Abstract
Introduction: Complement Factor H (CFH) is the major soluble regulatory protein that monitors activation and/or amplification of the complement system. Here, we assess serum levels of complement factor H (CFH) among patients, who were diagnosed with bronchiectasis.
Methods: 115 subjects of 80 patients and 35 healthy volunteers were recruited for the study. Blood samples were collected and subjected to centrifugation in order to obtain the serum. The sensitive sandwich ELISA technique, specific for CFH was used for evaluation of CFH in the serum.
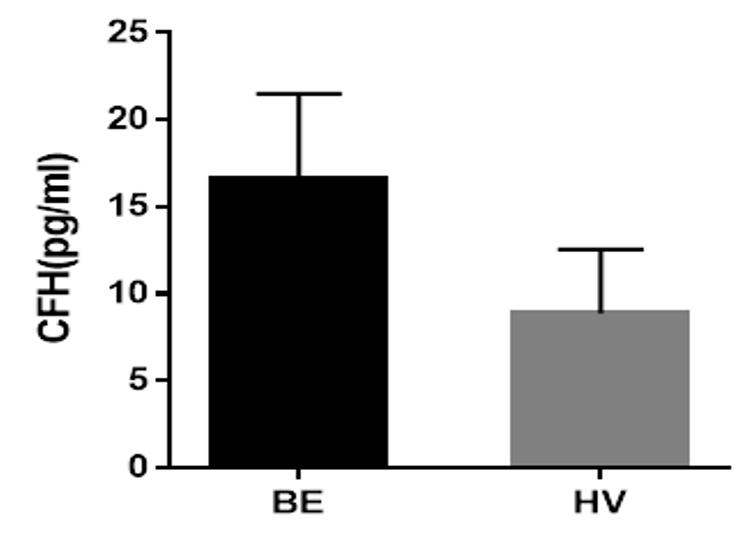
Results: The age and Body Mass Index (BMI) (expressed as Mean+/-SEM (range)) of the observed bronchiectasis patients and healthy volunteers were 66+/-1.13, (30-86) years, 54+/-2.37 (27-84) years and 26.14 kg/m2, 27.4 kg/m2 respectively. CFH was detected in 27.0% of all subjects examined. It was found to be more common among bronchiectasis patients (18.26%) compared to healthy volunteers (8.70%) (F=0.9362; df=1; p<0.05). Mean CFH concentration was 17.0pg/ml and 9.0pg/ml in bronchiectasis patients and healthy volunteers respectively. FEV1% Pre. FEV1/VC% and CFH levels were found to decrease with increasing severity of bronchiectasis. The association between lung function, CFH levels and severity of disease among bronchiectasis patients was negative, strongly linear (r= -0.9316, r2 =0.8678) and statistically significant (p<0.0001).
Conclusion: As such, it can be inferred that low level of compliment factor H is related to the progression of bronchiectasis.
Introduction
Bronchiectasis is a chronic disease and aetiologically heterogeneous, which is characterized by a vicious circle of impaired mucociliary clearance, bronchial infection and inflammation, resulting in a structurally abnormal and permanently widened airways1. Pathologically, it is the depiction of lung damage, such as inflamed thick bronchial wall and dilated bronchi. Such damage can have different causes that can be either inherited or acquired, both of which result in inflammation and infection. Following the introduction of antibiotic therapy and immunization programs in the developed countries, post-infectious damage (as a result of a virus, or bacteria, similar to tuberculosis and non-tuberculous mycobacterial infection) is no longer the common culprit of bronchiectasis. It is still, cystic fibrosis remains the most common cause of inherited bronchiectasis in white Caucasians. Regardless of the primary cause, bacterial species are found to colonize the respiratory tracts o f patients with bronchiectasis2.
A prevalence rate of 0.7% was reported in a study, consisting of 27,258 individuals diagnosed with bronchiectasis. The overall incidence rate was 18 cases/100,000 person s in 2004, and 32 cases/100,000 persons in the UK in the year 2011. The trend of the prevalence was higher in older age group (greater than 60 years of age) and was shown to be higher among women than men 3. Bronchiectasis has been associated with deficiencies in immunoglobulin, complement proteins and chronic granulomatous diseases. IgG, IgM and IgA deficiency has been reported to increase patient risk of recurrent pulmonary infections, that eventually resulted in bronchiectasis 4. α1 Antitrypsin (AAT) absence, or its deficiency was strongly correlated with air way infection in bronchiectasis5.
Complement factor H (CFH)/factor H (FH), a type of soluble complement protein which is activated through an alternative pathway is one of the most significant plasma inhibitors, that regulates complement activation in solution by binding and protecting host cells. It is described as a linear glycoprotein of approximately 155 KDa, consisting of twenty globular domains, known as complement control protein (CCP), or short consensus repeat (SCR), or short complement regulator domain. CFH prevents the complement cascade by blocking activating binding sites in C3b, which acts as a cofactor for factor I, a serum protease that cleaves and inactivates C3b 6,7. The CFH coding gene is located on the chromosome 1q32 within the Regulation of Complement Activation (RCA) gene cluster. It is expressed in the liver and systematically circulates in the body fluids. It is also produced outside the liver by many different types of cells which includes monocytes, fibroblast, endothelial cells, keratinocytes, thrombocytes and retinal pigment epithelial cells8.
Deficiency of CFH may lead to uncontrolled activation of the alternative pathway (AP) with resultant reduction of plasma C3 9. As the lack of factor H also correlates to low levels of C3, factor H deficiency can also be associated with higher susceptibility to infectious diseases10. Bystander damage in host cells and tissues can occur as a result of complement activation, because CFH and membrane-associated proteins have evolved to regulate and control the system11. Therefore, if CFH levels are abnormally low, this could result in a lower threshold for activation of the complement system. This is a consequence of oxidative stress, leading to increased likelihood of autoantibody-mediated lung destruction. C3 glomerulopathy (C3G), atypical haemolytic uremic syndrome (aHUS), IgA nephropathy (IgAN), systemic lupus erythematous (SLE), and age-related macular degeneration (AMD) — all are the diseases, associated with genetic variations in the CFH related proteins 12.
Considering the association between levels of complement factor H in blood and the optimal regulation of the immune response, we chose to investigate serum CFH levels among bronchiectasis patients. We hypothesize that the progression of bronchiectasis disease is associated with lower levels of CFH in sera. This could lead to an uncontrolled activation of the complement system, making the host more susceptible to infection, paving the way for the aggravation of bronchiectasis disease.
Material-Methods
Study population and Sample collection
This is a cross-sectional study, where 115 subjects were randomly selected. 80 subjects were patients, diagnosed with bronchiectasis, attending Sir William Leech Centre for Lung Research, Freeman Hospital, UK. 35 subjects were healthy volunteers. After collection of the whole blood samples from subjects, the samples were allowed clotting by leaving them undisturbed at room temperature. This usually takes 15 – 30 minutes. The clot was removed by centrifuging at 1,000 – 2,000 x g for 10 minutes in a refrigerated centrifuge13. The resulting supernatant is the serum, which was collected and used for the study.
Enzyme-Linked Immunorsorbent Assay (ELISA)
A sandwich ELISA kit, specific for CFH was used to determine the levels of CFH in serum from both patients and control. A Sino Biological ELISA pair set (SEK 10714) was used for the purpose of this research; it is an expression of a solid phase sandwich ELISA. It employs CFH specific monoclonal antibody, coated on a 96-well plate. Both serum samples and standards were added to the wells, using appropriate dilutions and CFH binds to the immobilized antibody if present. The wells were then washed and a mouse anti-CFH monoclonal antibody, conjugated to horseradish peroxidase (HRP) was added, hence antibody antigen antibody sandwich. The wells are again washed for the third time and a 3, 3', 5, 5'-Tetramethylbenzidine (TMB) substrate solution was added. This produces colour, proportional to the amount of CFH, present in the sample. The stop solution was then added and the optical density was read at 450nm.
A standard curve was plotted for each standard on the y-axis against the concentration on the x-axis. The concentration of the CFH for each sample was determined by identifying the mean absorbance value on the y-axis. A horizontal line was drawn to the standard curve; at the point of intersection, a vertical line was drawn on the x-axis and the concentration was read. We then multiplied the concentration by the dilution factor, that had been used to get the overall CFH concentration in each sample and control.
Statistical Analysis
The results were analysed, using analysis of variance (ANOVA), included in Microsoft excel statistical package. Pearson correlation analysis was carried out accordingly and all analyses were considered at a significance level of 0.05.
Ethical approval
Ethical approval was obtained from the Nation al Research Ethics Service, UK (Reference number: 12/NE/0248).
Results
CFH was detected in 31 (27.0%) of the 115 subjects examined. It was more common among bronchiectasis patients (18.26%), compared to the healthy volunteers (8.70%). Differences between bronchiectasis patients and healthy volunteers in relation to CFH levels w ere statistically significant (F=0.9362; df=1; p<0.05) (Table 1).
| CFH (pg/ml) | Subjects | Total (%) | |
| Bronchiectasis Patients (%) | Healthy Volunteers (%) | ||
| Detected | 21 (18.26) | 10 (8.70) | 31 (27.00) |
| Not detected | 59 (51.30) | 25 (21.70) | 84 (73.00) |
| Total (%) | 80 (70.00) | 35 (30.00) | 115 (100) |
CFH concentration was determined by plotting the mean absorbance (nm) against sample concentration (ml). Final CFH concentration (pg/ml) was the product of observed concentration and the sample dilution factor. The standard curves used to calculate the CFH concentrations are shown in Figure 1. The sample concentration was found to be directly proportional to the zero standard subtracted optical density.
Mean CFH concentration observed was 17.0 pg/ml and 9.0 pg/ml for bronchiectasis patients and healthy volunteers respectively. The maximum concentration being 226 pg/ml and 108 pg/ml for bronchiectasis patients and healthy volunteers respectively, while the minimum concentration was 0.00pg/ml for both groups (Figure 1 Table 2).
| Variable | Subjects | |
| Bronchiectasis Patients | Healthy Volunteers | |
| Mean | 17.0 | 9.0 |
| Minimum | 0.0 | 0.0 |
| Maximum | 226 | 108.0 |
| Kurtosis | 12.8 | 12.7 |
| Skewness | 3.5 | 3.3 |
| Count | 80.0 | 35.0 |
| STD | 44.0 | 22.0 |
The demographic properties of the subjects were expressed as mean±SEM. The age of subjects was comparatively higher among bronchiectasis patients (66±1.13) than healthy volunteers (54±2.37). The correlation between CFH levels and Age of subjects was weak (r=0.09483; r2 =0.008994 for bronchiectasis patients. r=0.09579; r2 =0.009176 for healthy volunteers) and statistically not significant (p>0.05); Body Mass Index was higher among healthy volunteers (27.0±0.85) than bronchiectasis patients (26.14±0.67). The correlation between CFH levels and BMI among bronchiectasis patients was weak and linear (r=0.03969; r2 =0.001575), but statistically not significant (p>0.05); However, the correlation between CFH levels and BMI among healthy volunteers was linear, moderately positive (r=0.4755; r2 =0.2261) and was statistically significant (p>0.05). FEV1/VC% was higher among the healthy volunteers (82.0±1.54) than bronchiectasis patients (66.2±1.63) and serum CFH was higher among the bronchiectasis patients (17±4.9), relative to healthy volunteers (9±3.71) (Table 3).
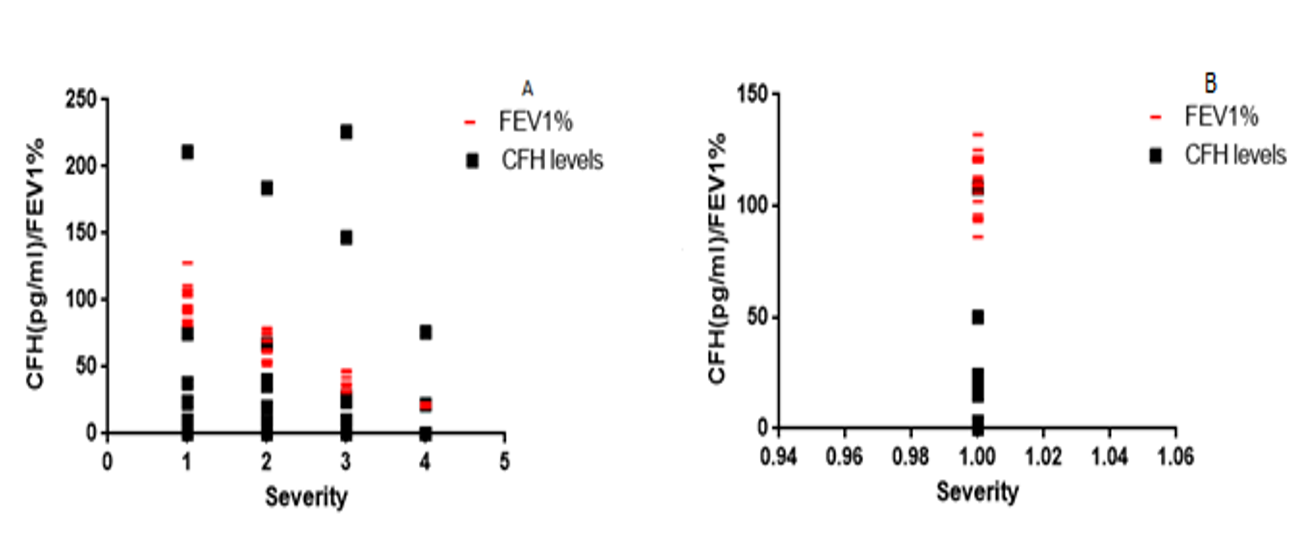
The lung function test shows, that compared to the healthy volunteers, among bronchiectasis patients FEV1% decreases with increasing severity of the disease. CFH levels also decrease with increasing severity of the disease among bronchiectasis patients, compared to healthy volunteers. The association between CFH levels and severity of disease among bronchiectasis patients was negative, perfectly linear (r= -0.9316, r2 =0.8678) and statistically significant (p<0.0001) (Figure 3).
| Variable | Subjects | |
| Bronchiectasis Patients | Healthy Volunteers | |
| Number of Subjects | 80 | 35 |
| Age, mean ±SEM (range), years | 66±1.13 (30-86) | 54±2.37 (27-84) |
| Gender (male/female) | 26/54 | 10/25 |
| BMI, mean ±SEM kg/m 2 | 26.14±0.67 (16.4-40.5) | 27.0±0.85 (20.70-36.70) |
| FEV1% Pre. mean ±SEM (range) | 68.0±3.05 (20.0-128.0) | 111.2±2.06 (86.0-132.0) |
| FEV1/VC% mean ±SEM (range) | 66.2±1.63 (37.0-100.0) | 82.0±1.54 (67.7-99.0) |
| Pack years, mean ±SEM (range) | 7.4±13.0 (0-50) | 7.0±15.0 (0-50) |
| Serum CFH, mean ±SEM pg/ml | 17±4.9 | 9±3.71 |
Discussion
The pivotal role, played by CFH in the regulation of complement activity in a host cannot be overemphasized. A change in the optimal activity of Factor H can lead to various clinical manifestations. High levels of factor H may lead to an under-activation, resulting in reduced complement activity on pathogenic cells, which increases host susceptibility to infections. Low levels of factor H may enable an over-activation, leading to an increased complement activity on healthy host cells. Such activity result s in an inflammation and/or autoimmune diseases, both are quite detrimental to the host. In this study, no trace of CFH was detected in 73.80% of the examined bronchiectasis patients. The average concentration detected (17.0pg/ml) among bronchiectasis patients (18.20%) was lower than the optimal level required for complement regulation (116 – 562 μg/ml) in serum14,15. Low level of CFH is therefore reported in the diagnosed population. Thus, our hypothesis that patients with bronchiectasis had low levels of serum CFH has been supported. Low CFH levels contribute to an increased risk of lung destruction, induced by a cycle of chronic progressive inflammation.
Lower level of serum CFH is characterized by excessive activation of the alternative pathway, probably caused by both increased amounts of reactive oxygen species (ROS) and reactive nitrogen species (RNS), released from leukocytes and macrophages. Those, in turn, are involved in the inflammatory process, especially in the lungs of COPD subjects, which are the consequences of oxidative stress16. It has also been reported that low levels of CFH, or deficiencies may result in an uncontrolled activation of the alternative pathway, with a resultant decrease in plasma C3 levels and its regulatory properties. This could trigger susceptibility to infections and the destruction of host cells in the lung as a result of hypersensitivity 9.
Contrary findings have also been reported17,18. It was observed, that factor H and SC5b-9 levels in sputum of stable chronic obstructive pulmonary disease (COPD) patients were high; sputum CFH concentrations and CFH/protein ratio in asthmatic patients were elevated. Genetic variation in the expression of factor H gene could explain the differences observed in the target population for the two studies. The sample type examined and sample size used could also be factors.
It was observed that the relationship between serum CFH levels and lung function among bronchiectasis patients was inversely proportional. A decrease in lung function was observed with increasing severity of bronchiectasis disease ; CFH levels also decrease with increasing severity of bronchiectasis disease. The normal lung function and the establishment of lung obstruction and restriction vary due to the forced expiratory volume in one second FEV1% and vital capacity (VC). According to NICE ’ s partial updated guideline on the assessment and classification of severity of airflow obstruction, the diagnosis is made, when FEV1/FVC is <0.7 and FEV1% predicted is <80%. The severity is classified as mild (stage1), moderate (stage 2), severe (stage 3) and very severe (stage 4) with ≥ 80%, 50-79%, 30-49% and <30% FEV1% respectively19. This study showed, that 17 bronchiectasis patients had a severe lung obstruction (stage 3), while a larger number of the patients had moderate (stage 2) lung obstruction. The correlation between bronchiectasis disease severity and decline in lung function was statistically significant (P<0.0001), with a strong negative linear correlation (r = − 0.931).
The age distribution of participants in this study ranges from 27 - 86 years and the mean averages among bronchiectasis patients and healthy volunteers were 66 and 54 years respectively. With respect to the sex of subjects examined, it was observed that the highest number of patients with bronchiectasis were females. The study concurs with a 2012 report which states, that females are at higher risk of developing respiratory linked conditions. Yet, the reason is unknown20. Similar finding was reported in an eight-year prevalence study in the UK, where it was observed that the higher prevalence and incidence rates of bronchiectasis were more common among women3.
The classification of human Body M ass Index (BMI) categorised individuals as underweight (≤ 20.0 kg/m2), normal weight (20.0-24.9 kg/m2), overweight (25.0-29.9 kg/m2) and obese (≥30.0 kg/m2) 21. Several studies have shown a strong association between decrease in lung function and BMI. In this study, the average BMI among bronchiectasis patients and healthy volunteers was 26.16 kg/m2 and 27.0 kg/m2 respectively, all classified as overweight. BMI among bronchiectasis patients indicate, that the majority of them are overweight. Only one-third of healthy volunteers were either overweight or obese. Although the association between BMI and CFH levels was not significant among bronchiectasis patients, it was statistically significant among the healthy volunteers (p<0.0126; r = 0.4755). An increase in BMI, as observed in this study, was slightly associated with decrease in lung function among bronchiectasis patients, which is in agreement with the Coronary Artery Risk Development in young Adults (CARDIA) study of 2008. The study found an established association between increased BMI and lung function 22.
Conclusions
In this study, we state that bronchiectasis is associated with low levels of complement factor H in serum. We have revealed, that lung function and serum levels of complement factor H are inversely proportional to the severity of bronchiectasis disease, and that increased BMI among bronchiectasis patients is associated with severe cases of the disease and decrease in lung function.
Competing interests
The authors declare that they have no competing interests.
Authors' Contributions
AS was responsible for the experimental design of this study, sample collection and processing and writing the initial draft of the manuscript. MMI was responsible for data analysis and writing the final draft of the manuscript. All authors have read and approved of the final draft of the manuscript.
References
-
Ringshausen
F.C.,
de Roux
A.,
Pletz
M.W.,
Hämäläinen
N.,
Welte
T.,
Rademacher
J.,
Bronchiectasis-associated hospitalizations in Germany, 2005-2011: a population-based study of disease burden and trends. PLoS One.
2013;
8
(8)
:
e71109
.
View Article PubMed Google Scholar -
Kim
C.,
Kim
D.G.,
Bronchiectasis. Tuberc Respir Dis (Seoul).
2012;
73
(5)
:
249-57
.
View Article PubMed Google Scholar -
Quint
J.K.,
Millett
E.,
Hurst
J.R.,
Time Trends in Incidence and Prevalence of Bronchiectasis in the UK. Thorax.
2012;
67
(2)
:
138-138
.
-
Al-Shirawi
N.,
Al-Jahdali
H.H.,
Al Shimemeri
A.,
Pathogenesis, etiology and treatment of bronchiectasis. Ann Thorac Surg.
2006;
1
(1)
:
41-51
.
-
Fregonese
L.,
Stolk
J.,
Hereditary alpha-1-antitrypsin deficiency and its clinical consequences. Orphanet J Rare Dis.
2008;
3
(16)
:
16
.
View Article PubMed Google Scholar -
Goldsby
R.A.,
Kindt
T.J.,
Osborne
B.A.,
Kuby ImmunologyW. H. Freeman and Company: New York; 2000.
Google Scholar -
Loeven
M.A.,
Rops
A.L.,
Berden
J.H.,
Daha
M.R.,
Rabelink
T.J.,
van der Vlag
J.,
The role of heparan sulfate as determining pathogenic factor in complement factor H-associated diseases. Mol Immunol.
2015;
63
(2)
:
203-8
.
View Article PubMed Google Scholar -
Bhattacharjee
A.,
Oeemig
J.S.,
Kolodziejczyk
R.,
Meri
T.,
Kajander
T.,
Lehtinen
M.J.,
Structural basis for complement evasion by Lyme disease pathogen Borrelia burgdorferi. J Biol Chem.
2013;
288
(26)
:
18685-95
.
View Article PubMed Google Scholar -
Falcão
D.A.,
Reis
E.S.,
Paixão-Cavalcante
D.,
Amano
M.T.,
Delcolli
M.I.,
Florido
M.P.,
Deficiency of the human complement regulatory protein factor H associated with low levels of component C9. Scand J Immunol.
2008;
68
(4)
:
445-55
.
View Article PubMed Google Scholar -
S Reis
E.,
Falcão
D.A.,
Isaac
L.,
Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand J Immunol.
2006;
63
(3)
:
155-68
.
View Article PubMed Google Scholar -
Hageman
G.S.,
Anderson
D.H.,
Johnson
L.V.,
Hancox
L.S.,
Taiber
A.J.,
Hardisty
L.I.,
A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA.
2005;
102
(20)
:
7227-32
.
View Article PubMed Google Scholar -
Józsi
M.,
Tortajada
A.,
Uzonyi
B.,
Goicoechea de Jorge
E.,
Rodríguez de Córdoba
S.,
Factor H-related proteins determine complement-activating surfaces. Trends Immunol.
2015;
36
(6)
:
374-84
.
View Article PubMed Google Scholar -
Harris
R.J.,
Blood separation and plasma fractionationWiley publishers: New York, USA; 1991.
Google Scholar -
Esparza-Gordillo
J.,
Soria
J.M.,
Buil
A.,
Almasy
L.,
Blangero
J.,
Fontcuberta
J.,
Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics.
2004;
56
(2)
:
77-82
.
View Article PubMed Google Scholar -
de Córdoba
S.R.,
de Jorge
E.G.,
Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clin Exp Immunol.
2008;
151
(1)
:
1-13
.
View Article PubMed Google Scholar -
Anderson
D.,
Macnee
W.,
Targeted treatment in COPD: a multi-system approach for a multi-system disease. Int J Chron Obstruct Pulmon Dis.
2009;
4
:
321-35
.
View Article PubMed Google Scholar -
Weiszhár
Z.,
Gálffy
G.,
Lázár
Z.,
Bikov
A.,
Losonczy
G.,
Horváth
I.,
Elevated sputum complement factor H levels in COPD; Relationship with disease severity. Eur Respir J.
2012;
40
(56)
:
794
.
-
Weiszhár
Z.,
Bikov
A.,
Gálffy
G.,
Tamási
L.,
Ungvári
I.,
Szalai
C.,
Elevated complement factor H levels in asthmatic sputa. J Clin Immunol.
2013;
33
(2)
:
496-505
.
View Article PubMed Google Scholar -
Chronic obstructive pulmonary disease: management of chronic pulmonary disease in adults in primary and secondary care (partial update). National Institute for Health and Care Excellence.
2010
.
-
Athanazio
R.,
Airway disease: similarities and differences between asthma, COPD and bronchiectasis. Clinics (S{ã}o Paulo).
2012;
67
(11)
:
1335-43
.
View Article PubMed Google Scholar -
Organization
World Health,
BMI classification 2013. World Health Organization.
2013
.
-
Thyagarajan
B.,
Jacobs
D.R.,
Apostol
G.G.,
Smith
L.J.,
Jensen
R.L.,
Crapo
R.O.,
Longitudinal association of body mass index with lung function: the CARDIA study. Respir Res.
2008;
9
(1)
:
31
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 7 (2019)
Page No.: 3286-3292
Published on: 2019-07-07
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 7343 times
- Download PDF downloaded - 1978 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress