Abstract
Background: In recent years, plerixafor, a CXCR4 chemokine receptor inhibitor, has emerged as a promising agent for the mobilization of hematopoietic stem cells (HSCs) when combined with other mobilizers such as granulocyte colony-stimulating factor (G-CSF) and chemotherapy in patients with multiple myeloma and lymphoma undergoing autologous peripheral blood stem cell transplantation (APBSCT). Our facility has recently implemented plerixafor as a specialized rescue treatment in lymphoma patients who are at risk or have experienced mobilization failure with G-CSF.
Case Series: We present five cases of lymphoma in young adult patients (26 to 49 years old), comprising two cases of Hodgkin lymphoma and three cases of diffuse large B-cell lymphoma. All five patients presented with advanced stage IV disease. Three patients received plerixafor following initial mobilization failure with G-CSF-based protocols, one patient received plerixafor preemptively, and one patient received it as an upfront treatment strategy.
Outcomes: All five cases achieved a collection of CD34+ cells exceeding 2 × 10⁶ cells/kg (ranging from 2.67 to 3.95 × 10⁶ cells/kg) after a single mobilization involving plerixafor, and no adverse reactions were reported.
Conclusion: Our findings highlight the significant enhancement of HSCs mobilization achieved with plerixafor compared to traditional methods. Plerixafor is not only highly effective but also safe for use in lymphoma patients. These case series findings underscore its value as a key tool in optimizing HSCs collection for successful APBSCT.
Introduction
Autologous peripheral blood stem cell transplantation (APBSCT) and salvage chemotherapy continue to be the recommended treatment approach for aggressive non-Hodgkin lymphoma (NHL) patients in the high to intermediate risk category and for those with relapsed or refractory Hodgkin lymphoma (HL)1, 2. In order to conduct a successful APBSCT, it is imperative to collect an adequate quantity of peripheral blood haematopoietic stem cells (PBSCs), where a minimum of 2.0×10⁶ cells/kg of CD34⁺ is necessary for engraftment, and 5.0×10⁶ cells/kg is associated with faster engraftment3. Successful mobilization is defined as the collection of a CD34⁺ dose of ≥2.0×10⁶ cells/kg by leukapheresis after a single mobilization procedure4 . The standard approach for mobilizing HSCs involves the use of granulocyte colony-stimulating factor (G-CSF) in combination with chemotherapy agents. Nevertheless, this method has been linked to a significant failure rate in mobilization5.
Since 2008, the European Medicines Agency (EMA) and the Food and Drug Administration have granted authorization for the use of plerixafor, a CXCR4 chemokine receptor antagonist, in conjunction with G-CSF and chemotherapy for more rapid and successful HSC mobilization6. The efficacy of plerixafor in haematological malignancy stems from its ability to disrupt the interaction between malignant cells and their protective environment. By inhibiting the binding of CXCL-12 to its receptor CXCR4, plerixafor interferes with the interaction between tumours and their stroma, thereby hindering the signalling that sustains the survival and protection of leukaemia stem cells within the stem cell niche. This mobilization of leukaemia cells from the protective stromal environment renders them more susceptible to cytotoxic therapy, potentially augmenting the effectiveness of treatment7.
Our facility recently implemented plerixafor at the end of 2022 as a specialized rescue treatment in lymphoma patients planned for APBSCT who have encountered limited success with G-CSF-based HSC mobilization. In this report, we present a collection of cases illustrating the successful mobilization of HSCs through the administration of plerixafor in combination with G-CSF and chemotherapy in lymphoma patients planned for APBSCT, and who are at risk of or have previously experienced mobilization failure with G-CSF.
Case Presentation
Case 1
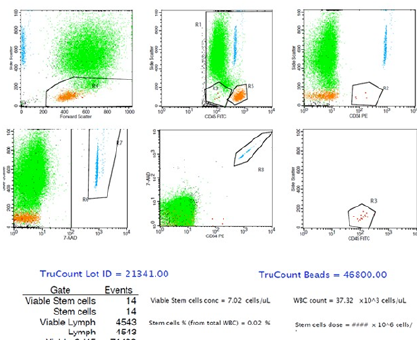
A 26-year-old gentleman was diagnosed with stage IV nodular sclerosing HL. He underwent two rounds of chemotherapy, which consisted of doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) and ifosfamide, cyclophosphamide, etoposide (ICE). Throughout the chemotherapy treatment, he did not experience any complications. The first mobilization with G-CSF (up to 900 µg/day) and etoposide (3248 mg once daily [OD]) without plerixafor was unsuccessful; the highest peripheral blood (PB) CD34+ cell concentration (on day 16 of mobilization) was 7.66 cells/µL, with only a total CD34+ cell dose of 0.82 × 106 cells/kg collected within two days using the COM.TEC® apheresis system (Fresenius, Lake Zurich, Illinois). Subsequently, 20 mg plerixafor was given on day 12 of mobilization, along with etoposide (3248 mg OD) and G-CSF (up to 900 µg/day), during the second mobilization period. The highest PB CD34+ cell concentration achieved was 158.27 cells/µL (on day 14 of mobilization), resulting in a CD34+ cell dose of 3.26 × 106/kg in a single leukapheresis using the same apheresis system as the first mobilization, with normal apheresis volume approaches (2.5 to 3 times the patient’s total blood volume) (Figure 1). He underwent APBSCT and successfully engrafted without any complications.
Case 2
A 27-year-old gentleman was diagnosed with stage IV classical HL. He completed two rounds of chemotherapy prior to mobilization and did not experience any complications. He underwent the first mobilization while on third-line chemotherapy consisting of the ICE regimen. The first mobilization using G-CSF (500 µg/day) and ICE was unsuccessful, where the highest PB CD34+ cell concentration was only 10.34 cells/µL (on day 16 of mobilization). However, harvesting did not proceed as anticipated, and collection was unsuccessful. The second mobilization was then carried out with an additional 20 mg plerixafor on day 14 and day 15 of mobilization, along with G-CSF (500 µg/day) and ICE. The highest PB CD34+ concentration was 21.07 cells/µL (on day 16 of mobilization), yielding 3.47 × 106/kg of harvested cells (within two days) using the COM.TEC® apheresis system (Fresenius, Lake Zurich, Illinois) with normal apheresis volume approaches. Until this reported case, he has not yet consented to PBSC infusion, despite the disease being stable. The harvested products remain in storage for future PBSC infusion once the patient provides consent, and he continued with regular follow-up for disease monitoring and treatment.
Case 3
A 49-year-old Chinese gentleman had extranodal diffuse large B-cell lymphoma (DLBCL), stage IV with bone marrow involvement. He completed two rounds of the ABVD regimen and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone (BEACOPP) prior to the first mobilization. He experienced unsuccessful mobilization using G-CSF (up to 900 µg/day) and RICE, achieving a highest PB CD34+ cell concentration of 12.17 cells/µL (day 20 of mobilization) and only 0.93 × 106/kg of CD34+ cells after two days of collection with the COM.TEC® apheresis system (Fresenius, Lake Zurich, Illinois) under normal apheresis volume approaches. A repeated PET-CT scan showed residual active disease in the pelvis, and he was advised to undergo third-line chemotherapy. Simultaneously, the second mobilization with plerixafor, along with G-CSF and etoposide, was successfully attempted, resulting in a highest PB CD34+ concentration of 38.46 cells/µL (day 17 of mobilization) with 3.12 × 106 cells/kg of CD34+ cells collected in a single leukapheresis using the Amicus® Cell separator system (Fresenius, Lake Zurich, Illinois), again with normal apheresis volume approaches. He underwent APBSCT and successfully engrafted without any complications.
Case 4
A 49-year-old male patient was diagnosed with stage IV DLBCL. He was initially treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) for six cycles. The patient experienced recurrent febrile neutropenia during the third cycle; thus, subsequent R-CHOP cycles were reduced to 75%. During the fifth cycle, the patient also had a perforation of the sigmoid colon, a rare complication of chemotherapy. Considering the disease progression detected by a positron emission tomography (PET) scan upon completion of the R-CHOP regimen, one cycle of RICE and three cycles of rituximab, dexamethasone, high-dose cytarabine, cisplatin (R-DHAC) were initiated. No severe known complications of chemotherapy were reported. Initially, the PB CD34 count was 13.10 cells/µL on day 17 while mobilized with RICE and G-CSF alone. Due to the limited circulating PB CD34+ cells, an additional 20 mg plerixafor was given on day 17 of mobilization, increasing the PB CD34 count to 60.19 cells/µL. The collection dose for PBSC harvesting (on day 18 of mobilization) was 3.76 × 106 cells/kg in a single leukapheresis using the COM.TEC® apheresis system (Fresenius, Lake Zurich, Illinois) with normal apheresis volume approaches. However, the patient succumbed to complications of the disease before undergoing APBSCT.
Case 5
A 45-year-old male was diagnosed with stage IV DLBCL and was started on frontline chemotherapy of R-CHOP for six cycles. A PET scan revealed active lymphoma in the cervical lymph nodes, and he continued with RICE for four cycles. The initial mobilizing agents used were RICE and G-CSF. Stem cell collection via the COM.TEC® apheresis system (Fresenius, Lake Zurich, Illinois) only yielded 0.62 and 0.67 × 106 cells/kg of CD34+ cells on two attempts. However, a PET scan following second-line chemotherapy indicated active lymphomatous disease in the cervical, abdominal, and pelvic lymph nodes. Subsequently, three cycles of R-DHAC were administered. R-DHAC and G-CSF were the second mobilizing agents. On day 12 and day 14, two doses of 20 mg plerixafor were added to the mobilization protocol. On day 15 of mobilization, the PB CD34 count was 57.10 cells/µL. The PBSCs were successfully collected, giving a dose of 3.95 × 106 cells/kg in a single leukapheresis using the Amicus® Cell separator system (Fresenius, Lake Zurich, Illinois), with normal apheresis volume approaches. Until this reported case, he has not yet undergone APBSCT due to active disease and is currently receiving salvage chemotherapy. The harvested products were stored for future PBSC infusion once a partial response is achieved following salvage chemotherapy.
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Age (years) | 26 | 27 | 49 | 49 | 45 |
| Gender | Male | Male | Male | Male | Male |
| Diagnosis | HL | Classical HL | DLBCL | DLBCL | DLBCL |
| Stage at diagnosis | IV | IV | IV | IV | IV |
| Marrow infiltration | Unknown | No | Yes | No | No |
| Body weight (kg) | 87 | 52 | 53 | 75.5 | 78 |
| Chemotherapy | |||||
| 1 st line | ABVD | ABVD | ABVD | R-CHOP | R-CHOP |
| 2 nd line | ICE | BEACOPP | ICE | RICE | RICE |
| 3 rd line | - | ICE | DAC | R-DHAC | R-DHAC |
| Number of mobilizations | 2 | 2 | 2 | 1 | 1 |
| H/o irradiation | No | No | No | No | No |
| Mobilizer agent | |||||
| 1 st mobilization | G-CSF + etoposide | G-CSF + ICE | G-CSF + RICE | Plerixafor + G-CSF + RICE | RICE + G-CSF |
| 2 nd mobilization | Plerixafor + G-CSF + etoposide | Plerixafor + G-CSF + ICE | Plerixafor + G-CSF + etoposide | Not related | Plerixafor + R-DHAC + G-CSF |
| Reason of plerixafor usage | Re-mobilization in previously unsuccessful mobilization | Pre-emptive use | Upfront employment on 2 nd mobilization | ||
| Volume of blood processed (mL)* | |||||
| 1 st mobilization | 11,500 | 11,000 | 11,500 | 11,500 | 11,000 |
| 2 nd mobilization | 11,500 | 11,000 | 12,000 | 11,500 | 12,000 |
| Maximum PB CD34 + count (cells/ml) | |||||
| 1 st mobilization | 7.66 | 10.34 | 12.17 | 60.19 | 37.38 |
| 2 nd mobilization | 158.27 | 21.07 | 38.46 | Not related | 57.10 |
| CD34 + dosage (x10 6 cells/kg) | |||||
| 1 st mobilization | 0.82 (2 days) | Unsuccessful | 0.93 (2 days) | 3.76 (1 day) | 1.29 (2 days) |
| 2 nd mobilization | 3.26 (1 day) | 3.47 (2 days) | 3.12 (1 day) | Not related | 3.95 (1 day) |
| Name of apheresis collection system used | |||||
| 1 st mobilization | COMTEC® apheresis system | Not related | COMTEC® apheresis system | COMTEC® apheresis system | COMTEC® apheresis system |
| 2 nd mobilization | COMTEC® apheresis system | COMTEC® apheresis system | Amicus® separator system | Amicus® separator system | |
| Infusion status | Yes | No | Yes | No (died) | No |
| Engraftment status | Yes | Not related | Yes | Not related | Not related |
| Neutrophil | Day 12 | - | Day 14 | - | - |
| Platelet | Day 21 | - | Day 20 | - | - |
Table 1 provides a comprehensive summary of six case series, offering detailed insights into each series, including the type and stage of the disease, the chemotherapy initiated, and the mobilizer agent used. Additionally, the table outlines key data points related to mobilization procedures, such as the maximum white blood cell (WBC) count, maximum PB CD34 count, and CD34+ cell dosage for both the first and second mobilizations. This structured presentation allows for a clear understanding of the varied aspects of each case series, facilitating a comprehensive analysis of the mobilization outcomes and associated parameters.
Discussion
According to a review conducted by the European Group for Blood and Marrow Transplantation focusing on autologous hematopoietic stem cell mobilization in myeloma and lymphoma patients, several factors influence the outcomes of mobilization procedures. These factors encompass various parameters, including older age, disease staging, prior chemotherapy (such as fludarabine treatment), and the count of CD34+ cells in peripheral blood before apheresis, particularly in autologous cases8. Lanza et al. elucidated in their study that the predictive factors influencing successful mobilization with plerixafor are a baseline platelet count of more than 150 x 10⁹/L, the absence of prior radiotherapy, and the non-utilization of fludarabine as significant determinants9.
In the realm of autologous transplantation, the application of plerixafor can be categorized into three specific strategies: delayed re-mobilization, pre-emptive use, and upfront utilization. Delayed re-mobilization is employed when a previous mobilization cycle has yielded unsatisfactory results, as shown in four of the presented cases (Cases 1 to 3), and it is not affordable for all patients due to its high cost. Pre-emptive use is typically reserved for cases where there is a limited number of circulating PB CD34+ cells (generally <10 cells/µL) prior to initiating leukocytapheresis, as shown in Case 410. On the other hand, upfront utilization is chosen in situations where there is an anticipated likelihood of mobilization failure, as shown in Case 5.
The utilization of plerixafor has demonstrated an improvement in CD34 yield, as indicated by a literature review conducted by Zhuang et al. In their study, the initial-day collection yields before administering plerixafor ranged from 0.19 to 2.38 (median 1.67) x 10⁶ CD34+ cells/kg recipient weight. Following the administration of plerixafor, the collection yield increased significantly by approximately 10-fold, ranging from 1.61 to 7.85 x 10⁶ CD34+ cells/kg recipient weight11. Similarly, in all cases described in the case series, five of the six cases showed an improved CD34 yield to >3.0 x 10⁶ cells/kg after plerixafor administration, indicating successful mobilization.
According to DiPersio JF's study examining plerixafor safety and effectiveness in mobilizing hematopoietic stem cells for APBSCT among 298 NHL patients and 302 patients with multiple myeloma, it was discovered that combining plerixafor with G-CSF resulted in a significantly higher proportion of patients achieving the desired CD34+ cell threshold for transplantation in fewer apheresis days compared to using only placebo and G-CSF. Additionally, 90% of patients in the plerixafor group underwent transplantation after initial mobilization. The study also underscored the well-tolerated nature of plerixafor and G-CSF, with gastrointestinal disorders and injection site reactions being the most common adverse events associated with plerixafor12, 13. In addition, Lanza et al. reported that pairing biosimilar filgrastim with plerixafor shows promise, demonstrating effectiveness comparable to, if not greater than, that of the originator filgrastim and plerixafor combination in mobilizing stem cells for high-risk patients14.
Plerixafor has proven effective in mobilizing hematopoietic stem cells. In another study aimed at comparing the yield of the CD34 product with and without the use of plerixafor, the author discovered that the average CD34/kg product obtained in the non-plerixafor group was 0.2 x 10⁶ cells. None of the patients in this group received an adequate product (≥ 2 x 10⁶ cells/kg) for subsequent autologous transplantation. However, when plerixafor was utilized, the average CD34/kg product obtained was 2.3 x 10⁶ cells. As in our cases, all patients yielded adequate CD34 products with the use of plerixafor15. Prior research conducted in Turkey focused on 20 patients with lymphoma and myeloma who had previously experienced unsuccessful mobilization attempts using either G-CSF alone or in combination with chemotherapy. Their study found that when plerixafor was administered alongside G-CSF, it successfully facilitated the collection of the minimum required CD34+ stem cells in 70% of the patients. Consequently, 80% of these individuals were able to advance to autologous stem cell transplantation16.
The use of G-CSF in combination with chemotherapy agents has been linked to a significant failure rate in mobilization. This is primarily due to the inability of G-CSF to stimulate the proliferation of long-term repopulating HSCs, contributing to the elevated rate of transplant failures5. However, when combined with G-CSF, the failure rate of mobilization dropped to 4% from 25%, as plerixafor antagonizes the CXCR4 receptor, which further inhibits the retention of HSCs within the bone marrow niche6. This was shown in the first four cases of the series, where adding plerixafor to G-CSF boosted HSC collection in a shorter amount of time. A previous study also supported these findings, showing no increase in the number of adverse events and reporting no treatment-related deaths17.
The inclusion of plerixafor has been shown to reduce the number of leukaphereses and remobilizations while increasing the yield of CD34+ cells18. This was observed in three of the six patients in this case series (Cases 1, 3, and 5), all of whom required only a single leukapheresis to achieve an adequate CD34+ dose (>3 x 10⁶ cells/kg), compared to previous mobilization with G-CSF and chemotherapy alone. Furthermore, the pre-emptive use of plerixafor in Case 4 demonstrated successful HSC collection with a single mobilization.
However, despite its effectiveness, the high upfront cost of plerixafor is a significant concern, especially in low- and middle-income countries, including Malaysia. As mentioned in a previous study, despite the predictable response resulting in the target of CD34+ cells, the cost of plerixafor has restricted its use, as the average wholesale package price for one 1.2 mL vial of plerixafor is $8652.6819, 20. The actual mobilization cost of plerixafor was not analyzed in these reported cases. Future studies are recommended to evaluate the cost-effectiveness of on-demand plerixafor use as a PBSC-mobilizing agent. Therefore, plerixafor should be used for patients who are poor mobilizers or have not succeeded with initial mobilization attempts. The current standard of care for HSC mobilization includes a risk-adapted strategy, incorporating plerixafor "just in time" as needed. Zanetti et al. demonstrated that the on-demand addition of plerixafor is both safe and effective for stem cell mobilization in myeloma patients21. In addition to being an effective mobilization regimen for lymphoma patients who experience mobilization failure with G-CSF, plerixafor also appears to be safe and effective for patients with non-hematologic diseases who struggle with insufficient mobilization22.
The limited sample size of just five cases may not accurately represent the true safety and effectiveness of plerixafor for our population. Although all five patients in these case series indicate that plerixafor is a potentially safe and effective mobilizing agent for optimizing HSC collection for successful APBSCT, successful mobilization with plerixafor does not necessarily translate into a successful APBSCT. Patients may succumb to complications of the primary disease before transplantation or reinfusion of harvested PBSC products, as seen in Case 4. Additionally, a delay in reinfusion due to active disease (Case 5) or a patient not being ready for reinfusion (Case 2) leaves the clinical outcome uncertain. Larger prospective studies are recommended to confirm these findings in the future.
Conclusion
In all current instances, because of multiple lines of chemotherapy and extended treatment durations, the quantity of CD34+ cells has been minimal. Nonetheless, with the addition of plerixafor, the collected CD34+ cells increased significantly compared to the initial collection attempt. Furthermore, no significant rise in adverse events was observed during or before the collection process. These findings indicate that incorporating plerixafor not only enhanced the count of CD34+ cells obtained but also demonstrated potential safety and effectiveness.
Abbreviations
ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine); APBSCT (Autologous peripheral blood stem cell transplant); BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone); CD34+ (CD34 positive); G-CSF (Granulocyte colony stimulating factor); HL (Hodgkin lymphoma); HSCs (Haematopoietic stem cells); ICE (ifosfamide, cyclophosphamide, etoposide); NHL (Non-Hodgkin lymphoma); PB (Peripheral blood); PBSCs (Peripheral blood stem cells); PET (positron emission tomography); R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone); R-DHAC (rituximab, dexamethasone, high-dose cytarabine, cisplatin); and WBC (White blood cell).
Acknowledgments
We would like to extend our gratitude to Haematology ward staff, Hospital USM, especially Sister Noor Hasney Remli, for their assistance in providing patient information for this publication.
Author’s contributions
RHZ, MNH: The conception and design of the case report. RHZ, NISS, MAS: Acquisition of data, analysis and interpretation of data. RHZ, MNH, MA: Drafting the article. NANA, AH, ADA: Revising it critically for important intellectual content. MNH. AH, ADA: Final approval of the version to be submitted. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The case series was granted an exemption from ethical review by the Institutional review boards, under the reference number USM/JEPeM/KK/2502023.
Consent for publication
Written informed consents were obtained from the patients for the publication of this case series and any accompanying images. Copies of the written consents are available for review by Editor-in-chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Lahoud
O.B.,
Sauter
C.S.,
Hamlin
P.A.,
Dahi
P.B.,
High-Dose Chemotherapy and Autologous Stem Cell Transplant in Older Patients with Lymphoma. Current Oncology Reports.
2015;
17
(9)
:
42
.
View Article PubMed Google Scholar -
Zahid
U.,
Akbar
F.,
Amaraneni
A.,
Husnain
M.,
Chan
O.,
Riaz
I.B.,
A Review of Autologous Stem Cell Transplantation in Lymphoma. Current Hematologic Malignancy Reports.
2017;
12
(3)
:
217-26
.
View Article PubMed Google Scholar -
Giralt
S.,
Costa
L.,
Schriber
J.,
Dipersio
J.,
Maziarz
R.,
McCarty
J.,
Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biology of Blood and Marrow Transplantation.
2014;
20
(3)
:
295-308
.
View Article PubMed Google Scholar -
Musto
P.,
Simeon
V.,
Grossi
A.,
Gay
F.,
Bringhen
S.,
Larocca
A.,
Predicting poor peripheral blood stem cell collection in patients with multiple myeloma receiving pre-transplant induction therapy with novel agents and mobilized with cyclophosphamide plus granulocyte-colony stimulating factor: results from a Gruppo Italiano Malattie EMatologiche dell'Adulto Multiple Myeloma Working Party study. Stem Cell Research & Therapy.
2015;
6
(1)
:
64
.
View Article PubMed Google Scholar -
Patterson
A.M.,
Pelus
L.M.,
G-CSF in stem cell mobilization: new insights, new questions. Annals of Blood.
2017;
2
:
10
.
View Article PubMed Google Scholar -
Clercq
E. De,
Mozobil\textregistered (Plerixafor, AMD3100), 10 years after its approval by the US Food and Drug Administration. Antiviral Chemistry & Chemotherapy.
2019;
27
.
View Article PubMed Google Scholar -
Lanza
F.,
Gardellini
A.,
Laszlo
D.,
Martino
M.,
Plerixafor: what we still have to learn. Expert Opinion on Biological Therapy.
2015;
15
(2)
:
143-7
.
View Article PubMed Google Scholar -
Mohty
M.,
Hübel
K.,
Kröger
N.,
Aljurf
M.,
Apperley
J.,
Basak
G.W.,
Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplantation.
2014;
49
(7)
:
865-72
.
View Article PubMed Google Scholar -
Lanza
F.,
Lemoli
R.M.,
Olivieri
A.,
Laszlo
D.,
Martino
M.,
Specchia
G.,
Factors affecting successful mobilization with plerixafor: an Italian prospective survey in 215 patients with multiple myeloma and lymphoma. Transfusion.
2014;
54
(2)
:
331-9
.
View Article PubMed Google Scholar -
Romon
I.,
Castillo
C.,
Cid
J.,
Lozano
M.,
Use of plerixafor to mobilize haematopoietic progenitor cells in healthy donors. Vox Sanguinis.
2022;
117
(1)
:
6-16
.
View Article PubMed Google Scholar -
Zhuang
L.,
Lauro
D.,
Wang
S.,
Yuan
S.,
Addition of plerixafor in poorly mobilized allogeneic stem cell donors. Journal of Clinical Apheresis.
2022;
37
(4)
:
388-94
.
View Article PubMed Google Scholar -
DiPersio
J.F.,
Micallef
I.N.,
Stiff
P.J.,
Bolwell
B.J.,
Maziarz
R.T.,
Jacobsen
E.,
Investigators
3101,
Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology.
2009;
27
(28)
:
4767-73
.
View Article PubMed Google Scholar -
DiPersio
J.F.,
Stadtmauer
E.A.,
Nademanee
A.,
Micallef
I.N.,
Stiff
P.J.,
Kaufman
J.L.,
Investigators
3102,
Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood.
2009;
113
(23)
:
5720-6
.
View Article PubMed Google Scholar -
Lanza
F.,
Saraceni
F.,
Pezzi
A.,
Martino
M.,
Bosi
A.,
Cascavilla
N.,
(Italian Society for Transplantation)
GITMO,
A comparative analysis of biosimilar vs. originator filgrastim in combination with plerixafor for stem cell mobilization in lymphoma and multiple myeloma: a propensity-score weighted multicenter approach. American Journal of Hematology.
2017;
92
(9)
:
557-9
.
View Article PubMed Google Scholar -
Camps
R.R.,
Pareja
M.O.,
Mancha
I.V.,
Castillo
I.M.,
Mazo
A.I.,
Effectiveness of Plerixafor in Patients Undergoing Mobilization Autologous Haematopoietic Progenitor Cell. Farmacia Hospitalaria.
2013;
37
(4)
:
317-21
.
PubMed Google Scholar -
Tekgündüz
E.,
Altuntaş
F.,
Şıvgın
S.,
Ş.Z. Akı,
Dönmez
A.,
Topçuoğlu
P.,
Plerixafor use in patients with previous mobilization failure: A multicenter experience. Transfusion and Apheresis Science.
2012;
47
(1)
:
77-80
.
View Article PubMed Google Scholar -
Yang
X.,
Wan
M.,
Yu
F.,
Wang
Z.,
Efficacy and safety of plerixafor for hematopoietic stem cell mobilization for autologous transplantation in patients with non-Hodgkin lymphoma and multiple myeloma: A systematic review and meta-analysis. Experimental and Therapeutic Medicine.
2019;
18
(2)
:
1141-8
.
View Article PubMed Google Scholar -
Mesquita Augusto Passos
R.,
Feldens
T.K.,
Marcolino
M.A.,
Gouvêa
A.S.,
Dos Santos Oliveira
L.,
Menardi Nasser
L.,
Economic evaluation of plerixafor addition in the mobilization and leukapheresis of hematopoietic stem cells for autologous transplantation: a systematic review. Expert Review of Pharmacoeconomics {&}amp; Outcomes Research.
2023;
23
(1)
:
15-28
.
View Article PubMed Google Scholar -
Sinha
S.,
Gastineau
D.,
Micallef
I.,
Hogan
W.,
Ansell
S.,
Buadi
F.,
Predicting Peripheral Blood Stem Cell Harvest Failure Using Circulating CD34 Levels: Developing Target-Based Cut-Points for Early Intervention. Bone Marrow Transplantation.
2011;
46
(7)
:
943-9
.
View Article PubMed Google Scholar -
Wehr
V.H.,
Comeau
J.M.,
Efficacy and Cost Analysis of a Plerixafor Protocol for Peripheral Blood Stem-Cell Mobilization in Patients with Multiple Myeloma or Non-Hodgkin Lymphoma. Journal of Hematology Oncology Pharmacy.
2016;
6
(4)
:
139-43
.
-
Zannetti
B.A.,
Saraceni
F.,
Cellini
C.,
Fabbri
E.,
Monaco
F.,
Guarini
A.,
Low-Dose Cyclophosphamide versus Intermediate-High-Dose Cyclophosphamide versus Granulocyte Colony-Stimulating Factor Alone for Stem Cell Mobilization in Multiple Myeloma in the Era of Novel Agents: A Multicenter Retrospective Study. Transplantation and Cellular Therapy.
2021;
27
(3)
.
View Article PubMed Google Scholar -
Worel
N.,
Apperley
J.F.,
Basak
G.W.,
Douglas
K.W.,
Gabriel
I.H.,
Geraldes
C.,
European data on stem cell mobilization with plerixafor in patients with nonhematologic diseases: an analysis of the European consortium of stem cell mobilization. Transfusion.
2012;
52
(11)
:
2395-400
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 12 No 4 (2025)
Page No.: 7295-7303
Published on: 2025-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 388 times
- PDF downloaded - 125 times
- XML downloaded - 39 times
 Biomedpress
Biomedpress


