Abstract
Introduction: C-reactive protein (CRP) has shown associations with multiple cardiovascular disorders, including atrial fibrillation (AF). Similarly, the neutrophil-to-lymphocyte ratio (NLR) is gaining recognition as a potential prognostic factor in cardiovascular health. Although AF has been widely studied, much of the current research emphasizes individuals of White ethnicity, underscoring the need for further investigation across more ethnically diverse populations.
Methods: To address this gap, this study utilized a Korean hospital database to examine the association of AF with CRP and NLR in a Korean patient population. A retrospective cohort study was conducted on 164 patients, equally divided between AF and normal sinus rhythm groups. CRP levels and NLR were measured using standard assays, and electrocardiography data confirmed AF diagnosis. Statistical analysis, including receiver operating characteristic curve evaluation, was performed to assess the discriminatory power of these biomarkers.
Results: Analysis of the study data revealed a significant difference in CRP levels between patients with AF and those with a normal sinus rhythm, indicating a pronounced inflammatory response associated with AF. Nevertheless, NLR did not demonstrate a significant difference between the AF and control groups.
Conclusion: While CRP could be a dependable marker for identifying inflammation in AF patients, NLR may not offer the same level of differentiation within this population. This study underscores the importance of evaluating inflammatory markers in AF within Korean individuals and highlights the need for broader, ethnicity-specific research in cardiovascular disease.
Introduction
Atrial fibrillation (AF) is the most prevalent arrhythmia observed in clinical practice, and its incidence has markedly risen over the past years. AF is independently associated with increased cardiovascular morbidity and mortality, thus representing a major public health concern1. AF is a type of supraventricular tachyarrhythmia characterized by disorganized atrial activation and ineffective atrial contraction. Its electrocardiographic features include irregular R-R intervals (when atrioventricular conduction is intact), the lack of distinct P waves, and erratic atrial activity, often referred to as fibrillatory waves2. In normal cardiac rhythm, impulses are generated by the sinoatrial node and travel smoothly through the atria. AF, however, arises from electrophysiological disruptions that affect impulse generation or from structural irregularities within cellular connections that would otherwise support rapid, coordinated conduction. Commonly, AF originates from abnormal electrical activity triggered by ectopic action potentials, typically in the pulmonary veins of the left atrium, or through reentrant circuits facilitated by non-uniform conduction pathways due to interstitial fibrosis3. As the average age of the population continues to rise, the incidence of AF is also increasing. Recently, the prevalence of AF has also notably risen among young individuals, highlighting the need for further research.
The infiltration of proteins and immune cells that facilitate the inflammatory response within cardiac tissue and circulatory pathways is closely associated with AF. Inflammation is an essential biological process that mammals utilize in response to injuries as a means of protecting vulnerable tissue4. Markers of inflammation have been linked to various aspects of AF, including its onset, persistence, severity, recurrence following cardioversion, effectiveness of electrical cardioversion in cases of persistent AF, and the prothrombotic condition associated with the disease5. Inflammatory response mediators have the potential to alter structural substrates and atrial electrophysiology, thereby increasing susceptibility to AF. Additionally, inflammation influences calcium homeostasis and connexins, which are linked to the triggers of AF and the heterogeneity of atrial conduction6. Corroborating these findings, numerous meta-analyses provide compelling evidence that inflammatory markers, such as C-reactive protein (CRP) and the neutrophil-to-lymphocyte ratio (NLR), have been effective in defining the degree of inflammation associated with AF7.
Peripheral blood CRP is an acute-phase protein, predominantly synthesized in hepatocytes, which acts as a prototype marker of inflammation8. CRP levels rise rapidly following the onset of inflammatory stimuli, making it a reliable biomarker for assessing inflammatory states in various clinical settings9. Elevated CRP levels have been widely utilized in clinical practice to detect, monitor, and predict the severity of numerous inflammatory and cardiovascular diseases10. AF is frequently associated with increased systemic inflammation11; such inflammation may not only be a consequence of AF but could also contribute to its onset and perpetuation12. The relevance of CRP in AF stems from its potential role in promoting atrial structural remodeling, fibrosis, and electrical abnormalities, which are key mechanisms in the pathogenesis of AF13. Several studies have examined the prognostic utility of blood CRP levels in patients with AF. Tanaka et al. found that an elevated CRP level was significantly associated with AF14.
The NLR is a readily accessible and cost-effective inflammatory marker derived from the differential count of neutrophils and lymphocytes in peripheral blood15. NLR reflects the balance between the innate immune response (represented by neutrophils) and adaptive immune response (represented by lymphocytes)16. As a marker of systemic inflammation, NLR has garnered attention for its potential role in AF17. Elevated NLR levels may reflect an inflammatory milieu conducive to the development and maintenance of AF, offering insights into the underlying inflammatory processes that contribute to this arrhythmia18. Consequently, NLR has been proposed as a potentially valuable biomarker for identifying patients at higher risk of AF and predicting adverse cardiovascular outcomes in those with established AF19. Bhat et al.20 identified NLR as an independent indicator for predicting both short- and long-term mortality in individuals with acute coronary syndromes. Similarly, Buonacera et al.16 found that a high NLR was related to bacterial and fungal infection, acute myocarditis, and post-op complications. Shao et al. 21 showed that a high NLR was associated with an increased risk of AF occurrence/recurrence.
The potential to investigate the association between inflammation and AF based on inflammation marker levels, such as CRP, is of great interest to researchers. However, most previous studies have been conducted in White/Caucasian populations, limiting the generalizability of their findings to other ethnic groups22. Prior research on inflammatory markers in AF has largely overlooked Korean individuals, creating a gap in knowledge regarding how CRP and NLR function as biomarkers in this population.
To address this limitation, our study utilized a Korean hospital database to evaluate the association between AF and two key inflammatory markers, CRP and NLR, in a Korean cohort. By analyzing these markers within this specific ethnic group, we aim to enhance the understanding of inflammation-related AF pathogenesis in Koreans and assess whether CRP and NLR serve as effective biomarkers for AF within this demographic.
Methods
Study Design
This retrospective study enrolled 82 patients with normal sinus rhythm (NSR) and 82 patients with atrial fibrillation (AF) who attended a doctor's appointment at a cardiovascular hospital in Seoul, Korea. This study adhered to the principles set forth in the Declaration of Helsinki and received approval from Dankook University’s ethics committee (DKU 2024-06-028-002). The need for informed consent was waived because only anonymized retrospective data were used.
Participant Selection
Individuals younger than 18 years, including middle and high school students, were excluded in alignment with Article 2, Paragraph 2, of the Regulations on the Implementation of Bioethics Law. The final group of 200 participants was randomly selected, without restrictions on age or sex. Additionally, pediatric cardiac interpretations were excluded, as they require consultation with pediatric doctors. Therefore, patients who visited the pediatric cardiology department were excluded from this study. To maintain the homogeneity of the study cohort, patients who were receiving or had a history of taking medications such as immunosuppressants, immunoglobulins, immunomodulators, NSAIDs, statins, and similar agents affecting the immune system were excluded from the study.
Electrocardiographic Evaluation
A 12-lead electrocardiograph (MAC5500HD) was used with all participants at rest in the supine position. Electrocardiograms (ECGs) obtained from all participants were sent to the MUSE system for analysis. AF was identified based on standard 12-lead ECG criteria, including irregular R-R intervals, the absence of distinct P waves, and the presence of fibrillatory waves. To ensure the reliability of ECG interpretation, two independent board-certified cardiologists reviewed all ECGs. In cases of discrepancy, a third senior cardiologist provided adjudication to reach a consensus. This approach minimized interobserver variability and enhanced the accuracy of AF diagnosis. Additionally, all ECG findings were cross-referenced with documented physician-diagnosed AF history from follow-up surveys to confirm consistency.
Laboratory and Demographic Data
Biochemical parameters were measured using automated laboratory analyzers to ensure accuracy and reproducibility, with the aid of the Beckman Coulter LH-750 Hematology Analyzer (Beckman Coulter, Inc., Fullerton, CA, USA). The CRP levels were evaluated using standard methods. Anticoagulated whole blood samples were processed on Sysmex NX-9000 (TOA Medical Electronics Co., Kobe, Japan) to determine complete blood cell counts and differential leukocyte counts. The NLR was determined by dividing the absolute count of neutrophils by the absolute count of lymphocytes. To maintain analytical accuracy, the laboratory adhered to Clinical and Laboratory Standards Institute (CLSI) guidelines for quality assurance. Additionally, both internal and external quality control programs were implemented, with participation in a national laboratory proficiency testing program to validate assay performance against standardized reference materials.
All statistical analyses were conducted using MedCalc 13.1.1.0 (Mariakerke, Belgium). Continuous variables were expressed as mean ± standard deviation (SD). For comparisons between the AF and NSR groups, independent t-tests were used to compare continuous variables, such as CRP levels and NLR. Receiver operating characteristic (ROC) curve analysis was performed to assess the discriminatory ability of CRP and NLR for distinguishing between AF and NSR. A p-value of <0.05 was considered statistically significant for all tests.
Results
Clinical Characteristics of Patients with AF and NSR
A total of 164 patients were included in this study, with 82 patients in the AF group and 82 in the NSR group. The mean age of the AF group was significantly higher than that of the NSR group (71.5 ± 8.4 years vs. 61.3 ± 9.1 years; p < 0.001). As age is a well-established risk factor for AF, this difference was addressed in the subsequent analysis by including age as a covariate in multivariate logistic regression models to account for its potential confounding effect.
| Variables | NSR (n = 82) | AF (n = 82) |
| Age (years) | 61.341 | 71.548 |
| Male (n) | 41 | 44 |
| Heart rate (bpm) | 71.4 | 78.5 |
| LVH (n) | 3 | 10 |
| RBBB (n) | 1 | 7 |
| LBBB (n) | 0 | 3 |
| LAFB (n) | 0 | 3 |
Patients in the NSR group had an average heart rate of 71.4 beats per minute (bpm), while those in the AF group had a higher average heart rate of 78.5 bpm. A higher proportion of patients in the AF group (n = 10) presented with left ventricular hypertrophy (LVH) than in the NSR group (n = 3). In the NSR group, 1 patient had right bundle branch block (RBBB). The AF group had 7 patients with RBBB, 3 patients with left bundle branch block (LBBB), and 3 with left anterior fascicular block (LAFB). No left-sided conduction abnormality was observed in the NSR group (Table 1).
In the NSR cohort, the clinical diagnoses included 16 individuals with coronary artery disease (CAD), 9 reporting chest discomfort, 3 with a history of myocardial infarction (MI), and 4 diagnosed with aortic stenosis. Additionally, 1 patient had undergone aortic valve replacement, 3 had mitral valve replacement, and 1 had tricuspid valve replacement. Heart failure was present in 6 individuals, cardiomyopathy in 4, and angina pectoris in 15. Tachycardia was noted in 9 patients, Takayasu disease in 1, hypertension in 6, and a history of cancer in 4. In the AF cohort, diagnoses were distributed as follows: 45 patients with AF, 2 with CAD, 7 with chest discomfort, 4 with MI, 5 with aortic stenosis, 8 with mitral valve replacement, 2 with tricuspid valve replacement, 5 with heart failure, 2 with cardiomyopathy, and 2 with angina pectoris (Table 2).
| Variables | NSR (n = 82) | AF (n = 82) |
| Atrial fibrillation | 0 | 45 |
| Coronary artery disease | 16 | 2 |
| Chest discomfort | 9 | 7 |
| Myocardial Infarction | 3 | 4 |
| Aortic stenosis | 4 | 5 |
| Aortic valve replacement | 1 | 0 |
| Mitral valve replacement | 3 | 8 |
| Tricuspid valve replacement | 1 | 2 |
| Heart failure | 6 | 5 |
| Cardiomyopathy | 4 | 2 |
| Angina pectoris | 15 | 2 |
| Tachycardia | 9 | 0 |
| Takayasu disease | 1 | 0 |
| Hypertension | 6 | 0 |
| Cancer | 4 | 0 |
| Total | 82 | 82 |
CRP
The average CRP level among patients with NSR was 4.92 mg/L, with 68 individuals falling within the normal range (0–8 mg/L) and 14 exceeding it. The mean CRP level was 28.56 ± 6.84 mg/L in the AF group and 4.92 ± 1.73 mg/L in the NSR group. The mean difference in CRP levels between the two groups was 23.64 mg/L, with a 95% confidence interval (CI) of 19.87–27.41 mg/L (p = 0.002) (Figure 1).
NLR
The average NLR value in patients with NSR was 2.51, with 66 individuals falling within the normal range (1–3). Furthermore, 6 individuals had NLR values below the lower threshold of normalcy, while 10 surpassed the upper limit of the normal range. The mean NLR was 2.74 ± 1.12 in the AF group and 2.51 ± 0.98 in the NSR group. The mean difference in NLR was 0.23, with a 95% CI of -0.14 to 0.60 (p = 0.3161), suggesting a lack of statistical significance in differentiating between the two groups (Figure 2).
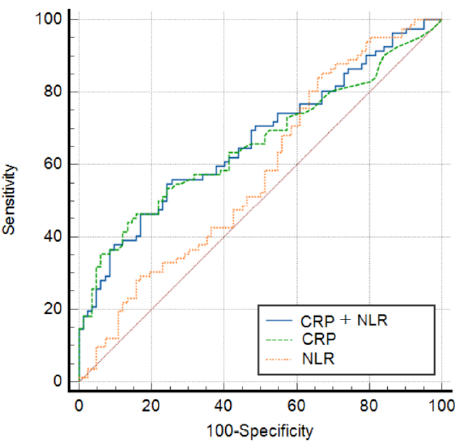
The ROC curve analysis demonstrated an area under the curve (AUC) of 0.658 for CRP, with a corresponding sensitivity (SE) of 0.0431. For NLR, the AUC was 0.577, with an SE of 0.0449. For CRP+NLR, the AUC improved to 0.667, with an SE of 0.0422 (Figure 3 and Table 3).
| Variable | AUC | SE | 95% CI |
| CRP | 0.658 | 0.0431 | 0.583 – 0.732 |
| NLR | 0.577 | 0.0449 | 0.489 – 0.665 |
| CRP+NLR | 0.667 | 0.0422 | 0.593 – 0.741 |
Discussion
This study showed that CRP levels in Koreans were lower than those reported in individuals of White/Caucasian populations and that CRP concentrations in Koreans were significantly associated with the prevalence of AF.
CRP
The significant difference observed in CRP levels between patients with AF and those with NSR underscores the potential role of inflammation in AF pathogenesis. This finding aligns with that of previous studies suggesting an association between elevated CRP levels and the development, progression, and recurrence of AF. Numerous epidemiological and mechanistic investigations have highlighted the multifaceted relationship between inflammation and AF, implicating CRP as a promising biomarker for risk stratification and prognostication in patients with AF.
Existing research has consistently demonstrated elevated CRP levels in patients with AF compared to those in individuals with NSR, implicating systemic inflammation in the pathophysiology of AF. Tanaka et al. 14 found that an elevated CRP level was significantly associated with AF. Hijazi et al. 23 showed that elevated IL-6 levels were related to old age, persistent and permanent AF, and heart failure. The prognostic significance of CRP in AF is further supported by its association with adverse cardiovascular outcomes, including stroke, heart failure, and mortality.
These findings highlight the clinical relevance of CRP as a biomarker for identifying high-risk individuals, guiding therapeutic interventions, and monitoring disease progression in AF. Incorporating CRP measurements into risk prediction models may enhance risk stratification strategies and facilitate personalized management approaches in patients with AF.
NLR
Despite the lack of statistically significant differences between patients with AF and NSR, the clinical relevance of NLR in AF management nevertheless merits consideration. While NLR has been implicated in various inflammatory conditions and cardiovascular diseases, its utility as a prognostic or diagnostic marker in AF remains uncertain. However, given the complex pathophysiology of AF, exploring the potential role of NLR as a supplementary biomarker in risk stratification and treatment response assessment could be valuable.
Several factors may contribute to the observed lack of significance in NLR levels between AF and NSR groups. These include the heterogeneous nature of AF, with diverse underlying etiologies and comorbidities that may influence inflammatory marker profiles differently. Additionally, variations in patient characteristics, such as age, sex, and disease duration, could confound the association between NLR and AF. Methodological considerations, including assay techniques and sample handling procedures, may also impact the accuracy and reproducibility of NLR measurements, potentially attenuating the observed differences between groups.
While the nonsignificant finding underscores the limitations of NLR as a standalone biomarker for discriminating between patients with AF and NSR, it highlights the need for a comprehensive multimodal approach to risk assessment and patient management in AF. Integrating clinical, demographic, and biochemical parameters, including NLR, CRP, and other inflammatory markers, may enhance risk stratification models and improve patient outcomes. Future studies employing longitudinal designs and larger sample sizes are warranted to validate these findings and elucidate the clinical implications of inflammatory marker profiling in AF management.
The combined marker, referred to as CRP+NLR, demonstrated the highest AUC and SE compared to CRP and NLR individually. These findings suggest that the integration of CRP and NLR values into a single combined marker can enhance the ability to stratify risk among patients with AF and provide a better indicator of inflammatory status.
SE
It is noteworthy that both CRP and NLR yielded statistically significant SE, indicating that their association with AF was unlikely random. Additional studies involving larger sample sizes and varied patient demographics are needed to confirm these findings and to better understand the mechanisms contributing to the observed associations.
Limitations
In interpreting the findings of this study, several limitations must be acknowledged. First, the relatively small sample size may have constrained the statistical power, potentially diminishing the ability to detect significant differences in biomarkers between the AF and NSR groups. Furthermore, the cross-sectional nature of the study precludes the establishment of a causal relationship between elevated CRP and NLR levels and AF. Confounding variables such as comorbidities or other inflammatory conditions were not fully controlled. Future research should aim to address these limitations by conducting studies with larger and more diverse patient populations to validate the observed associations and to enhance the generalizability of the findings. Prospective longitudinal studies would be particularly valuable in determining whether changes in biomarkers over time are predictive of AF onset or recurrence, helping in clarifying the causal relationship between CRP and NLR levels and AF. Additionally, exploring the combined use of CRP and NLR in a composite biomarker model across different clinical settings may provide further insights into their utility in AF risk stratification and management.
Conclusions
Based on the outpatient data collected from patients, the assessment of inflammatory markers, CRP and NLR, revealed significant disparities between patients with AF and NSR. CRP levels exhibited a notable difference, underscoring the potential role of inflammation in the pathogenesis of AF and highlighting CRP as a promising biomarker for risk stratification and prognostication in these patients. Conversely, NLR levels demonstrated minimal variance between the two groups, suggesting limited utility as a discriminatory marker in this context. These findings describe a significant association between inflammation and AF pathogenesis.
Future studies involving larger, multicenter cohorts with diverse populations are essential to validate these results and explore the clinical utility of inflammatory markers in AF diagnosis and management. Expanding research in this area could provide deeper insights into the mechanistic underpinnings of inflammation in AF and support the development of personalized therapeutic strategies for patients with this arrhythmic disorder.
Abbreviations
AF (Atrial Fibrillation); AUC (Area Under the Curve); bpm (beats per minute); CAD (Coronary Artery Disease); CI (Confidence Interval); CLSI (Clinical and Laboratory Standards Institute); CRP (C-Reactive Protein); DKU (Dankook University); ECG (Electrocardiogram); LAFB (Left Anterior Fascicular Block); LBBB (Left Bundle Branch Block); LVH (Left Ventricular Hypertrophy); MAC5500HD (Electrocardiograph model); MI (Myocardial Infarction); MUSE (ECG analysis system); NLR (Neutrophil-to-Lymphocyte Ratio); NSR (Normal Sinus Rhythm); NX-9000 (Hematology Analyzer model); p-value (Probability value); RBBB (Right Bundle Branch Block); ROC (Receiver Operating Characteristic); SD (Standard Deviation); SE (Sensitivity).
Acknowledgments
None.
Author’s contributions
Ji Yeon Chang: Conceptualization, Methodology, Data Curation, Writing – Original Draft; Bo Kyeung Jung: Conceptualization, Methodology, Formal Analysis, Writing – Original Draft; Jae Kyung Kim: Supervision, Writing – Review & Editing, Project Administration, Corresponding Author; All authors read and approved the final manuscript. Ji Yeon Chang and Bo Kyeung Jung contributed equally to this work.
Funding
None.
Availability of data and materials
Source data for this study are not publicly available owing to privacy or ethical restrictions. The source data will be made available to verified researchers upon reasonable request to the corresponding author.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Liu
T.,
Li
G.,
Li
L.,
Korantzopoulos
P.,
Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. Journal of the American College of Cardiology.
2007;
49
(15)
:
1642-8
.
View Article Google Scholar -
Lambiase
P.D.,
Maclean
E.,
Review of the National Institute for Health and Care Excellence guidelines on the management of atrial and ventricular arrhythmias. Heart.
2024;
110
(5)
:
313-22
.
View Article Google Scholar -
Joglar
J.A.,
Chung
M.K.,
Armbruster
A.L.,
Benjamin
E.J.,
Chyou
J.Y.,
Cronin
E.M.,
2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology.
2024;
83
(1)
:
109-279
.
View Article Google Scholar -
Scott
L.,
Li
N.,
Dobrev
D.,
Role of inflammatory signaling in atrial fibrillation. International journal of cardiology.
2019;
287
:
195-200
.
View Article Google Scholar -
Wu
N.A.,
Xu
B.,
Xiang
Y.,
Wu
L.,
Zhang
Y.,
Ma
X.,
Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. International journal of cardiology.
2013;
169
(1)
:
62-72
.
View Article Google Scholar -
Hu
Y.F.,
Chen
Y.J.,
Lin
Y.J.,
Chen
S.A.,
Inflammation and the pathogenesis of atrial fibrillation. Nature Reviews Cardiology.
2015;
12
(4)
:
230-43
.
View Article Google Scholar -
Gutierrez
A.,
Wagoner
D.R. Van,
Oxidant and inflammatory mechanisms and targeted therapy in atrial fibrillation: an update. Journal of cardiovascular pharmacology.
2015;
66
(6)
:
523-9
.
View Article Google Scholar -
Lee
Y.,
Park
H.C.,
Shin
J.H.,
Lim
Y.H.,
Shin
J.,
Park
J.K.,
Single and persistent elevation of C-reactive protein levels and the risk of atrial fibrillation in a general population: The Ansan-Ansung Cohort of the Korean Genome and Epidemiology Study. International journal of cardiology.
2019;
277
:
240-6
.
View Article Google Scholar -
Sproston
N.R.,
Ashworth
J.J.,
Role of C-reactive protein at sites of inflammation and infection. Frontiers in immunology.
2018;
9
:
754
.
View Article Google Scholar -
Banait
T.,
Wanjari
A.,
Danade
V.,
Banait
S.,
Jain
J.,
Role of high-sensitivity C-reactive protein (Hs-CRP) in non-communicable diseases: a review. Cureus.
2022;
14
(10)
:
e30225
.
View Article Google Scholar -
Korantzopoulos
P.,
Letsas
K.P.,
Tse
G.,
Fragakis
N.,
Goudis
C.A.,
Liu
T.,
Inflammation and atrial fibrillation: a comprehensive review. Journal of arrhythmia.
2018;
34
(4)
:
394-401
.
View Article Google Scholar -
Phillips
K.P.,
Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. Journal of atrial fibrillation.
2013;
6
(3)
:
935
.
View Article Google Scholar -
Everett
T.H.,
Olgin
J.E.,
Atrial fibrosis and the mechanisms of atrial fibrillation. Heart rhythm.
2007;
4
(3)
:
S24-7
.
View Article Google Scholar -
Tanaka
M.,
Imano
H.,
Kubota
Y.,
Yamagishi
K.,
Umesawa
M.,
Muraki
I.,
Serum high-sensitivity C-reactive protein levels and the risk of atrial fibrillation in Japanese population: the circulatory risk in communities study. Journal of Atherosclerosis and Thrombosis.
2021;
28
(2)
:
194-202
.
View Article Google Scholar -
Regolo
M.,
Vaccaro
M.,
Sorce
A.,
Stancanelli
B.,
Colaci
M.,
Natoli
G.,
Neutrophil-to-lymphocyte ratio (NLR) is a promising predictor of mortality and admission to intensive care unit of COVID-19 patients. Journal of clinical medicine.
2022;
11
(8)
:
2235
.
View Article Google Scholar -
Buonacera
A.,
Stancanelli
B.,
Colaci
M.,
Malatino
L.,
Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. International journal of molecular sciences.
2022;
23
(7)
:
3636
.
View Article Google Scholar -
Xiao
H.,
Lv
X.,
Zhou
S.,
Ren
Q.,
Zhang
Z.,
Wang
X.,
Association of systemic inflammatory markers with postoperative arrhythmias in esophageal cancer: a propensity score matching. Journal of Cardiothoracic Surgery.
2024;
19
(1)
:
142
.
View Article Google Scholar -
Zhang
J.L.,
Yang
R.,
Zhu
Y.,
Shao
Y.,
Ji
Y.,
Wang
F.F.,
Association between the neutrophil-to-lymphocyte ratio and risk of in-hospital heart failure and arrhythmia in patients with acute myocardial infarction. Frontiers in Cardiovascular Medicine.
2023;
10
:
1275713
.
View Article Google Scholar -
Fagundes
A.,
Ruff
C.T.,
Morrow
D.A.,
Murphy
S.A.,
Palazzolo
M.G.,
Chen
C.Z.,
Neutrophil-lymphocyte ratio and clinical outcomes in 19,697 patients with atrial fibrillation: Analyses from ENGAGE AF-TIMI 48 trial. International journal of cardiology.
2023;
386
:
118-24
.
View Article Google Scholar -
Bhat
T.,
Teli
S.,
Rijal
J.,
Bhat
H.,
Raza
M.,
Khoueiry
G.,
Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert review of cardiovascular therapy.
2013;
11
(1)
:
55-9
.
View Article Google Scholar -
Shao
Q.,
Chen
K.,
Rha
S.W.,
Lim
H.E.,
Li
G.,
Liu
T.,
Usefulness of neutrophil/lymphocyte ratio as a predictor of atrial fibrillation: a meta-analysis. Archives of medical research.
2015;
46
(3)
:
199-206
.
View Article Google Scholar -
Kwon
C.H.,
Kang
J.G.,
Lee
H.J.,
Kim
N.H.,
Sung
J.W.,
Cheong
E.,
C-reactive protein and risk of atrial fibrillation in East Asians. EP Europace.
2017;
19
(10)
:
1643-9
.
View Article Google Scholar -
Hijazi
Z.,
Aulin
J.,
Andersson
U.,
Alexander
J.H.,
Gersh
B.,
Granger
C.B.,
Biomarkers of inflammation and risk of cardiovascular events in anticoagulated patients with atrial fibrillation. Heart.
2016;
102
(7)
:
508-17
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 12 No 3 (2025)
Page No.: 7184-7190
Published on: 2025-03-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 717 times
- PDF downloaded - 219 times
- XML downloaded - 50 times
 Biomedpress
Biomedpress





