 1,027 (79.74%) | Ref. | - | - | - | Ref. | - | - | - |
| Cardia | 261 (20.26%) | 1.33 | 1.05 | 1.68 | 0.017 | 0.95 | 0.51 | 1.79 | 0.885 | |
| Stage | I | 27 (13.99%) | Ref. | - | - | - | Ref. | - | - | 0.000 |
| II | 96 (49.74%) | 8.35 | 1.13 | 16.44 | 0.037 | 7.15 | 1.00 | 13.06 | 0.051 | |
| III | 52 (26.94%) | 4.27 | 3.72 | 5.89 | 0.000 | 2.22 | 1.51 | 3.01 | 0.003 | |
| IV | 18 (9.33%) | 4.66 | 3.83 | 5.67 | 0.000 | 3.84 | 1.38 | 3.33 | 0.001 | |
| Grade | I | 171 (13.32%) | Ref. | - | - | - | Ref. | - | - | 0.000 |
| II | 112 (8.72%) | 3.99 | 2.04 | 7.79 | 0.000 | 2.47 | 0.75 | 8.14 | 0.135 | |
| III | 213 (16.59%) | 7.35 | 4.42 | 13.72 | 0.000 | 4.73 | 1.82 | 12.28 | 0.001 | |
| IV | 788 (61.37%) | 6.06 | 3.40 | 10.81 | 0.000 | 2.36 | 0.95 | 5.87 | 0.064 | |
| Morphology | AC, Intestinal Type | 665 (51.63%) | 1.19 | 0.93 | 1.51 | 0.158 | 0.83 | 0.42 | 1.67 | 0.607 |
| AC, Diffuse Type | 380 (29.50%) | 1.18 | 0.91 | 1.54 | 0.213 | 0.82 | 0.40 | 1.69 | 0.591 | |
| Others | 243 (18.87%) | Ref. | - | - | - | Ref. | - | - | ||
Survival Analysis
Of the 2,631 cases, survival analysis was performed for 1,288 cases (we did not have access to contact information for the other 1,343 cases). From these, 514 (39.90%) cases died and overall survival was 60.1% for the 5-year survival analysis. The 1-year to 5-year survival proportions were 91.61%, 64.21%, 58.53%, 30.14% and 24.77%, respectively. The overall mortality incidence rate was 17.19 per 100,000 people, and mean follow-up time was 23.22 (range of 6 - 36) months.
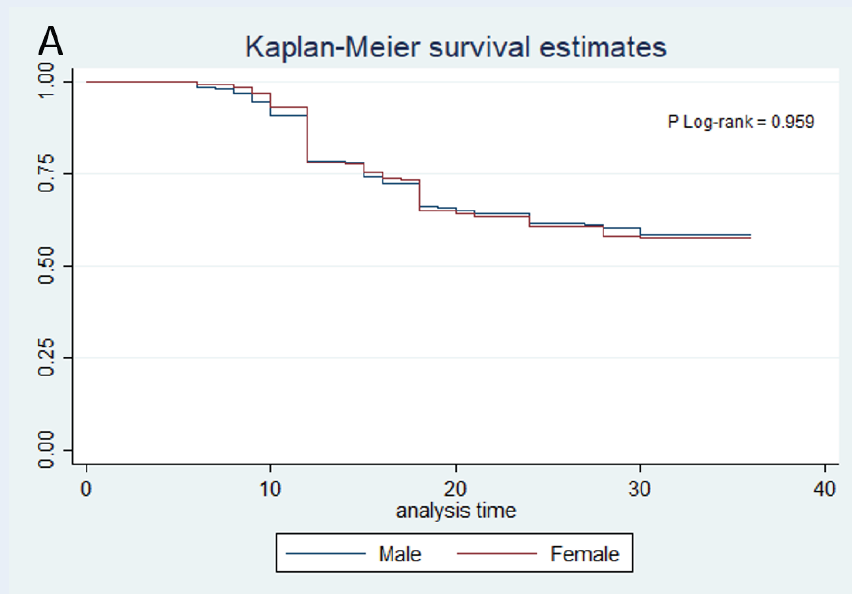
Log-rank test for equality of survival functions did not show any significant difference among cases ≤ 65 years and > 65 years (P log-rank = 0.881). There was not any significant difference of survival functions between males and females (P log-rank = 0.959), but survival functions showed significant differences with respect to grade of tumor (P log-rank = 0.000) and stage of cancer (P Log-rank = 0.000), as higher stages and grades had worse survival functions. The morphological type of cancer did not have a significant effect on survival function (P log-rank = 0.316); however, cases with cardia tumors had worse survival than non-cardia tumors (P log-rank = 0.012) (Figure 1 ).
Cox regression analysis at univariate level showed that the hazard of mortality was 1.33 times higher in cardia than non-cardia tumors (HR = 1.33; 95% CI: 1.05-1.68; P = 0.017). In addition, higher stage and grade significantly increased the hazard of mortality compared with lower stage and grade. For example, HR = 4.6 (95% CI: 3.83-5.67; P = 0.000) for stage IV vs. stage I, and HR=6.06 (95% CI: 3.40-10.81; P = 0.000) for grade IV vs. grade I.
At multivariate level, after adjusting all variables, regression analysis showed that grade of cancer significantly increased the HR, as HR in tumors with grade 4 was 2.36 times higher than those with grade 1 (HR = 2.36; 95% CI;0.95 - 5.87; P = 0.064). Those with grade 3 were 4.73 times higher than those with grade 1 (HR = 4.73; 95% CI:1.82 - 12.28; P = 0.001), and those with grade 2 had a HR of 2.47 (95% CI: 0.75 - 8.14: P = 0.135) compared to those with grade 1 cancer. Age, gender, and morphological type did not impart any statistical differences among the groups with respect to mortality hazards. The results were presented in detail in Table 3.
Discussion
According to the last published results of the EA-PBCR, gastric cancer was the most common cancer among men (ASR 29.7) and the third common in women (ASR 13.3). Therefore, we aimed to evaluate the epidemiologic profile of gastric cancer- along with 1- to 5-year gastric cancer specific survival analysis- in East Azerbaijan, the northwest region of Iran. During the 3 years of study, 2,631 newly diagnosed gastric cancer cases were registered in East Azerbaijan. The most common age group was the 7th decade- with 531 (31.2%) gastric cancer cases. Non-cardia gastric cancer frequency was markedly higher (85% of cases), with 53.70% of cases in stage II and 67.81% with grade IV disease. The overall mortality incidence rate was 17.19 per 100,000 people, and the overall survival proportion was 60.1% - with mean follow-up time of 23.22 (range of 6 - 36) months for a 5-year study. The 1- to 5-year survival proportions were 91.61%, 64.21%, 58.53%, 30.14% and 24.77%, respectively. Cardia cancer had a 1.33-fold higher mortality hazard ratio than non-cardia cancer. Survival functions showed a significant difference with the grade of tumor (P log-rank = 0.000) and stage of cancer (P log-rank = 0.000), as higher stages and grades had worse survival functions.
According to the GLOBOCAN 2018 report, gastric cancer is the 5th most common cancer and the 3rd leading cause of cancer-related deaths, accounting for 8.2% of all cancer deaths worldwide 1. Gastric cancer is the most common cancer among males- in terms of incidence and mortality- in Western Asian countries, including Iran, Turkmenistan, and Kyrgyzstan. The highest age-standardized incidence rates were reported in Korea for both genders 1. Gastric cancer patients have an overall survival of about 90% in the early stages of disease; the 5-year survival proportions are 94% and 88% for stages IA and IB, respectively. However, in cases with advanced stage, the prognosis is poor. Recently, the average 5-year survival rate was 31% in the United States, 19% in the United Kingdom, and 26% in Europe 16, 17. Gastric cancer is ranked as the leading cause of cancer-related deaths in Iran, accounting for 16.1% of cancer-related deaths. East Azerbaijan of Iran is a high-risk region of gastric cancer for both males and females 7. Previous reports have revealed extremely high incidence and mortality rates for gastric cancer in East Azerbaijan, with gastric cancer being the 2nd leading cause of death (10.4% of all deaths) 12, 13, 18.
The estimated gastric cancer specific Age-Standardized Mortality Rates (ASMRs) in 29 different provinces of Iran revealed extremely high mortality rates. There is a 6-fold difference between the northern (ASMR = 29.1 per 100,000) and southern (ASMR = 5.0 per 100,000) regions of Iran, with an average ASMR of 15 for males and 8.1 for females11. Iran is a large and geographically diverse country with several ethnic groups. Therefore, the variation in ASMRs and the high gastric cancer mortality rate in Iran may be linked to gene-environmental factors. However, different environmental risk factor exposures, including H. pylori infections and patterns, may also contribute to these variations. The overall 5-year mortality rate in our study was 17.19 per 100,000; this rate has been on a tremendous decline during the last decade. According to the results of a study by Zendehdel et al., the ASMR in Iran in 2012 was 27.6 per 100,000 in men (ranking as the 2nd highest ASMR), and 13.6 per 100,000 in women (ranking as the 4th highest ASMR) 11.
Our results showed better 1- to 5-year survival proportions compared with previous reports from Iran19, 20, 21, 22. According to other European reports, Iran still have lower survival rates than most developed countries 23. In our study, since more than half of the cases were in stage II of disease, and non-cardia gastric cancer was more common than cardia (85% vs. 15%), these results may have led to better prognosis. Cancers of the gastric cardia have epidemiological characteristics which are more similar to those of esophageal adenocarcinoma, and with poor prognosis; incidentally, the incidence of cardia cancers has been increasing, particularly in high-income countries 24, 25. Evidence from previous studies have shown that the prognosis is better for non-cardia than cardia cancers 26, 27, 28. There was a significant difference in survival function between cardia and non-cardia gastric adenocarcinoma in this study as well, with cardia cases having worse survival than non-cardia cases. Indeed, gastric cardia cancers had a 1.33-fold higher mortality hazard ratio than non-cardia cancers. Similar findings to ours have been reported in other studies conducted in Iran 19, 20, 21, 22.
Regardless of the overall incidence of gastric cancer, the proportion of non-cardia to cardia involvement in different population types seems to have different patterns 7. Malekzadeh et al. emphasized this theory of higher rates of gastric cardia vs. non-cardia in the north and northwest regions of Iran, and vice versa for the south region and low risk areas of Iran, such as Khuzestan7. However, the results of our study showed an obvious higher incidence of non-cardia cancers in East Azerbaijan; the 85% prevalence of H. pylori infection in the East Azerbaijan population probably explains the higher incidence of non-cardia cancer in the present study 29, 30.
Gastric cancer is more common in men than women in most countries. The male to female ratio has ranged from 2.2:1 in developed countries, to 1.83:1 in developing countries 16. In the present study, about 70% of gastric cases were males, and the male to female ratio was 2.35:1. Although the gastric cancer mortality rate is 2-3 times higher in men than women 1, 31, there was no significant difference in survival function among genders in this study. While most other studies from Iran have shown lower overall survival in males 19, 20, gender did not have any significant impact on mortality hazard of gastric cancer, according to the results of our study.
Strengths and Limitations of the Study
Population-based cancer registries are the best resources for epidemiological and surveillance systems and studies. In this study, we utilized one of the best and most reliable population-based cancer registry data in recent years. Indeed, the East Azerbaijan population-based cancer registry (EA-PBCR) has been established with high quality and timeliness in the region.
EA-PBCR is in its first step toward improving the quality indicators, including comparability, validity, timeliness, and completeness of cancer data. However, we performed the survival analysis only on approximately half of the registered gastric cancer cases in this study because we could not access the contact information of the others. Moreover, the stage of the disease is not a mandatory variable in the recent Iranian National Cancer Registry program; therefore, the stage was not available for most of gastric cancer cases. We hope to achieve better and more complete data concerning the stage and grade of cancers since a nationwide program has been recently instated in Iran for cancer staging, and is an ongoing plan at present.
Conclusion
In conclusion, non-cardia cancer is still the dominant subsite of gastric cancer in East Azerbaijan. However, we had higher 1- to 5- year survival proportions in East Azerbaijan, with improvement in mortality rates in the region. Additional studies of gastric cancer, particularly with respect to cancer subsite and cancer morphology, would be most informative.
Abbreviations
APC: Annual percentage change
ASMR: Age-standardized mortality rate
ASR: Age-standardized incidence rate
CI: Confidence Interval
EA-PBCR: East Azerbaijan Population-Based Cancer Registry
HR: Hazard Ratio
ICD-O: International Classification of Disease for Oncology
NID: National identification
OS: Overall Survival
SDI: Socio demographic index
Acknowledgments
We would like to thank you from The East Azerbaijan Population Based Cancer Registry (EAPBCR) for providing the data of this study.
Author’s contributions
PJ, RD: substantial contributed to conception and design, acquisition of data, and analysis and interpretation of data; PJ, RD: drafting the article, and revised manuscript critically for important intellectual content; MHS, HMA: revised manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by Tabriz University of Medical Sciences as a confirmed research project [Grant number: 5/d/4876, 1395/2]; and Ministry of Health and Medical Education, Deputy of Research and Technology for manuscript submission (Grant number: 700/1480, 1395.10.4). The funding body didn’t have any role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Availability of data and materials
Data and materials used and/or analysed during the current study are available from the corresponding author on reasionable request.
Ethics approval and consent to participate
The East Azerbaijan Population Based Cancer Registry (EAPBCR) provided the data of this study. The ethics committee of Tabriz University of Medical Sciences has been approved this project, and all patients information and records are confidential (Grant Number: IR.TBZMED.REC.1395.1333). We followed ethics rules of EAPBCR for manuscript publication.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin.
2018;
68
(6)
:
394-424
.
View Article PubMed Google Scholar -
Roshandel G, Ghanbari-Motlagh A, Partovipour E, Salavati F, Hasanpour-Heidari S, Mohammadi G, et al. Cancer incidence in Iran in 2014: Results of the Iranian National Population-based Cancer Registry. Cancer Epidemiol.
2019;
61
:
50-58
.
View Article PubMed Google Scholar -
Global Burden of Disease Cancer C, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol.
2019;
5
(12)
:
1749-1768
.
View Article Google Scholar -
Rastaghi S, Jafari-Koshki T, Mahaki B, Bashiri Y, Mehrabani K, Soleimani A. Trends and Risk Factors of Gastric Cancer in Iran (2005-2010). Int J Prev Med.
2019;
10
:
79
.
View Article PubMed Google Scholar -
Kavousi A, Bashiri Y, Mehrabi Y, Etemad K, Teymourpour A. Identifying high-risk clusters of gastric cancer incidence in Iran, 2004 - 2009. Asian Pac J Cancer Prev.
2014;
15
(23)
:
10335-10337
.
View Article PubMed Google Scholar -
Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. . Cancer Epidemiol Biomarkers Prev.
2014;
23
(5)
:
700-713
.
View Article PubMed Google Scholar -
Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med.
2009;
12
(6)
:
576-583
.
-
Abdi E, Latifi-Navid S, Zahri S, Yazdanbod A, Safaralizadeh R. Helicobacter pylori genotypes determine risk of non-cardia gastric cancer and intestinal- or diffuse-type GC in Ardabil: A very high-risk area in Northwestern Iran. Microb Pathog.
2017;
107
:
287-292
.
View Article PubMed Google Scholar -
Leylabadlo HE, Kafil HS, Yousefi M. Gastric cancer mortality in a high-incidence area (Ardabil Province, Northwest Iran): What risk factors are causative?. Eur J Cancer Prev.
2016;
25
(6)
:
573-574
.
View Article PubMed Google Scholar -
Sadjadi A, Malekzadeh R, Derakhshan MH, Sepehr A, Nouraie M, Sotoudeh M, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer.
2003;
107
(1)
:
113-118
.
View Article PubMed Google Scholar -
Zendehdel K, Marzban M, Nahvijou A, Jafari N. Six-fold difference in the stomach cancer mortality rate between northern and southern Iran. Arch Iran Med.
2012;
15
(12)
:
741-746
.
-
Somi MH, Dolatkhah R, Sepahi S, Belalzadeh M, Naghashi S, Asghari Jafarabadi M. A 12-year trend analysis of the incidence of gastrointestinal cancers in East Azerbaijan: last updated results of an ongoing population-based cancer registry. BMC Cancer.
2019;
19
(1)
:
782
.
View Article PubMed Google Scholar -
Somi MH, Dolatkhah R, Sepahi S, Belalzadeh M, Sharbafi J, Abdollahi L, et al. Cancer incidence in the East Azerbaijan province of Iran in 2015-2016: results of a population-based cancer registry. BMC Public Health.
2018;
18
(1)
:
1266
.
View Article PubMed Google Scholar -
Moradzadeh R, Anoushirvani AA. Trend of Gastric Cancer Incidence in an Area Located in the Center of Iran: 2009-2014. J Gastrointest Cancer.
2020;
51
(1)
:
159-164
.
View Article PubMed Google Scholar -
Moradzadeh R, Nadrian H, Najafi A. Trend of gastric cancer in a province in Western Iran: A population-based study during 2001-2014. J Res Med Sci.
2020;
25
:
12
.
View Article PubMed Google Scholar -
Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol.
2019;
14
(1)
:
26-38
.
View Article PubMed Google Scholar -
Feng Q, May MT, Ingle S, Lu M, Yang Z, Tang J. Prognostic Models for Predicting Overall Survival in Patients with Primary Gastric Cancer: A Systematic Review. Biomed Res Int.
2019;
2019
:
5634598
.
View Article PubMed Google Scholar -
Somi MH, Golzari M, Farhang S, Naghashi S, Abdollahi L. Gastrointestinal cancer incidence in East Azerbaijan, Iran: update on 5 year incidence and trends. Asian Pac J Cancer Prev.
2014;
15
(9)
:
3945-3949
.
View Article PubMed Google Scholar -
Karimi JM, Gholami A, Cheragian B, Abolghasemi J, Solaymani-dodaran M, Madani AH, et al. Survival rate of patients with gastric cancer . Med J Islam Repub Iran.
2019;
33
:
74
.
View Article PubMed Google Scholar -
Akhondi-Meybodi M, Ghane M, Akhondi-Meybodi S, Dashti G. Five-year Survival Rate for Gastric Cancer in Yazd Province, Central Iran, from 2001 to 2008. Middle East J Dig Dis.
2017;
9
(1)
:
39-48
.
View Article PubMed Google Scholar -
Movahedi M, Afsharfard A, Moradi A, Nasermoaddeli A, Khoshnevis J, Fattahi F, et al. Survival rate of gastric cancer in Iran. J Res Med Sci.
2009;
14
(6)
:
367-373
.
-
Veisani Y, Delpisheh A. Survival rate of gastric cancer in Iran; a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench.
2016;
9
(2)
:
78-86
.
-
Pabla BS, Shah SC, Corral JE, Morgan DR. Increased Incidence and Mortality of Gastric Cancer in Immigrant Populations from High to Low Regions of Incidence: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol.
2020;
18
(2)
:
347-359-e5
.
View Article PubMed Google Scholar -
Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut.
2015;
64
(12)
:
1881-1888
.
View Article PubMed Google Scholar -
Lagergren F, Xie SH, Mattsson F, Lagergren J. Updated incidence trends in cardia and non-cardia gastric adenocarcinoma in Sweden. Acta Oncol.
2018;
57
:
1173-1178
.
View Article PubMed Google Scholar -
Asplund J, Kauppila JH, Mattsson F, Lagergren J. Survival Trends in Gastric Adenocarcinoma: A Population-Based Study in Sweden. Ann Surg Oncol.
2018;
25
(9)
:
2693-2702
.
View Article PubMed Google Scholar -
Ghidini M, Donida BM, Totaro L, Ratti M, Pizzo C, Benzoni I, et al. Prognostic factors associated with survival in a large cohort of gastric cancer patients resected over a decade at a single Italian center: the Cremona experience. Clin Transl Oncol.
2020;
22
:
1004-1012
.
View Article PubMed Google Scholar -
Cao X, Cao D, Jin M, Jia Z, Kong F, Ma H, et al. CD44 but not CD24 expression is related to poor prognosis in non-cardia adenocarcinoma of the stomach. BMC Gastroenterol.
2014;
14
(157)
.
View Article PubMed Google Scholar -
Ghotaslou R, Milani M, Akhi MT, Nahaei MR, Hasani A, Hejazi MS, et al. Diversity of Helicobacter Pylori cagA and vacA Genes and Its Relationship with Clinical Outcomes in Azerbaijan, Iran. Adv Pharm Bull.
2013;
3
(1)
:
57-62
.
-
Hosseini E, Poursina F, de Wiele TV, Safaei HG, Adibi P. Helicobacter pylori in Iran: A systematic review on the association of genotypes and gastroduodenal diseases. J Res Med Sci.
2012;
17
(3)
:
280-292
.
-
Casamayor M, Morlock R, Maeda H, Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience.
2018;
12
:
883
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 11 (2020)
Page No.: 4114-4121
Published on: 2020-11-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8447 times
- Download PDF downloaded - 2149 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress



