Abstract
Introduction: Lavandula angustifolia L. (Lamiaceae family) displays notable cytotoxic properties against bacteria and fungi, as well as antioxidant activity. Recently, drug delivery systems for cancer therapy have focused on novel carrier designs that demonstrate high efficiency and reduced side effects. The encapsulation and delivery of bioactive agents through essential oils has emerged as a new strategy in cancer research. This study aimed to investigate the effects of nanoparticles synthesized using L. angustifolia essential oil on MCF-7 and SK-BR-3 breast cancer cell lines.
Methods: Physical and chemical parameters—specifically, particle size, zeta potential, and polydispersity index—were optimized using various techniques. Chemical composition analyses via GC/MS identified thirteen components in the essential oil. Eucalyptol, (+)-2-Bornanone, and endo-Borneol were the major fractions, while acetic acid and p-Cymen-7-ol appeared in smaller amounts. Zeta potential measurements revealed the electrical charge on particle surfaces, and FTIR analysis displayed distinct peaks corresponding to functional groups. Cell viability was assessed via MTT assay to evaluate nanoparticle toxicity against MCF-7 and SK-BR-3 cells.
Results: The synthesized nanoliposome showed notable cytotoxic effects on MCF-7 and SK-BR-3 cells, with IC50 values of 305 ± 0.29 and 400 ± 0.39 µg/ml, respectively. Expression of the caspase-3 gene increased approximately 2-fold in both treated cell lines at 48 hours after treatment, while Bcl2 expression decreased. The control group showed the lowest level of caspase-3 expression.
Conclusion: These findings suggest that nanoparticles synthesized from L. angustifolia essential oil, particularly those enriched with Eucalyptol and Bornanone, can enhance the precision of chemotherapeutic targeting in breast cancer. This nanoliposome formulation could be a promising novel candidate for pharmaceutical applications and improved cancer therapies.
Introduction
Different types of cancer, accounting for approximately 17% of global mortality, cause serious financial, emotional, and physical challenges in all communities. Breast cancer, defined as unregulated cell growth, is the most prevalent cancer, especially in mature women in many Asian countries1, 2. In Iran, the high incidence and mortality rates of breast cancer have risen as a major public health concern3. Currently, the use of synthetic and chemical anticancer drugs has shown various side effects, including diarrhea, constipation, indigestion, extreme fatigue, and compromised immune function4. Therefore, due to convenient access to natural products, their higher efficacy, fewer adverse effects, and diverse chemical composition, the use of medicinal plants in cancer therapy has gained more attention5.
The application of nanomaterials as a highly efficient method for drug delivery in cancer treatment has recently attracted significant interest. Therefore, the use of a drug delivery system based on medicinal plants aims to ultimately reduce the hazardous effects caused by conventional cancer therapy methods, such as surgery, radiotherapy, and chemotherapy. To overcome these obstacles, numerous researchers have been interested in applying nanotechnology research to the treatment of breast cancer6. To date, the anticancer effects of nanoparticles derived from various medicinal plants have been investigated in several studies7, 8, 9, but the mechanism of cell growth and inhibition has received less attention and has been the subject of only a few studies.
Lavandula angustifolia Mill., from the Lamiaceae family, is one of the most important medicinal herbs in the Mediterranean region. This genus and its related species have been applied as spasmolytic, carminative, stomachic, or diuretic10. The L. angustifolia essential oil also possesses many biological activities, including anticancer and antioxidant effects, as well as the ability to scavenge free radicals and stimulate the immune system11, 12. The Caspase cascade genes, including both upstream and downstream genes, play a crucial role as key mediators of this event. Caspase proteins cleave their substrates by targeting the carboxyl side of the aspartate residue13. BCL2 gene positivity is observed in 20% of breast cancer patients, and the BCL2 gene is notably overexpressed in these receptors. SK-BR-3 is a HER2-positive human breast cancer cell line that can exhibit an epithelial morphology in tissue culture14.
In cancer therapy, resistance to drugs and chemical treatment is a primary challenge. Therefore, the development of novel therapeutic agents to overcome treatment resistance is vital and important. This study aimed to investigate the cellular and molecular changes in MCF-7 and SKBR-3 cell lines treated with the nanoparticle of L. angustifolia essential oil.
Methods
Extraction of Lavender Essential Oils (LEO)
The dried tissues of Lavandula angustifolia Mill. were crushed using an electric mill before being dissolved in a 1:10 (w/v) solution of 80% ethanol and distilled water. The solutions were shaken over the course of one day. Distilled water was stirred for one day at 25 °C using a magnetic stirrer. The mixture was then filtered through filter paper. After freeze-drying to obtain a liquid extract, the extracts were concentrated using a vacuum evaporator (Heidolphe, Germany). For further use, the essential oil was extracted and stored at 4 °C6.
Gas Chromatography-Mass Spectrometry
Bioactive natural compounds of lavender essential oil were determined using Gas Chromatography-Mass Spectrometry (Agilent Technologies, Palo Alto, CA, USA). An HP–5MS 5% column coated with methyl silicone served as the stationary phase, and helium was used as the carrier gas at a constant flow rate of 10 mL/min15.
Encapsulation Process and Nanoliposomes
Cholesterol, polyethylene glycol (PEG), and soybean phosphatidylcholine (SPC) (70:31:1) were used to construct nanoliposomes in chloroform solvent. The solution was then combined with lavender essential oil dissolved in ethanol. A rotary evaporator was used at 34 °C under reduced pressure to vaporize the solvent, forming liposomes. An ultrasonic process at 60 °C for 10 min in a water bath was applied to the suspensions of lavender nanoparticles. Morphological and structural characterization of the constructed liposome formulation was investigated using an atomic force microscopy instrument14. Dynamic Light Scattering (DLS) was employed to determine the average particle size of LEO-loaded liposomes at room temperature. To estimate the drug release rate, approximately 100 mL of the LEO-encapsulated liposomal sample was placed in a dialysis bag (molecular weight cut-off 12 kDa). The bag was immersed in 100 mL of PBS (pH 7.4) at 37 °C with gentle, consistent agitation at 100 rpm on a rotary apparatus and incubated for 72 h at 37 °C. At different time intervals, 1 mL of the release medium was removed for UV spectrophotometric assay at a wavelength of 270 nm15.
Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR spectroscopy of L. angustifolia essential oil before and after encapsulation was investigated using a VIR-100/200/300 (Jasco, France) spectrometer. The absorption FT-IR spectra of the particles and extract were observed in the range of 400–4000 cm−1 at room temperature16.
Cell Lines and Cell Culture
MCF7 (Cat N. IF.Y10021) and SKBR-3 (Cat N. IF.Y.WC10022) cell lines were obtained from the Infertility Research Center at the University of Shahid Sadoughi Medical Sciences in Iran. The cell lines were cultured in RPMI (Gibco). The culture medium was supplemented with 10% (v/v) fetal bovine serum (Sinaclon, Iran), 5% fetal bovine serum, and penicillin-streptomycin (100 U/mL). The cells were then incubated at 37 °C with 5% CO2.
Cell Viability and Toxicity Assays
The cytotoxic effect of lavender essential oil and LEO nanoparticles on two cell lines, MCF7 and SKBR3, was studied using the MTT assay method. One hundred trypsinized cells were seeded into 96-well microplates at a concentration of 10⁴ cells/mL per well, containing 100 μL of complete culture medium. They were then incubated for 24 h at 37 °C with 5% CO2 and humidified air, and were regularly monitored using an inverted microscope. The cells were treated for 24 h, 48 h, and 72 h with different concentrations of lavender essential oil and LEO-nanoparticles, ranging from 5 to 100 μg/mL, dissolved in an appropriate solvent. The cytotoxic effect was measured by adding 100 μL of culture medium containing MTT formazan (5 mg/mL) and incubating for 4 h at 37 °C. The produced formazan crystals were dissolved using 10% DMSO solution, and the absorbance at the three time points was recorded on an ELISA plate reader (Drawell Dnm-9602, China) at 570 nm9. Non-treated cells in culture medium served as a control group. The percentage of cell survival (cell viability = OD average value of treatment group / OD average value of the control group) was calculated by comparing the optical density (OD) to the control group.
The trypan blue dye exclusion technique was also used to determine the percentage of viable cells in suspension treated with lavender essential oil nanoparticles. Here, 50 μL of the cell suspension was mixed with an equal volume of trypan blue. A hemocytometer slide was used to count the transparent (live) cells, while dead cells appeared blue17.
RNA Extraction, cDNA Synthesis, and qRT-PCR
Cells treated with the IC50 dose of the synthesized LEO-nanoparticle and cells treated with lavender essential oil were subjected to RNA extraction. The targeted cells were washed with PBS (Sinaclon, Iran) and lysed directly in the culture flask prior to RNA isolation. Total RNA was isolated using RNX-Plus (Sinaclon, Bioscience, Iran) following the manufacturer’s instructions. The quantity and quality of the extracted RNA were determined using a NanoDrop 2000 Spectrophotometer and 1.5% agarose gel electrophoresis. Superscript II reverse transcriptase (Qiagen, Invitrogen) was used for first-strand cDNA synthesis. Real-time PCR amplification was performed on an ABI PRISM 7700 Sequence Detection System (Thermo Scientific, Waltham, MA, USA) using 96-well plates. Real-time PCR was carried out in a total volume of 20 μL containing 0.5 μL of synthesized cDNA from each sample, 1× buffer (10X), 5 mM MgCl₂, 200 μM dNTPs, 300–600 nM of each primer, 0.2 U/μL enzyme, and SYBR Green I. A solution buffer containing ROX dye served as a passive reference to normalize fluorescence in real-time PCR. PCR amplification was performed in triplicate, using a thermocycler program of 95 °C for 10 min for DNA denaturation, followed by 35–45 cycles of 95 °C for 20 s and 60 °C for 1 min. At the end of each PCR assay, the melting curve was analyzed to verify primer specificity, yielding a single melting peak for the amplified product. The real-time amplification was done with the same sample concentrations (equal dilution), using primers for the housekeeping gene (GAPDH) and the target genes to measure relative efficiency.
| Gene Name | Gene ID | Amplicon size(bp)/ Concentration (nm) | Cytogenetic Location | Primer Sequence |
| HER-2 | NM_001005862.2 | 131 | 17q12 | F (5'- CCTCTGACGTCCATCATCTC -3') |
| 300 | R (5'- ATCTTCTCGTGCCGTCGCTT -3') | |||
| CASP3 | NM_001354777.1 | 241 | 4q35.1 | F (5'- AAGCGAATCAATGGACTCTGG -3') |
| 420 | R (5'- CTGTACCAGACCGAGATGTC -3') | |||
| GAPDH | NM_001256799.3 | 195 | 12p13.31 | F (5'- CCATGAGAAGTATGACAAC -3') |
| 500 | R (5'- GAGTCCTTCCACGATACC -3') |
Statistical Analysis
Each in vitro experiment was repeated at least three times to verify reproducibility. The SPSS program was used to analyze the data obtained. All results were expressed as the mean and standard deviation of the mean (SD). A Student’s t‑test or one-way ANOVA was performed to compare the means between two independent groups. All graphs were designed using GraphPad Prism® 5 software (version 5.04). *P < 0.05 or **P < 0.01. *P < 0.05 and **P < 0.01 were considered statistically significant.
| Compound | Composition (%) |
|---|---|
| Acetic acid, 1,7,7-trimethyl-bicyclo[2,2,1]hept-2-yl ester | 0.15 |
| p-Cymen-7-ol | 0.24 |
| 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, cis | 0.25 |
| β-Pinene | 0.43 |
| Bicyclo[3,1,1]hept-2-ene-carboxaldehyde,6,6-dimethyl- | 0.44 |
| Camphene | 0.65 |
| α-Pinene | 1.41 |
| Cyclohexene, 1-methyl-4-(1-methylethyl)- | 1.74 |
| Terpinen-4-ol | 1.43 |
| o-Cymene | 1.47 |
| endo-Borneol | 29.2 |
| (+)-2-Bornanone | 30.5 |
| Eucalyptol (1,8-Cineole) | 30.74 |
Results
Composition of L. angustifolia essential oil
Using GC/Mass analysis, 13 different compounds were identified. Among the detected fractions, Eucalyptol (1,8-cineole) was found to be the major component (30.74%), followed by (+)-2-Bornanone (30.50%) and endo-Borneol (29.2%). The concentrations of the other components were detected at less than 2% (Table 2).
Characterization of encapsulated essential oil of L. angustifolia in liposomes Dynamic light scattering (DLS) was used to evaluate the particle size of the vesicles. The results showed an average particle size of around 60 nm for the synthesized nanoliposomes containing lavender essential oil, similar to that of blank nanoliposomes (Figure 1). The zeta-potential values indicated the electrical charge on their surface. Zeta potential measurements of blank and LEO-nanoparticles ranged from -2 to -4.5 mV, indicating a neutral surface charge (Figure 1). The average electrophoretic mobility was -0.000015 cm/Vs, and the dispersion medium viscosity was 0.891 mPa·s, respectively.
The LEO-loaded nanoliposomes were incubated in PBS buffer solution at 37°C. The in vitro release behavior of the nanoliposomes was tested for 72 hours, and the results were presented as a cumulative release percentage in Figure 2. The release of essential oil as a drug from nanoliposomes was distinctly slower in comparison with a crude solution of lavender essential oil. The release rate of the essential oil components from nanoliposomes was 63.98% after 30 min and continued at the same rate after 1 h, whereas the release rate from pure lavender essential oil at this time was recorded as 87.63% (Figure 2). Based on these observations, we could claim that the encapsulation of lavender essential oil in nanoliposomes could be an effective way to continuously supply bioactive plant components in the body.
FTIR analysis of the essential oil and its nanoparticle revealed numerous distinctly different locations under the applied wavelengths. These peaks were assigned to the following groups: the functional group -OH, the stretching vibration of -CH, the aliphatic ester (stretching vibration of C=O), and the bending vibration of -CH2 and -CH2CH3. Peaks for the stretching vibrations of the functional groups P=O and P-O-C were observed at 1214.88 cm-1 and 982.51 cm-1, respectively. The FTIR spectra of LEO-loaded and control nanoliposomes were compared, and it was found that there were some distinct peaks at 3372.41 cm-1, 2295.87 cm-1, 1641.68 cm-1, and 1058.15 cm-1, corresponding to the functional groups -OH, -PH, C=O, and C-O, respectively18 (Figure 3).
Under the experimental conditions applied in this work, our data clearly showed that the lavender essential oil was completely encapsulated. In particular, the ratio between the oil volume and the lipid volume was 0.1, indicating that the encapsulated oil was not able to disrupt the liposome packing.
| Treatment type | IC 50 values (µg/ml) | |
| MCF-7 cells | SK-BR-3 cells | |
| Free essential oil (24h) | 425 ± 0.45 | 550 ± 0.32 |
| Free essential oil (48h) | 355 ± 0.36 | 450 ± 0.41 |
| Free essential oil (72h) | 305 ± 0.29 | 400 ± 0.39 |
| Encapsulated essential oil (24h) | 240 ± 0.42 | 350 ± 0.44 |
| Encapsulated essential oil (48h) | 170 ± 0.52 | 225 ± 0.13 |
| Encapsulated essential oil (72h) | 100 ± 0.34 | 175 ± 0.12 |
Cell toxicity assay
The MTT assay was used to investigate the anticancer activity of lavender essential oil and LEO-loaded nanoliposomes on MCF-7 and SK-BR-3 cells after 24, 48, and 72 hours. The lavender essential oil and LEO-nanoparticles at concentrations ranging from 5 to 100 µg/ml were used to evaluate and compare cell toxicity. The IC50 values of the essential oil on MCF-7 cells were 425, 355, and 305 µg/ml at 24, 48, and 72 hours of treatment, respectively. However, the IC50 values of the essential oil on SK-BR-3 cells were 550 ± 0.32, 450 ± 0.41, and 400 ± 0.39 µg/ml, respectively. The IC50 values of LEO-nanoparticles were significantly lower in both cell lines compared to the crude essential oil (Table 3). A comparison of the measured IC50 values showed that MCF-7 and SK-BR-3 cells were much more sensitive to the essential oil. A time- and dose-dependent effect was observed in both MCF-7 and SK-BR-3 cell lines.
Percentage cell viability using crude lavender essential oil and synthesized nanoparticles at equal concentrations over a 72-hour period after treatment showed that SKBR3 cells exhibited greater resistance to both types of treatment compared to the MCF7 cell line. In both cell lines, the viability percentage of cells treated with nanoparticles was lower than that of those treated with the crude plant extract (Table 4).
| Cell line | Treatment | Live cell count | Total cell count | Viability (%) |
| MCF7 | LEO | 2.52×10 7 | 3.22×10 7 | 78% |
| LEO NP | 2.12×10 7 | 3.20×10 7 | 65% | |
| SKBR3 | LEO | 2.92×10 7 | 3.32×10 7 | 87% |
| LEO NP | 2.62×10 7 | 3.15×10 7 | 83% |
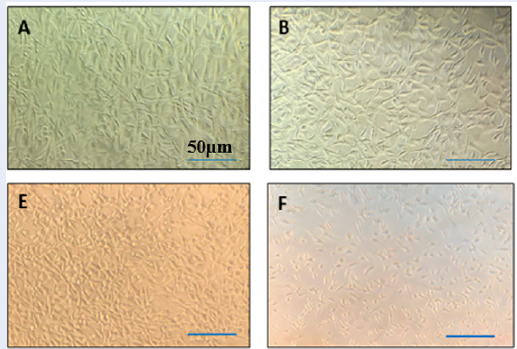
More than 90% of MCF7 and SKBR3 cells survived after being treated with various quantities of liposomes in the absence of the essential oil, suggesting that the liposomes were not hazardous to the cells. The outcomes showed that the suppression of the cell development process in response to LEO-loaded nanoliposomes was more pronounced than it was with the essential oil in its free form (p-value <0.01). The induction of cell death, however, was shown to be time-dependent for both the free form of the essential oil and the encapsulated essential oil, and the suppression of cell proliferation increased noticeably from 24 hours to 72 hours (Figure 4, Figure 5 ). The treatment of both cell lines with liposomes loaded with LEO showed a significant reduction in cell viability compared with untreated cells in a time- and dose-dependent manner.
Gene expression assay
The expression levels of the Caspase3 and Bcl2 genes were measured in MCF-7 and SK-BR-3 cell lines treated with essential oil and encapsulated essential oil after three time intervals (24, 48, and 72 hours) at the determined IC50 dose. The Bcl2 gene expression level decreased in both cell lines (Figure 6 A, C) during the 72 h following treatment at the relative IC50 concentrations of 100 ± 0.34 and 175 ± 0.12, respectively. The results showed that, after treating MCF-7 and SK-BR3 cells with L. angustifolia essential oil for 72 h, the cellular caspase-3 and Bcl2 activities increased in a time-dependent manner (Figure 6B). Also, caspase3 activity was significantly higher than that of the Bcl2 gene in response to essential oil nanoparticles (Figure 6). Based on these findings, the essential oil of L. angustifolia may induce an apoptotic effect on MCF-7 cells through caspase-8 activity, which is also likely to be caspase-dependent.
In contrast, the activity of the Bcl2 gene did not show any significant increase in both cell lines after 72 h of treatment with the L. angustifolia nanoparticle compared to the caspase3 gene (P < 0.05). Real-time qPCR analysis is illustrated in Figure 6.
Discussion
Cancer is a complex, multifactorial disease characterized by uncontrolled cell proliferation, attacking surrounding tissues at distant metastasis sites19. It remains a major public health concern worldwide and in Iran20. Recent cancer therapies, including the use of chemical drugs, chemotherapy, radiotherapy, and even surgery, are often associated with multidrug resistance, serious side effects, and high costs. In recent years, researchers have uncovered interesting and beneficial chemicals in medicinal plants. The role of medicinal plants in the production and development of new, modern drugs is undeniable. However, the use of therapeutic and effective doses, the formulation of bioactive compounds, and drug release in the body and target tissues remain significant challenges, especially in the treatment of cancer21.
Compared to bulk materials and chemical drugs, nanoparticles have attracted attention due to their novel or enhanced physical and chemical properties. Different types of nanomaterials and nanoparticles, including liposomes, polymer particles, micelles, quantum dots, silver nanoparticles, etc., have been developed as effective modalities and tested for therapeutic applications22. Recently, nanoparticles have been introduced as an important structure in modern medicine, especially for overcoming multi-drug resistance. The release of coated drugs from nanoparticles to the target tissues at adequate doses, effective delivery, and reducing their accumulation in unintended sites are major challenges restricting the effective use of nanoparticles22.
The biological and pharmacological activities of Lavandula species have been studied23, 24, 25. The potential bioactivity of lavender essential oil has been demonstrated by numerous studies. This genus is widely utilized in aromatherapy, food, cosmetics, and perfume sectors, in addition to its use as a medicinal plant26. The anticancer activities of lavender plant against six human cancer cell lines were evaluated, and 16 bioactive components were recorded by GC/MS analysis of plant exudates27. Therefore, the present study aimed to identify the chemical constituents of the lavender essential oil encapsulated in liposomes and to assess the cell toxicity of the constructed nanoliposome compared to lavender essential oil.
First, the chemical composition of the synthesized nanoparticle was determined using GC/Mass spectrometry analysis, which showed thirteen components. Further, GC-MS analysis of the nanoparticles’ essential oil revealed that the major components were Eucalyptol (30.74%), (+)-2-Bornanone (30.50%), and endo-Borneol (29.20%), while the minor components were Acetic acid (0.15%), p-Cymen-7-ol (0.24%), and 2-Cyclohexen-1-ol (0.25%). Similar results were observed by Fahmy et al. (2022), who reported comparable abundances of these fractions in L. officinalis essential oil. Despite the differences in the values and ratios of these two components (Bornanone and β-Pinene) and their similarity to our results, it should be noted that the studied species are entirely different, and slight variations in values are to be expected.
Eucalyptol is a terpenoid compound isolated from Eucalyptus species, with strong antioxidant properties that can serve as a promising candidate in the treatment of human diseases and for use in modern pharmacology. In some experimental studies, the anticancer activity of eucalyptol (EU) has been demonstrated. In vitro and in vivo anti-metastatic activity of eucalyptol against skin cancer cells has been shown. The effects of eucalyptol in cells have been associated with the expression of the epithelial marker E-cadherin, indicating that it reverses the epithelial-to-mesenchymal transition28. Eucalyptol was also reported to be effective as an antioxidant in guinea pig brains, regulating pro-inflammatory cytokines and antioxidant enzymes. In particular, eucalyptol reduces the expression of PI3K/Akt/mTOR by using the PI3K inhibitor29. The GC/MS analysis of the nanoparticle showed that it is rich in 1,8-cineole, which is in agreement with previously published findings on Lavender (Lavandula stoechas L.) essential oil that demonstrated potent anti-inflammatory and anticancer properties30.
In this experimental study, the effect of lavender essential oil nanoparticles on cell viability was examined by exposing MCF7 and SKBR3 cell lines to different concentrations of the nanoparticles, and the viability of the exposed cells was measured using the MTT assay. Plant essential oils can influence oxidative stress levels in cells. Depending on the type and concentration, they may trigger peroxidation, mitochondrial dysfunction, and apoptosis, which lead to cytotoxic activity. However, most studies suggest that they are typically non-genotoxic, meaning they do not directly damage DNA31, 32. The cell viability test was conducted at similar concentrations for the lavender essential oil and synthesized nanoparticles. This evaluation method is safe, quick, reliable, and inexpensive, and is widely used to quantify cell viability and proliferation. The results displayed in Table 4 indicate that LEO has a highly cytotoxic effect on MCF7 cells, with 63% viability, compared to SKBR3 cells, which showed 83% viability.
Recorded data showed that cell viability was almost at its maximum at the 48-hour interval after treatment. At this time, by comparing the behavior of the two nanoparticle-treated cells, it was found that the SKBR3 cell line was more affected by the nanoparticle than the MCF7 cell line, and its viability rate was higher. The effect of hydroalcoholic extracts of Ephedra major and Momordica charantia species showed that the highest cell toxicity occurred 72 hours after treatment, while in this research, the highest effect was observed 48 hours after treatment, indicating a faster efficiency of the nanoparticle on cell viability33. The highest toxicity dose of nanoparticles corresponded to the concentration of 1000 ng/mL in both cell lines, indicating that the sensitivity of both lines to the nanoparticles is similar at high doses. However, the IC50 assessment showed that in SKBR3 cells, this IC50 rate was slightly higher than that for the MCF7 cell line, which may be due to cellular drug resistance and detoxification of the drug within the cells. Considering that the IC50 value differs at various treatment time intervals, it is suggested that there is a time-dose dependency for killing 50% of the cells. Therefore, we conclude that nanoliposomes, microscopic particles that can encapsulate LEO, provide a safe and efficient delivery method for this essential oil.
At present, the direct relationship and mode of action of plant essential oils and genes involved in cancer are not widely recognized. In cancerous cells, apoptosis processes can be enhanced by many mechanisms. Thus, identifying and applying agents that suppress the proliferation of malignant cells can serve as a beneficial strategy for cancer prevention and chemotherapy. The essential oil of lavender species, with antitumor and strong antioxidant properties, acts as an activator of some tumor suppressor genes34. The caspase group of cysteine proteases is involved in controlling inflammation and the apoptosis signaling pathway. The function of proteins associated with caspase genes in cellular destruction has been established35. Our observations revealed a remarkable increase in caspase-3 levels in the group receiving nanoparticles derived from lavender essential oil after 72 hours with the IC50 dose of the nanoparticle. In contrast, we observed a significant reduction in Bcl2 levels in the group administered the appropriate IC50 dose 72 hours after treatment, compared with the control group (essential oil). Similar to caspases, Bcl-2 protein subfamilies such as Bcl-2, Bcl-xL, Mcl-1, Bid, and Pum are essential and crucial in apoptotic regulation36.
Our results showed that cells receiving LEO-nanoparticles exhibited an increase in the level of caspase-3 expression. Considering the key role of the caspase gene in the apoptosis process, an increase in caspase-3 activity could potentially enhance the effectiveness of LEO in killing cancer cells. By modulating caspase-3 expression, novel strategies for cancer treatment may be developed. Further research is needed to explore the mechanisms by which LEO and nanoliposomes induce caspase-3 activation and ultimately lead to cancer cell death29, 37.
Conclusions
Our research has shown that Lavandula angustifolia essential oil is a promising candidate for further investigation in cancer treatment. Due to their improved solubility, stability, and bioavailability in in vitro and in vivo applications, drug delivery systems now provide a platform for anticancer drug development. Compared to the free form of essential oil, LEO-loaded nanoliposomes exhibited a stronger inhibitory effect on cancer cell proliferation. In the MCF-7 and SK-BR-3 cell lines, administration of both types of LEO increased the expression of caspase-3 and simultaneously decreased the expression of BCL2. Based on the results obtained in this study, it is suggested to use other forms of nanoparticles with lavender essential oil to investigate their effects on cell toxicity in various cancer cell lines under laboratory conditions. After achieving positive results, laboratory tests should be performed on cancerous mice, with the ultimate aim of introducing these formulations into clinical and pharmaceutical stages.
Abbreviations
IC50 (Half-maximal Inhibitory Concentration), PBS (Phosphate-Buffered Saline), DLS (Dynamic Light Scattering), FTIR (Fourier Transform Infrared Spectroscopy), GC/MS (Gas Chromatography-Mass Spectrometry), MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide, a cell viability assay), DMSO (Dimethyl Sulfoxide), ELISA (Enzyme-Linked Immunosorbent Assay), qRT-PCR (Quantitative Real-Time Polymerase Chain Reaction), cDNA (Complementary DNA), RNA (Ribonucleic Acid), ANOVA (Analysis of Variance), SD (Standard Deviation), SPSS (Statistical Package for the Social Sciences), LEO (Lavender Essential Oil), PEG (Polyethylene Glycol), SPC (Soybean Phosphatidylcholine), HER2 (Human Epidermal Growth Factor Receptor 2), BCL2 (B-cell Lymphoma 2, an anti-apoptotic protein), CASP3 (Caspase-3, an apoptosis-related gene), GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase, a housekeeping gene), PI3K/Akt/mTOR (Phosphoinositide 3-Kinase/Protein Kinase B/Mechanistic Target of Rapamycin, a signaling pathway), MCF7 (Michigan Cancer Foundation-7, a breast cancer cell line), SKBR3 (Sloan-Kettering Breast Cancer 3, a HER2-positive breast cancer cell line), RPMI (Roswell Park Memorial Institute culture medium), FBS (Fetal Bovine Serum), NP (Nanoparticle), LEO-NP (Lavender Essential Oil Nanoparticle), UV (Ultraviolet) and OD (Optical Density).
Acknowledgments
The authors would like to thank Dr. Babakhanzadeh, Dr. Azari, Ms. Mozhdeh for their valuable support.
Author’s contributions
E.M, S.S: doing the central phases of article and writing the manuscript; M.R: Gathering the samples; B.H: perform statistical tests. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
The Institutional Ethics Committee of the Yazd University approved the study design. All authors and participants in this article have given their consent for publication.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Singh
S.K.,
Singh
S.,
Lillard
J.W.,
Singh
R.,
Drug delivery approaches for breast cancer. International Journal of Nanomedicine.
2017;
12
:
6205-18
.
PubMed Google Scholar -
Siegel
R.L.,
Miller
K.D.,
Jemal
A.,
Cancer statistics, 2018. CA: a Cancer Journal for Clinicians.
2018;
68
(1)
:
7-30
.
PubMed Google Scholar -
Enayatrad
M.,
Amoori
N.,
Salehiniya
H.,
Epidemiology and trends in breast cancer mortality in iran. Iranian Journal of Public Health.
2015;
44
(3)
:
430-1
.
PubMed Google Scholar -
Yap
T.A.,
Carden
C.P.,
Kaye
S.B.,
Beyond chemotherapy: targeted therapies in ovarian cancer. Nature Reviews. Cancer.
2009;
9
(3)
:
167-81
.
PubMed Google Scholar -
Rayan
A.,
Raiyn
J.,
Falah
M.,
Nature is the best source of anticancer drugs: indexing natural products for their anticancer bioactivity. PLoS One.
2017;
12
(11)
:
e0187925
.
View Article PubMed Google Scholar -
Devi
V.K.,
Jain
N.,
Valli
K.S.,
Importance of novel drug delivery systems in herbal medicines. Pharmacognosy Reviews.
2010;
4
(7)
:
27-31
.
PubMed Google Scholar -
Sun
J.,
Bi
C.,
Chan
H.M.,
Sun
S.,
Zhang
Q.,
Zheng
Y.,
Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids and Surfaces. B, Biointerfaces.
2013;
111
:
367-75
.
PubMed Google Scholar -
Wang
L.,
Li
H.,
Wang
S.,
Liu
R.,
Wu
Z.,
Wang
C.,
Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech.
2014;
15
(4)
:
834-44
.
PubMed Google Scholar -
Wang
L.,
Wang
S.,
Chen
R.,
Wang
Y.,
Li
H.,
Wang
Y.,
Oridonin Loaded Solid Lipid Nanoparticles Enhanced Antitumor Activity in MCF-7 Cells. Journal of Nanomaterials.
2014;
2014
(1)
:
903646
.
View Article Google Scholar -
Basch
E.,
Foppa
I.,
Liebowitz
R.,
Nelson
J.,
Smith
M.,
Sollars
D.,
Lavender (Lavandula angustifolia miller). Journal of Herbal Pharmacotherapy.
2004;
4
(2)
:
63-78
.
PubMed Google Scholar -
Tabatabaei
S.M.,
Kianinodeh
F.,
Nasiri
M.,
Tightiz
N.,
Asadipour
M.,
Gohari
M.,
In Vitro Inhibition of MCF-7 Human Breast Cancer Cells by Essential Oils of Rosmarinus officinalis, Thymus vulgaris L., and Lavender x intermedia. Archives of Breast Cancer. Archives of breast Cancer.
2018;
:
81-9
.
View Article Google Scholar -
Jahangir
M.,
Nazari
M.,
Babakhanzadeh
E.,
Manshadi
S.D.,
Where do obesity and male infertility collide?. BMC Medical Genomics.
2024;
17
(1)
:
128
.
PubMed Google Scholar -
Dou
H.,
Yu
P.Y.,
Liu
Y.Q.,
Zhu
Y.,
Li
F.C.,
Wang
Y.Y.,
Recent advances in caspase-3, breast cancer, and traditional Chinese medicine: a review. Journal of Chemotherapy (Florence, Italy).
2024;
36
(5)
:
370-88
.
PubMed Google Scholar -
Brockhoff
G.,
Heckel
B.,
Schmidt-Bruecken
E.,
Plander
M.,
Hofstaedter
F.,
Vollmann
A.,
Differential impact of Cetuximab, Pertuzumab and Trastuzumab on BT474 and SK-BR-3 breast cancer cell proliferation. Cell Proliferation.
2007;
40
(4)
:
488-507
.
PubMed Google Scholar -
Smit
E.,
Rüger
C.P.,
Sklorz
M.,
Goede
S. De,
Zimmermann
R.,
Rohwer
E.R.,
Investigating the trace polar species present in diesel using high-resolution mass spectrometry and selective ionization techniques. Energy & Fuels.
2015;
29
(9)
:
5554-62
.
-
Huth
F.,
Govyadinov
A.,
Amarie
S.,
Nuansing
W.,
Keilmann
F.,
Hillenbrand
R.,
Nano-FTIR absorption spectroscopy of molecular fingerprints at 20 nm spatial resolution. Nano Letters.
2012;
12
(8)
:
3973-8
.
PubMed Google Scholar -
Phillips
H.J.,
Terryberry
J.E.,
Counting actively metabolizing tissue cultured cells. Experimental Cell Research.
1957;
13
(2)
:
341-7
.
PubMed Google Scholar -
Mohammadpanah
M.,
Mojodi
E.,
Ehsani
R.,
The synthesis and characterization of liposomal nano-carriers loading Lavandula angustifolia essential oil to affect breast cancerous cell-lines. Yafteh.
2020;
22
(1)
:
84-95
.
-
Coltri
P.P.,
Santos
M.G. Dos,
Silva
G.H. da,
Splicing and cancer: challenges and opportunities. Wiley Interdisciplinary Reviews. RNA.
2019;
10
(3)
:
e1527
.
View Article PubMed Google Scholar -
Saedi
S.,
Saedi
A.,
Ghaemi
M.M.,
Milani
F.M.,
Epidemiological Study of Breast Cancer in Iran: A Micro Review Study. Eurasian Journal of Science and Technology.
2022;
2
(3)
:
227-35
.
View Article Google Scholar -
Hussin
A.H.,
Adverse effects of herbs and drug-herbal interactions. Malaysian Journal of Pharmacy.
2002;
1
(2)
:
39-44
.
-
Barua
S.,
Mitragotri
S.,
Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today.
2014;
9
(2)
:
223-43
.
View Article PubMed Google Scholar -
Ghadiri
M.K.,
Gorji
A.,
Lavender for medicine: A brief review of clinical effects. Avicenna (Doha).
2002;
1
:
23-7
.
-
Cavanagh
H.M.,
Wilkinson
J.M.,
Lavender essential oil: a review. Australian Infection Control : Official Journal of the Australian Infection Control Association Inc.
2005;
10
(1)
:
35-7
.
View Article Google Scholar -
Fisher
A.D.,
Lavender
J.S.,
April
M.D.,
Hill
R.,
Bynum
J.,
Schauer
S.G.,
A descriptive analysis of supermassive transfusion recipients among US and coalition forces during combat operations in Afghanistan and Iraq. Military Medicine.
2023;
188
(5-6)
:
e1022-7
.
View Article PubMed Google Scholar -
Prusinowska
R.,
Śmigielski
K.B.,
Composition, biological properties and therapeutic effects of lavender L). A review. Herba Polonica.
2014;
60
(2)
:
56-66
.
View Article Google Scholar -
Fahmy
M.A.,
Farghaly
A.A.,
Hassan
E.E.,
Hassan
E.M.,
Hassan
Z.M.,
Mahmoud
K.,
Evaluation of the Anti-Cancer/Anti-Mutagenic Efficiency of Lavandula officinalis Essential Oil. Asian Pacific Journal of Cancer Prevention.
2022;
23
(4)
:
1215-22
.
View Article PubMed Google Scholar -
Rahaman
A.,
Chaudhuri
A.,
Sarkar
A.,
Chakraborty
S.,
Bhattacharjee
S.,
Mandal
D.P.,
Eucalyptol targets PI3K/Akt/mTOR pathway to inhibit skin cancer metastasis. Carcinogenesis.
2022;
43
(6)
:
571-83
.
View Article PubMed Google Scholar -
Chamcheu
J.C.,
Roy
T.,
Uddin
M.B.,
Banang-Mbeumi
S.,
Chamcheu
R.N.,
Walker
A.L.,
Role and therapeutic targeting of the PI3K/Akt/mTOR signaling pathway in skin cancer: a review of current status and future trends on natural and synthetic agents therapy. Cells.
2019;
8
(8)
:
803
.
View Article PubMed Google Scholar -
Boukhatem
M.N.,
Sudha
T.,
Darwish
N.H.,
Chader
H.,
Belkadi
A.,
Rajabi
M.,
A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules (Basel, Switzerland).
2020;
25
(16)
:
3671
.
View Article PubMed Google Scholar -
Russo
R.,
Corasaniti
M.T.,
Bagetta
G.,
Morrone
L.A.,
Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evidence-Based Complementary and Alternative Medicine.
2015;
2015
:
397821
.
View Article PubMed Google Scholar -
Luo
H.,
Jiang
B.,
Li
B.,
Li
Z.,
Jiang
B.-H.,
Chen
Y.C.,
Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. International journal of nanomedicine.
2012;
24
:
3951-9
.
View Article Google Scholar -
Sabbagh
S.K.,
Ghodrati
E.,
Hajibeiki
A.,
Mazaheri
M.,
Ardakani
M.R. Sarafraz,
Ardakani
Z. Shaker,
Effect of hydroalcoholic extract of ephedra major, momordica charantia, and resveratrol on cytotoxicity and caspase-3 genes expression level in mcf-7 breast cancer cell line. Gene, Cell and Tissue.
2021;
8
(3)
:
e110658
.
View Article Google Scholar -
Alhazmi
M.I.,
Hasan
T.N.,
Shafi
G.,
Al-Assaf
A.H.,
Alfawaz
M.A.,
Alshatwi
A.A.,
Roles of p53 and caspases in induction of apoptosis in MCF- 7 breast cancer cells treated with a methanolic extract of Nigella sativa seeds. Asian Pacific Journal of Cancer Prevention.
2014;
15
(22)
:
9655-60
.
View Article PubMed Google Scholar -
Henshall
D.C.,
Chen
J.,
Simon
R.P.,
Involvement of caspase-3-like protease in the mechanism of cell death following focally evoked limbic seizures. Journal of Neurochemistry.
2000;
74
(3)
:
1215-23
.
View Article PubMed Google Scholar -
Ichim
G.,
Tait
S.W.,
A fate worse than death: apoptosis as an oncogenic process. Nature Reviews. Cancer.
2016;
16
(8)
:
539-48
.
View Article PubMed Google Scholar -
Ghavami
S.,
Hashemi
M.,
Ande
S.R.,
Yeganeh
B.,
Xiao
W.,
Eshraghi
M.,
Apoptosis and cancer: mutations within caspase genes. Journal of Medical Genetics.
2009;
46
(8)
:
497-510
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 12 No 3 (2025)
Page No.: 7224-7235
Published on: 2025-03-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 893 times
- PDF downloaded - 271 times
- XML downloaded - 53 times
 Biomedpress
Biomedpress







