Abstract
Introduction: Allergic asthma is a prevalent disorder, in which eosinophilic inflammation is involved in the lungs. Asthma affects people all over the world, regardless of the country's level of development. Chronic allergen-induced fibrotic damage of the lungs is stimulated in 55 days, which results in significant tissue destruction constitutive to pulmonary tissues, in addition to extensive oxidative & inflammation-induced damage of small and large airways. To date, there is no cure for asthma, and symptoms are controlled using corticosteroids, which may cause systemic side effects. Flavonoids, like fisetin, are a class of secondary metabolites produced by plants, which are known to have numerous beneficial effects. Previous report demonstrated that fisetin has beneficial effects against various diseases such as cancers, tumors, diabetes, and alcohol-induced liver injury.
Methods: In the present study, chronic allergic disease (asthma) was developed in C57BL/6J mice, using intraperitoneal injection of ovalbumin for 54 days together with orally administered fisetin as a treatment strategy. Fisetin was administered 1 hour before intratracheal treatment. On day 55, treated animals were sacrificed, and tissues were collected for various assays.
Results: Fisetin was found to reduce the symptoms of asthma significantly. Reduction in total cell infiltration, eosinophil count, and the levels of serum IgE were observed. There was a down regulation in CD3+CD4+ TH cells, and a decrease in the deposition of collagen in the lung and airways.
Conclusion: From these observations, we conclude that fisetin is effective in the treatment of asthma, and a pathway by which fisetin acts was hypothesized.
Introduction
Asthma is a common chronic respiratory disease, which involve inflammation of the airways or the bronchial tubes of the lungs 1. It is a major non-communicable disease that affects over 200 million people all over the world 2. Asthma can be fatal if not treated properly (WHO, 2017)2. The prevalence of asthma can be traced back to ancient Egypt, when it was treated by drinking a mixture, called kyphi, made of several herbs and berries 3. Chronic allergic asthma involves inflammation of the airways, causing them to swell, and causes the muscles to become tight, obstructing the flow of air in the airways 1. Such obstruction along with bronchospasm and airway hyper-responsiveness (AHR) leads to asthma-related symptoms, such as bronchoconstriction, coughing, wheezing, tightness of chest and breathing shortness 1,4,5.
Although the major cause of asthma is unknown, a combination of environmental and genetic factors was shown to be involved in allergic diseases6. Preclinical studies have shown that there is an increase in the level of inflammatory cells such as eosinophils, mast cells, and type 2 T helper cells (TH2) in allergic asthma4,5. Cytokine levels, mainly interleukins IL-4, IL-5, and IL-13, which are secreted by CD4+ TH2 cells, are also affected 4,5,7. The transcription factor GATA3 plays an essential role in the expression of TH2 cytokines7. IL-4 is involved in the generation of IgE 5,8, IL-5 promotes airway eosinophilia 5,9 and IL-9 helps the induction, proliferation and differentiation of mast cells5. IL-13 is involved in the hypersecretion of mucus 5 and the activation of inducible nitric oxide synthase (iNOS), thus leading to the production of nitric oxide 7. Fibrosis of the airways is associated with asthma, in which eosinophilic infiltration and deposition of collagen lead to thickening of the epithelial basement membrane5. In this process, profibrotic cytokines, such as TGFβ, are involved5.
Flavonoids are a class of secondary metabolites with polyphenolic structures, produced by plants and fungi 10. Flavonoids are produced by plants for growth and protection of vegetables 10. Their beneficial effects are documented, and are attributed to their anti-inflammatory, antioxidative and anticarcinogenic properties, and the ability to regulate enzymes, such as xanthine oxidase (XO) and cyclo-oxygenase (COX) 10. The structure of flavonoids is comprised of a 15-carbon skeleton, including two phenyl rings (A and B) and one heterocyclic ring (ring C) 6 as shown in Figure 1.
According to the composition of the rings, flavonoids can be classified into flavones (e.g., luteolin), flavonols (e.g. fisetin), flavanones (e.g. hesperetin), isoflavones (e.g. daidzein), anthocyanidins (e.g. cyanidin) and flavanols (e.g. catechins) 6.
Fisetin (3, 7, 3’, 4’-tetrahydroxyflavone) is a flavonol with 4 hydroxyl groups at positions 3, 7, 3’ and 4’ of the flavonoid structure 11 as shown in Figure 1. It is commonly found in such as like apples, strawberries and grapes, and vegetables like tomatoes, cucumbers, and onions12,13. Strawberries have the highest content of fisetin as compared to other fruits and vegetables14. Fisetin bears the potential to be an effective antioxidant, anticarcinogenic, anti-inflammatory and immuno-suppressing agent12,13. It has been found that fisetin can suppress the secretion of TH2 cytokines (IL4, IL5, and IL13) from basophils, and prevent the activation of macrophages13. Fisetin can affect mast cells and inhibit their ability to release histamines and cytokines13. It can also inhibit the action of IκB-α kinase (IKK) in cancer cells, thereby inhibiting the activation of NFκB13.
Materials and methods
Ethical approval
Experiments were performed as per rules given by the departmental and institutional animal ethics committee. All animals were reared in the animal house (free of any pathogen) of the Department of Zoology under University of Calcutta.
Mice
Male C57BL/6J mice (8-10 weeks) of body weight 20-25 grams were classified into three groups (n=5 for each group):
Ovalbumin-induced asthma & fisetin treatment
The mice from Ova group were sensitized with the help of 100 µg of Ovalbumin (Ova) grade-V (Sigma-Aldrich), forming complex with Al(OH)3 in a 0.2 ml volume, and administered intraperitoneally on day 015,16,17. They were challenged with 250 µg intratracheal Ova on day 8 (intratracheally), and with 125 µg Ova on 15th, 18th, 21st, 24th, 27th, 30th, 33rd, 36th, 39th, 42nd, 45th, 48th, 51st and 54th day respectively 16. For intratracheal treatment, the mice were anesthetized for a short period by isoflurane inside a standard anesthesia chamber. Anesthetized mice were taken from the chamber, positioned in a supine posture, and tongue was extended with lined forceps. After this, they were sensitized with 50 µL of Ova (at concentrations as mentioned above) placed at the back of its tongue 16. Mice of the control group received normal saline.
Mice of Ova + F group were treated with oral 2 µM/Kg fisetin (50 nM) dissolved in DMSO one hour before each intratracheal challenge. (Source of Fisetin: Cellular Neurobiology Laboratory, Salk Institute for Biological Studies, USA). A diagram of the treatment regime is presented in Figure 2.
Airway hyperresponsiveness (AHR) measurement
By using whole body plethysmography in unrestrained mice, airway hyper-responsiveness (AHR) was measured on day 55 i.e. 24 hours after the last intratracheal treatment. Before recording the data, the chambers of the 2 mice whole body plethysmograph (emka Technologies, France) were calibrated with 5 ml air. Mice of each group were placed in the chambers, acclimatized for 10 minutes and exposed to increasing concentrations (0, 5, 10, 15, 20 mg/ml) of methacholine (TCI, Japan) in PBS for 1 minute. Enhanced pause (Penh) was recorded for 4 minutes after each challenge.
Sacrifice and collection of tissues
Mice were sacrificed on the 55th day by cervical dislocation after taking measurement of whole-body plethysmography, and the following tissues were collected.
Peripheral blood (PB)
After performing cardiac puncture, 1 ml of blood was collected in tubes that contain EDTA (1.5mg/ml) as an anticoagulant. To view under microscope, a blood smear was prepared on a microscopic slide.
Bronchoalveolar lavage fluid (BALF)
Both lungs were injected with 1 ml of cold PBS slowly and then by drawing it out, BALF was collected18. The BALF was centrifuged at 200X g at 4°C for 10 min. The cell pellet was resuspended in fetal bovine serum and a smear was made on a microscope slide.
Lung parenchyma
Immediately after collecting the BALF, the lungs were washed with PBS.
Assays
One lung was taken in a Petri dish and minced into digestible pieces. These pieces of lung were then digested using a 1X collagenase/ hyaluronidase cocktail (Stem Cell Technology) overnight at 37°C. To get a single cell suspension, it was filtered through a no. 60 sieve (Sigma-Aldrich).
Hydroxyproline estimation
Whole lung tissue was washed, minced and finally vacuum dried to remove oxygen and the hydroxyproline was estimated to measure the overall collagen content of the lung.
For gene & protein expression
For gene expression studies, whole lung was stored in RNA later solution (Ambion, Inc.) at -80˚C. For protein expression studies, whole lung was washed with PBS and stored at -80˚C.
For histology
One lung was collected in whole in 10% buffered formalin.
Total cell (TC) count
To determine cell viability, PB/ BALF was mixed with Trypan Blue dye (HIMEDIA, India) in 1:1 ratio. Further, it is observed under a microscope by using hemocytometer.
Differential cell (DC) count
Thin smear of cells was put on a slide. The PB/ BALF smears were immediately air-dried followed by methanol fixation (SRL, India). Staining was carried out using Wright- Giemsa (SRL, India) for 15 min, then washed under running distilled water, and observed under a light microscope (Dewinter Fluorex LED) at 40X magnification 19. Their nuclear morphology characterized different cell types.
Ova-specific serum IgE
Mouse purified IgE was purchased from BD Biosciences, USA and the provided protocol were followed (BD OptEIA Mouse IgE ELISA Set). 96-well microtitre plates were coated with (Corning, USA) 50 mg/ml ovalbumin in sterile PBS overnight at 4°C.
Plates were washed with PBS-Tween (0.05%) three times. Blocking was done by 5% skimmed milk in PBS for 1 hour at room temperature. After washing, 50 µl plasma samples (1:10 dilution in blocking buffer) were added per well and incubated for 90 min at 37°C. Wells were then washed 4 times with wash-buffer. Biotin-labeled 2 µg rat anti-mouse IgE (clone R35-118; BD Biosciences, USA) was added to each well for 1 hour at 37 °C. 100 µl avidin- HRP (BD Biosciences, diluted 1: 1000 in blocking buffer) was added in each well, followed by incubation for 90 mins at 37°C. TMB substrate (3, 3’, 5, 5’, tetramethylbenzidine; Sigma Aldrich) was added to each well at room temperature for 30 min. Color development was measured at 405 nm by using ELISA plate reader (Thermo Scientific). Serum containing IgE was determined by using a standard curve prepared using purified IgE (BD Biosciences).
CFU-c assay
To assess progenitors from all lineages, colony forming unit-cell assay was performed. Methylcellulose was used to make semisolid media (HIMEDIA, India). Semisolid media composed with IMDM (HIMEDIA, India), 30% FBS (HIMEDIA, India), 20 mg/ml BSA (Biosera), 1% Pen-Strep (HIMEDIA, India). Stem cell factor (Biovision) was added at the concentration of 5 ng/ml. All constituents were mixed thoroughly by vortexing. 1.5% methylcellulose (HIMEDIA, India) was added into this mixture. Each 24 well plates (Nest Biotech) was filled with 1 ml of semi-solid media. Next, 106 cells were added to each well based on treatment groups and control groups in triplicate and incubated at 37°C in a 5% CO2 humidified chamber (Thermo Fisher) for 7 days. Colonies were counted after 7 days and the clonogenic potential of the sample was measured by dividing the number of colonies obtained by the total number of plated cells.
Gene expression
RNA was isolated from lung tissues with TRIzol reagent (Life Technologies, USA). cDNA was prepared using reverse transcriptase III. Total mRNA was assessed by Reverse Transcriptase PCR (RT-PCR) and Real time PCR (qRT) as previously described20.
Real time (qRT)- PCR
Total mRNA were assessed by quantitative PCR (qPCR) analysis as previously mentioned20. The primers used for Real time PCR are listed in Table 1A, with β-actin as the internal control.
| Method | Gene | Type | Primer sequence (5’-3’) | ||
| A. | qRT- PCR primers | β-actin | House-keepinggene | Forward | GTGGGCCGCTCTAGGCACCAA |
| Reverse | CTCTTTGATGTCACGCACGATTTC | ||||
| IL3 | Cytokine genes | Forward | CCGTTTAACCAGAACGTTGAA | ||
| Reverse | CCACGAATTTGGACAGGTTT | ||||
| IL4 | Forward | GGCATTTTGAACGAGGTCAC | |||
| Reverse | AAATATGCGAAGCACCTTGG | ||||
| IL5 | Forward | ||||
| Reverse | AGCCCCTGAAAGATTTCTCC | ||||
| B. | RT- PCR primers | GAPDH | House-keepinggene | Forward | TGTGATGGGTGTGAACCACGA |
| Reverse | TGCTGTTGAAGTCGCAGGAGAC | ||||
| IL4 | Cytokine genes | Forward | TCGGCATTTTGAACGAGGTC | ||
| Reverse | GAAAAGCCCGAAAGAGTCTC | ||||
| IL5 | Forward | TCACCGAGCTCTGTTGACAA | |||
| Reverse | CCACACTTCTCTTTTTGGCG | ||||
| IL13 | Forward | GGTCCCTGGCAATATTACTGTAT | |||
| Reverse | CCCATTCACTACACATCACCTT | ||||
| IFNγ | Forward | CCTCTGAGACAATGAACGCT | |||
| Reverse | AAAGAGATAATCTGGCTCTGC | ||||
| TGFβ | Forward | ACCGCAACAACGCCATCTAT | |||
| Reverse | GTAACGCCAGGAATTGTTGC | ||||
| iNOS | Forward | CCCTTCCGAAGTTTCTGGCAGCAGC | |||
| Reverse | GGCTGTCAGAGCCTCGTGGCTTTGG | ||||
Reverse Transcriptase (RT)-PCR
mRNA expression of different cytokine genes were assessed by RT-PCR as previously described 20. Gene specific primers are listed in Table 1B and GAPDH was taken as a house-keeping gene.
Flow cytometry
Flow cytometry was carried out employing BD FACSVerse (BD Biosciences, USA), and the results were analyzed using the FACSSuite (BD Biosciences, USA) software for analysis. Cell surface staining was performed using the conjugated antibodies: CD45-conjugated with PerCPCy5.5 (BioLegend), CD3e-conjugated with PE (BD Biosciences, USA), CD4- conjugated with V450 (BD Biosciences, USA), CD8a-conjugated with Alexa Fluor 488 (BD Biosciences, USA), B220-conjugated with FITC (BD Biosciences, USA), GR-1-conjugated with FITC (MACS), and F4/80-conjugated with PE (Invitrogen). Among the CD45+ hematopoietic cells are T cells (CD45+CD3) and B (CD45+B220) cells. Of the CD3+ cells, CD3+CD4+ are helper T (TH) cells, and CD3+CD8+ are cytotoxic T (TC) cells. Neutrophils are CD45+Gr1+, monocytes are CD45+F4/80low+ and macrophages are CD45+F4/80high+ 16.
Analysis of protein expression by Western blot
Total cellular protein was isolated from lung tissues and primary antibodies were used to analyze the expression of proteins in allergen-sensitized mice. The antibodies were rabbit anti-mouse NFκB (Santa Cruz), rabbit anti-mouse TGFβ, rabbit anti-mouse STAT6 (Santa Cruz) and rabbit anti-mouse GAPDH (Santa Cruz). Western blot protocol was described previously 20.
Histology
Right lobes from the lungs were separated and fixed immediately in 10% neutral buffer formalin. Lung samples were processed, dehydrated, embedded in paraffin, and sectioned to 5mm thickness. Cellular infiltration and influx of inflammatory cells were visualized using standard histological techniques of hematoxylin and eosin staining as previously described (Paul et al. 2018). Collagen deposition and pulmonary fibrosis in the lungs were also identified by Masson’s trichrome staining 9.
Statistical analysis
Data from the present study were analyzed using Graph Pad Prism 6. All values are represented as Mean ± SEM, with p<0.05. Statistical significance has been calculated by t-test. * has been used to denote significance with respect to control samples, and # has been used to denote significance in comparison to Ova- treated samples.
Results
In this study, we have established ovalbumin-induced chronic allergic asthma in C57BL/J mice, and 2 µM/kg fisetin (50 nM) was administered orally one hour before each intratracheal challenge.
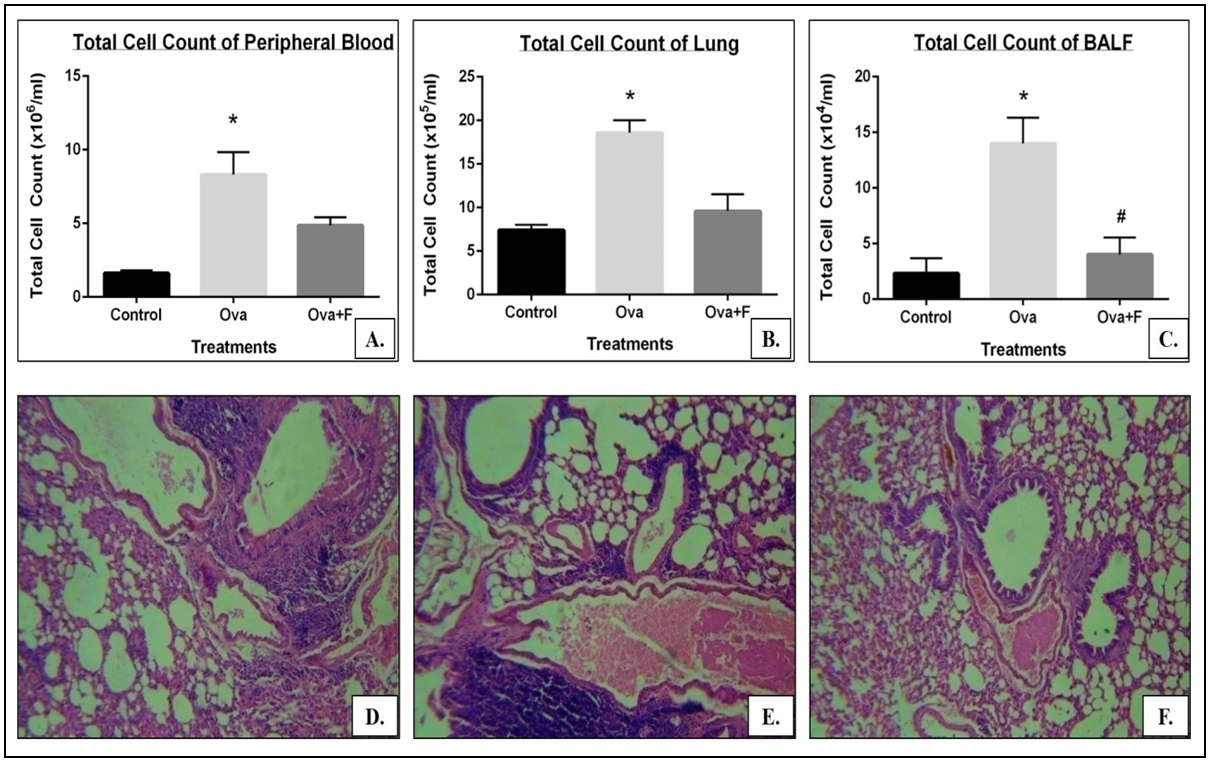
Induction of asthma was confirmed by the increase in the Penh value following exposure to methacholine (data not shown), and the infiltration of cells into the blood (by 5.15 folds; p<0.05; Figure 3A). Infiltration of cells was 6.01 folds higher in BALF compared to untreated control (p<0.05; Figure 3B) and in the lung, the value was 2.51 folds higher (p<0.05; Figure 3C, E).
Influx of eosinophils in the blood increased 3.50 folds (p<0.05; Figure 4A), and the BALF has 2.50 folds more than control group. (p<0.05; Figure 4B). In peripheral blood, there was a 5.12 folds more CD45+B220+ B cell population (Figure 5B, J), a 7.65 folds more CD45+CD3+ T cell population (p<0.05) (Figure 5E, J), a 7.19 folds more CD3+CD4+ TH cell population (p<0.05) (Figure 5E, J), a 9.48 folds more CD3+CD8+ TC cell population (p<0.05) (Figure 5E, J), a 3.95 fold more CD45+GR1+ neutrophil population (p<0.05) (Figure 5H, K), a 5.42 fold more CD45+F4/80low+ monocyte population (p<0.05) (Figure 5H, K) and a 8.98 fold more CD45+F4/80high+ macrophage population (p<0.05) (Figure 5H, K) compared to control group.
In the lung, CD45+B220+ B cell population displayed 3.34 fold increase (Figure 6B, J), while a 7.93 fold more CD45+CD3+ T cell population (Figure 6E, J), a 24.33 fold more CD3+CD4+ TH cell population (Figure 6E, J), a 29.70 fold more CD3+CD8+ TC cell population (Figure 6E, J), a 1.79 fold more CD45+GR1+ neutrophil population (Figure 6H, K), a 3.52 fold more CD45+F4/80low+ monocyte (Figure 6H, K) and a 9.36 fold more CD45+F4/80high+ macrophage population (Figure 6H, K) was observed compared to control group. Less than 0.05 of P value was considered statistically significant.
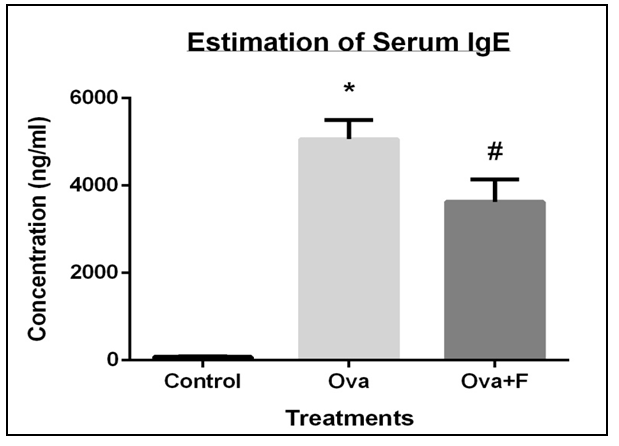
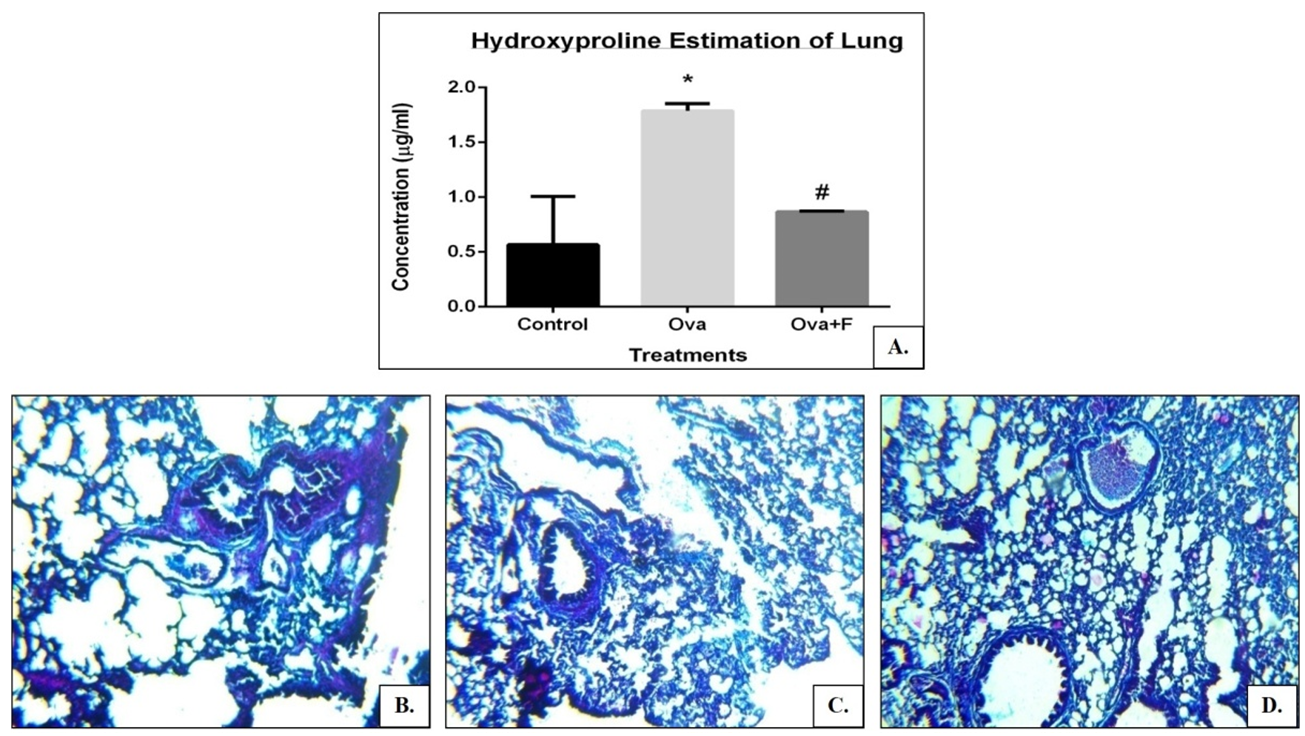
Serum IgE of the allergen-sensitized mouse was observed to be 63.88 folds higher (Figure 7) and a 3.16 folds higher collagen deposition in the lung (Figure 8A, C). Expression of TH2 genes like IL4 increased by 4.14 folds, IL5 increased by 1.56 folds and IL13 increased by 1.63 folds respectively. Expression of pro-inflammatory genes like IFNγ increased by 1.33 folds, TGFβ increased by 1.88 folds, iNOS increased by 1.89 folds, NFκB, and STAT6. The clonogenic potential of the blood, BM, and lung cells decreased by 1.95 folds, 1.40 folds, and 1.20 folds, respectively.
Taken together, the data suggest that fisetin has a protective role in oval bumin-sensitized lung inflammation via its signal molecules. Fisetin inhibited the infiltration of cells into the blood by 1.71 folds (Figure 3A), into the BALF by 3.47 folds (Figure 3B) and into the lung by 1.94 folds (Figure 3C, F). Eosinophilic infiltration decreased by 2.72 folds in the blood (Figure 4A), and by 2.50 folds in the BALF (Figure 4B). In the blood, fisetin led to a 10.51-fold reduction in B cells (Figure 5C, J), a 4.22-fold reduction in T cells (Figure 5F, J), a 5.54-fold decrease in the TH cells (Figure 5F, J), a 4.34-fold decrease in the TC cells (Figure 5F, J), a 2.27-fold decrease in monocytes (Figure 5I, K) and a 2.52-fold reduction in macrophages (Figure 5I, K). In the lung, fisetin led to a 2.94-fold reduction in B cells (Figure 6C, J), a 2.57-fold reduction in the T cells (Figure 6F, J), a 1.45-fold reduction in the TH cells (Figure 6F, J), a 5.60-fold reduction in the TC cells (Figure 6 F, J), a 6.28-fold reduction in monocytes (Figure 6I, K) and a 5.32-fold reduction in macrophages (Figure 6I, K).
Fisetin successfully reduced the serum IgE concentration by 1.40 folds (Figure 5), as well as the collagen deposition in the lungs by 2.05 folds (Figure 8A, D). Expression of the TH2 genes IL4 was reduced by 4.27 folds, IL5 reduced by 1.40 folds and IL13 reduced by 2.68 folds, as did the expression of TGFβ (by 1.07 folds), NFκB and STAT6 (Figure 9).
Fisetin, however, did not have a significant effect on the expression of IFNγ and iNOS. Clonogenic potential of the blood, BM, and lung cells were restored by 3.17 folds, 1.32 folds, and 1.24 folds respectively with fisetin (Figure 6).
Discussion
Bronchial inflammation and hyperresponsiveness in asthma are complex respiratory diseases. Over 15-20 million people are affected by asthma in India, and this number is rising with time2. To date, asthma cannot be completely cured, but it can be regulated using medications like corticosteroids2. Prolonged use of inhaled corticosteroids can lead to several systemic side effects such as osteoporosis, reduced bone density, glaucoma, cataracts, and bruising 21. We designed a study to use fisetin, a natural compound, to treat chronic allergic asthma to overcome these limitations.
Fisetin is a natural product, which is readily available. Fisetin is a potent antioxidant and is thought to exert its antioxidant effect by activating heme oxygenase-I (HO-I) under oxidative stress conditions22. It has also been shown that fisetin suppresses NFκB signaling pathway and MAPKs. It inhibits the degranulation of basophils and mast cells and reduces the expression of IL4, IL13, and other cytokines by basophils and mast cells 23.
In vitro, studies with fisetin has been proved for its ability to inhibit the secretion of cytokines by basophils and mast cells13. Previously, it has been reported that NFκB activity in bronchial epithelial cells is inhibited by using fisetin. Our data also support that IκB-α and nuclear translocation of p65 are blocked by fisetin. IkBα is activated by the action of IKK and dissociates into IkB, and later activates NFkB. NFkB translocates into the nucleus and further enables TLRs. Fisetin blocks IKK complex and finally inhibit NFkB activation 24.
C57BL/6J mice showed robust Th2 responses when they were treated with ovalbumin challenge 25,26. In ovalbumin-sensitized allergic asthma, IL4 production increases by the activation of mast cells17, followed by activation of B cells and finally synthesize IgE antibody.
When prolonged ovalbumin challenge was used, T-lymphocytes were activated, which further secreted Th2 cytokine IL-5 and regulated eosinophilic inflammation by proliferation and differentiation 27. Eosinophils are thought to influx into the airway mucosa and AHR. Our preclinical data emphasize that total number of cells increased in ovalbumin challenges in BALF as well as blood, and eosinophils and lymphocytes in ova challenged mice were upregulated, where the value was downregulated in the treated group. Following chronic allergen challenge, treated mice group showed reduced AHR and Th2 cytokines (IL-4 and IL-5) in gene expression profile. Furthermore, a histological study revealed the break-down of the alveolar wall, thickening of the smooth muscle layer, accumulation of infiltration of inflammatory cells deposited surrounding the inflamed region of the lung, and the accumulation of the mucin in the inner lumen of the bronchi, which result in a reduction of bronchiole lumen volume.
Similarly, the treated groups downregulated CD4+ T-lymphocytes promotes a high level of Th2 cytokine including IL-5, IL-3 which support our data. Nuclear factor kappa B (NF-κB) is the most important transcription factor which regulates multiple pro-inflammatory mediators and various interleukins. Activation of NF-κB in epithelial cells up-regulates the expression of TNF-α. We found the lung protein NF-κB expressed in OVA-treated mice and STAT6 phosphorylation, which are associated with IL-13 to develop airway remodeling. TGF-β is involved in native T-cell differentiation into Th17 cells, which induces neutrophil airway inflammation. Our study demonstrates the inhibitory effect of Fisetin on mice model of allergic asthma. Western blot showed a reduced expression of TGF-β in post-OVA mice lung homogenate. Published data highlighted the essential role of TGF-β in airway fibrosis, which promotes several signaling cascades including JNK, and p38 pathway 28,29.
Different progenitor cells of the allergen-exposed mice show consistently reduced number in post-ova group, which supports the previous data of chronic allergic model of mice. Based on this study, we present a possible therapeutic mechanism of orally administered fisetin (Figure 11).
Conclusions
While our studies have shown that fisetin is effective as an anti-inflammatory agent, which can be used as a therapeutic agent in asthma, its pharmacological properties are yet to be determined. With the rising occurrence of asthma all over the world, high cost and adverse effects of current drugs, the findings of this paper can be a step towards an economical and safer therapy for asthma.
Competing Interests
There is no conflict of interest among the authors.
Contribution of authors
PP performed the experiment and the assays, and analyzed the data. SM2 performed few assays. SM1 analyzed the data and wrote the manuscript. ERB initiated the project, designed the experiments, analyzed the data and wrote the manuscript. All authors approved the manuscript.
Acknowledgments
We would like to acknowledge the Indian Council of Medical Research (ICMR), UGC-UPE-II and UGC-SAP for providing the grant, contingency and fellowship for this work.
We acknowledge the University Grants Commission (UGC), New Delhi, for providing fellowship to SM. We also acknowledge the BD- CoE at the Centre for Research in Nanoscience & Nanotechnology (CRNN), University of Calcutta, Kolkata, for allowing us to use the BD FACSVerse.
References
-
Asthma. American Academy of Allergy, Asthma & Immunology.
2019
.
-
Asthma fact sheet No.307. World Health Organization.
2017
.
-
Manniche
L.,
Sacred luxuries: fragrance, aromatherapy, and cosmetics in ancient EgyptCornell University Press 1999.
Google Scholar -
Mitra
S.,
Paul
P.,
Mukherjee
K.,
Biswas
S.,
Jain
M.,
Sinha
A.,
Mesoporous Nano-carbon particle Loaded Fisetin has a Positive Therapeutic Effect in a Murine Preclinical Model of Ovalbumin Induced Acute Allergic Asthma. J Nanomedine Biotherapeutic Discov.
2015;
5
:
132
.
-
Barnes
P.J.,
Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond).
2017;
131
(13)
:
1541-58
.
View Article PubMed Google Scholar -
Tanaka
T.,
Takahashi
R.,
Flavonoids and asthma. Nutrients.
2013;
5
(6)
:
2128-43
.
View Article PubMed Google Scholar -
Ray
A.,
Raundhal
M.,
Oriss
T.B.,
Ray
P.,
Wenzel
S.E.,
Current concepts of severe asthma. J Clin Invest.
2016;
126
(7)
:
2394-403
.
View Article PubMed Google Scholar -
Henderson
W.R.,
Chi
E.Y.,
Maliszewski
C.R.,
Soluble IL-4 receptor inhibits airway inflammation following allergen challenge in a mouse model of asthma. J Immunol.
2000;
164
(2)
:
1086-95
.
View Article PubMed Google Scholar -
Cho
J.Y.,
Miller
M.,
Baek
K.J.,
Han
J.W.,
Nayar
J.,
Lee
S.Y.,
Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest.
2004;
113
(4)
:
551-60
.
View Article PubMed Google Scholar -
Panche
A.N.,
Diwan
A.D.,
Chandra
S.R.,
Flavonoids: an overview. J Nutr Sci.
2016;
5
:
e47
.
View Article PubMed Google Scholar -
Maher
P.,
Akaishi
T.,
Abe
K.,
Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci USA.
2006;
103
(44)
:
16568-73
.
View Article PubMed Google Scholar -
Pal
H.C.,
Pearlman
R.L.,
Afaq
F.,
Fisetin and Its Role in Chronic Diseases. Adv Exp Med Biol.
2016;
928
:
213-44
.
View Article PubMed Google Scholar -
Wu
Mei-Yao,
Hung
Shih-Kai,
Fu
Shu-Ling,
Immunosuppressive effects of fisetin in ovalbumin-induced asthma through inhibition of NF-$κ$B activity. Journal of agricultural and food chemistry.
2011;
59
(19)
:
10496-10504
.
-
Khan
Naghma,
Syed
Deeba N,
Ahmad
Nihal,
Mukhtar
Hasan,
Fisetin: a dietary antioxidant for health promotion. Antioxidants & redox signaling.
2013;
19
(2)
:
151-162
.
-
Shakeri
F.,
Boskabady
M.H.,
Anti-inflammatory, antioxidant, and immunomodulatory effects of curcumin in ovalbumin-sensitized rat. Biofactors.
2017;
43
(4)
:
567-76
.
View Article PubMed Google Scholar -
Banerjee
E.R.,
Jiang
Y.,
Henderson
W.R.,
Latchman
Y.,
Papayannopoulou
T.,
Absence of alpha 4 but not beta 2 integrins restrains development of chronic allergic asthma using mouse genetic models. Exp Hematol.
2009;
37
(6)
.
View Article PubMed Google Scholar -
Chauhan
Preeti S,
Dash
D,
Paul
BN,
Singh
Rashmi,
Intranasal curcumin ameliorates airway inflammation and obstruction by regulating MAPKinase activation (p38, Erk and JNK) and prostaglandin D2 release in murine model of asthma. International immunopharmacology.
2016;
31
:
200-206
.
-
Han
H.,
Ziegler
S.F.,
Bronchoalveolar Lavage and Lung Tissue Digestion. Bio Protoc.
2013;
3
(16)
:
e859
.
View Article PubMed Google Scholar -
Sood
R.,
Medical Laboratory Technology: Methods and InterpretationsJaypee Brothers Medical Publishers Pvt Ltd: India; 2009.
Google Scholar -
Paul
P.,
Majhi
S.,
Mitra
S.,
Banerjee
E.R.,
Immuno-modulatory and Therapeutic Effect of Curcumin in an Allergen-sensitized Murine Model of Chronic Asthma. J Clin Cell Immunol.
2018;
9
(03)
:
551
.
View Article Google Scholar -
Dahl
R.,
Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med.
2006;
100
(8)
:
1307-17
.
View Article PubMed Google Scholar -
Syed
Deeba N,
Adhami
Vaqar Mustafa,
Khan
Naghma,
Khan
Mohammad Imran,
Mukhtar
Hasan,
Exploring the molecular targets of dietary flavonoid fisetin in cancer. Seminars in cancer biology.
2016;
40
:
130-140
.
-
Nagai
K.,
Takahashi
Y.,
Mikami
I.,
Fukusima
T.,
Oike
H.,
Kobori
M.,
The hydroxyflavone, fisetin, suppresses mast cell activation induced by interaction with activated T cell membranes. Br J Pharmacol.
2009;
158
(3)
:
907-19
.
View Article PubMed Google Scholar -
Huang
W.,
Li
M.L.,
Xia
M.Y.,
Shao
J.Y.,
Fisetin-treatment alleviates airway inflammation through inhbition of MyD88/NF-κB signaling pathway. Int J Mol Med.
2018;
42
(1)
:
208-18
.
View Article PubMed Google Scholar -
Brewer
J.P.,
Kisselgof
A.B.,
Martin
T.R.,
Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med.
1999;
160
(4)
:
1150-6
.
View Article PubMed Google Scholar -
Whitehead
G.S.,
Walker
J.K.,
Berman
K.G.,
Foster
W.M.,
Schwartz
D.A.,
Allergen-induced airway disease is mouse strain dependent. Am J Physiol Lung Cell Mol Physiol.
2003;
285
(1)
:
32-42
.
View Article PubMed Google Scholar -
Yamaguchi
Y.,
Suda
T.,
Suda
J.,
Eguchi
M.,
Miura
Y.,
Harada
N.,
Tominaga
A.,
Takatsu
K.,
Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med..
1988;
167
(1)
:
43-56
.
-
Massagué
J.,
Blain
S.W.,
Lo
R.S.,
TGFbeta signaling in growth control, cancer, and heritable disorders. Cell.
2000;
103
(2)
:
295-309
.
View Article PubMed Google Scholar -
Imamichi
Y.,
Waidmann
O.,
Hein
R.,
Eleftheriou
P.,
Giehl
K.,
Menke
A.,
TGF beta-induced focal complex formation in epithelial cells is mediated by activated ERK and JNK MAP kinases and is independent of Smad4. Biol Chem.
2005;
386
(3)
:
225-36
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 7 (2019)
Page No.: 3262-3273
Published on: 2019-07-04
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 9438 times
- Download PDF downloaded - 2518 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress