Abstract
Objective: To characterize the clinical and paraclinical features of stage III breast carcinoma (BC) and evaluate the response to neoadjuvant chemotherapy (NAC).
Methods: A cross-sectional study was conducted on 108 patients diagnosed with stage III breast cancer, who were treated with NAC, consisting of four cycles of Doxorubicin and Cyclophosphamide (4AC), followed by four cycles of Paclitaxel (4T).
Results: The average age of the patients was 45.16 ± 9.98 years (median age: 45 years), ranging from 29 to 66 years. According to the AJCC's 8th edition (2017), the majority of patients were classified as stage IIIA (37.96%) and IIIB (38.89%), while 23.15% were in stage IIIC. Molecular subtypes included Luminal B in 60.66% of patients, HER2-positive in 42.59%, and triple-negative in 24.59%. The clinical response rate (based on RECIST 1.1 criteria) was 94.44% after four NAC cycles and improved to 97.22% after eight cycles. Histopathological assessment using the Chevallier classification showed a pathological complete response (pCR) rate of 29.63% and a near-complete response rate of 6.48%, with a residual disease (RD) rate of 63.89%.
Conclusion: The study revealed that stage III breast carcinoma predominantly affects women around the age of 45 (66.67% were under 50 years), with stage IIIB being the most common. Luminal B was the most prevalent molecular subtype, while HER2 positivity was high, accounting for 40.98%. The dose-dense NAC regimen (2-week cycles) of 4AC-4T demonstrated high efficacy, with over 97% of patients achieving a clinical response after eight cycles, based on RECIST 1.1 criteria. The pathological complete response (pCR) rate, according to the Chevallier classification, reached 29.63%, indicating significant tumor regression, while 70.37% exhibited residual disease, either invasive or in situ. These findings highlight the effectiveness of the dose-dense 4AC-4T NAC regimen in downstaging tumors and improving surgical outcomes in stage III breast cancer patients.
Introduction
In women, breast cancer (BC) is currently the most common cancer, with 2.3 million new cases each year (11.7%), accounting for 11.7% of cancer-related deaths. In Vietnam, GLOBOCAN 2020 reported approximately 21,555 new breast cancer cases in a single year, constituting 25% of all newly diagnosed cancers among women. The incidence rate is 34.2 per 100,000 people, with 9,345 deaths, accounting for 7.6% of cancer-related mortality1. These figures highlight the pressing situation of breast cancer in Viet Nam, where patients continue to be diagnosed at advanced stages, which increases the risk of recurrence or metastasis, reduces treatment effectiveness, and raises healthcare costs.
Stage III breast cancer is classified as locally advanced, where the tumor has spread beyond the breast to nearby lymph nodes and tissues but has not metastasized to distant organs. According to the AJCC 8th edition classification system (2017), stage III is further divided into three sub-stages: IIIA, IIIB, and IIIC, determined by tumor size and the degree of regional lymph node involvement. Neoadjuvant chemotherapy (NAC) has emerged as the standard treatment for the majority of patients at this stage, particularly for those ineligible for immediate surgery. NAC has demonstrated numerous favorable outcomes in such cases2, 3.
With the ongoing development of new anti-cancer drugs and treatment regimens, several chemotherapy protocols have become standard in breast cancer management. Recent studies indicate that dose-dense regimens, characterized by 2-week treatment cycles, may enhance treatment response in advanced breast cancer compared to traditional 3-week cycles4. This improvement is achieved without a substantial increase in adverse events and provides long-term therapeutic benefits. However, most existing studies are retrospective, and focused research on specific chemotherapy regimens remains limited, particularly in Vietnam. Additionally, the evaluation of NAC response in stage III breast cancer has primarily been derived from studies including patients at earlier (stage II) or later (stage IV) stages, leaving a gap in understanding the response specifically in stage III cases5. Consequently, we designed this study with two objectives: to describe the clinical and subclinical characteristics of stage III breast cancer and the response results of NAC for stage III breast cancer at Vietnam National Cancer Hospital.
Methods
Study Design and Participants
This cross-sectional descriptive study is part of a larger investigation involving 250 stage III breast cancer patients. The sample size was calculated using a descriptive sample size estimation formula based on the expected pathological complete response (pCR) rate of 16.8%, according to the study by Le Thanh Duc et al. (2023). From this cohort, a convenient sample of 108 patients who received the dose-dense NAC regimen (2-week interval cycles) consisting of 8 chemotherapy cycles (4AC-4T: four cycles of Doxorubicin and Cyclophosphamide, followed by four cycles of Paclitaxel) was selected for analysis.
Eligible participants included women aged 18 years or older who were diagnosed with stage III breast cancer based on the TNM of the AJCC's 8th classification (2017)6. Exclusion criteria included incomplete medical records, failure to complete the treatment regimen, severe comorbidities, pregnancy, or a history of other cancers. The study was conducted at the Vietnam National Cancer Hospital from August 2021 to August 2022, with most patients undergoing total mastectomy following NAC treatment.
Research Procedures
Clinical and Subclinical Evaluation
Patient data, including age at diagnosis, menstrual status, and family history, were collected. Tumor characteristics and regional lymph nodes were assessed through clinical examinations and imaging techniques (ultrasound, mammography, and chest CT scans). Tumor staging and lymph node involvement were classified using the TNM system.
Histopathological diagnosis was made through core needle biopsy, recording histological type and immunohistochemistry results, including estrogen receptor (ER), progesterone receptor (PR), HER2 status, and the Ki-67 proliferation index. Molecular classification was based on the St. Gallen consensus of 2015 to guide treatment decisions7.
NAC Treatment and Monitoring
The NAC regimen included eight cycles administered biweekly (dose-dense schedule), with the first four cycles consisting of Doxorubicin (60 mg/m²) and Cyclophosphamide (600 mg/m²), followed by four cycles of Paclitaxel (175 mg/m²). Prophylactic granulocyte colony-stimulating factor (G-CSF) was given before each cycle to prevent neutropenia.
Adverse events were recorded, including cardiovascular effects, hematological changes (hemoglobin levels, white blood cells, platelets), and liver and kidney function. Additionally, unwanted side effects such as fatigue, loss of appetite, weight loss, and hair loss were documented. Adverse events were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 criteria8.
Response Evaluation
Clinical and subclinical responses were evaluated after four cycles and again upon completing eight cycles before surgery. Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, which categorizes outcomes as Complete Response (CR), Partial Response (PR), stable disease (SD), and progressive disease (PD)9.
Pathological response was assessed post-surgery using the Chevallier classification, which defines four categories10:
Data Analysis
All data were analyzed using SPSS 26. Descriptive statistics were applied to summarize patient demographics and clinical characteristics. T-tests and analysis of variance (ANOVA) were used to compare means, with statistical significance set at p < 0.05.
Ethical Considerations
This study is part of the research project titled "Evaluation of Neoadjuvant Chemotherapy and Surgical Treatment of Stage III Breast Cancer," which was reviewed and approved by the Biomedical Research Ethics Council at K Hospital (certification number 1951/BVK-HĐĐ, dated August 1, 2022). Patients were fully informed and consented to participate in the study. The research was conducted according to strict ethical standards, and the authors declare no conflicts of interest in conducting or interpreting the study results.
Results
Patient Characteristics
The study included 108 patients with an average age of 45.16 ± 9.98 years. Most patients (34.26%) were aged 30 - 40, and 33.33% were over 50. Over half (56.48%) were diagnosed within the past 3 months, and 78.70% were still menstruating.
TNM Classification: Tumor staging revealed that 53.70% of patients had T4 tumors, with 70.37% showing cN2 nodal involvement. Stages IIIA and IIIB represented 37.96% and 38.89% of the cases, respectively.
Most tumors were of the non-special type (NST) (90.74%), and Grade II tumors were the most common (48.15%). Luminal B was the dominant molecular subtype (29.63%), followed by HER2-positive (24.07%) and triple-negative breast cancer (TNBC) (18.52%).
| Characteristics | n =108 | Ratio (%) |
| Age Distribution | ||
| Under 30 | 5 | 4.63 |
| 30-40 | 37 | 34.26 |
| 40-50 | 30 | 27.78 |
| Over 50 | 36 | 33.33 |
| Duration Since Diagnosis | ||
| Under 3 months | 61 | 56.48 |
| 3-6 months | 21 | 19.44 |
| More than 6 months | 26 | 24.07 |
| Menstrual Status | ||
| Menstruating | 85 | 78.70 |
| Menopausal | 26 | 24.08 |
| TNM classification | n =108 | Ratio (%) |
| cT Staging | ||
| cT0 | 2 | 1.85 |
| cT1 | 1 | 0.93 |
| cT2 | 27 | 25.00 |
| cT3 | 20 | 18.52 |
| cT4 | 58 | 53.70 |
| cN Staging | ||
| cN1 | 7 | 6.48 |
| cN2 | 76 | 70.37 |
| cN3 | 25 | 23.15 |
| Cancer Stage | ||
| III A | 41 | 37.96 |
| III B | 42 | 38.89 |
| III C | 25 | 23.15 |
| Histological Subtype | n =108 | Ratio (%) |
| NST | 98 | 90.74 |
| Invasive Lobular | 1 | 0.93 |
| Invasive Ductal | 7 | 6.48 |
| Metastatic Carcinoma | 2 | 1.85 |
| Tumor Grade | ||
| Unknown | 32 | 29.63 |
| Grade I | 2 | 1.85 |
| Grade II | 52 | 48.15 |
| Grade III | 22 | 20.37 |
| Molecular Subtypes | ||
| Luminal A | 10 | 9.26 |
| Luminal B/HER2(-) | 32 | 29.63 |
| Luminal B/HER2(+) | 26 | 24.07 |
| HER2(+) | 20 | 18.52 |
| TNBC | 20 | 18.52 |
| Changes before and after treatment of | Mean | SD | 95% CI | p | |
| Tumor Size (at 4- Cycles) | 28.29 | 22.69 | 23.92 | 32.66 | 0.00 |
| Node Size (at 4- Cycles) | 10.78 | 9.18 | 9.03 | 12.53 | 0.00 |
| Tumor Size (at 8-Cycles) | 38.21 | 24.51 | 33.47 | 42.95 | 0.00 |
| Node Size (at 8-Cycles) | 13.94 | 9.23 | 12.17 | 15.70 | 0.00 |
Results of response to neoadjuvant chemotherapy
After four chemotherapy cycles, the tumor size was reduced by 28.29 mm, and lymph node size by 10.78 mm. After eight cycles, the tumor size was further reduced by 38.21 mm, and lymph nodes by 13.94 mm (p < 0.05). The complete clinical response (CR) increased from 6.48% to 19.44% between four and eight cycles. The pathological complete response (pCR) rate was 28.70%, with a residual disease (RD) rate of 71.29%.
At the 4-cycle evaluation, 7 out of 108 patients (6.48%) achieved a complete clinical response, which increased to 21 out of 108 patients (19.44%) by the 8-cycle evaluation. The number of patients with stable or progressive disease decreased from 3 out of 108 (2.78%) to 1 out of 108 (0.93%) cases and 2 out of 108 (1.85%), respectively. The overall response rate improved from 102 out of 108 patients (94.44%) after four cycles to 105 out of 108 patients (97.22%) after eight cycles.
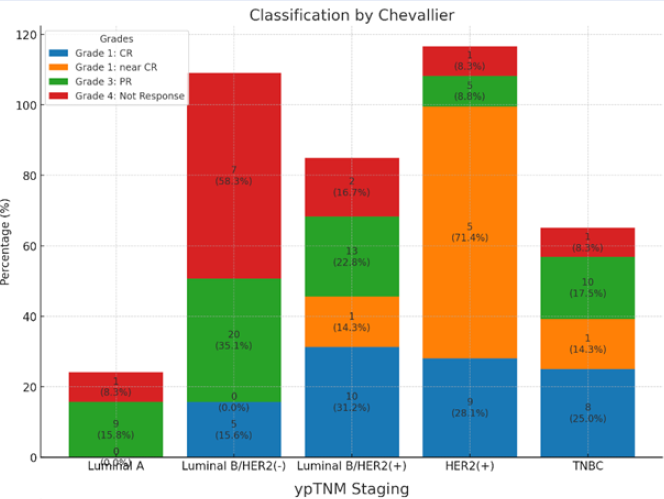
Classification by Chevallier: the rate of pathological complete response (pCR) was 29.63% (32/108), and 6.48% showed a near-complete response. Partial responses were observed in 52.77% of patients, and 11.11% showed no response. Each bar shows the distribution of grades (CR, near CR, PR, No Response) across different cancer subtypes (Luminal A, Luminal B/HER2(-), Luminal B/HER2(+), HER2(+), and TNBC). Both the percentage and actual counts are displayed on the bars.
ypTNM Staging: This chart shows the distribution of TNM stages (0, IA, IIA, IIB, IIIA, IIIB, IIIC) for the same cancer subtypes, with both percentages and counts provided.
Adverse Effects
Most adverse effects were mild to moderate, including fatigue (81.48%) and hair loss (88.89%). Hematologic toxicity included mild anemia (16.67%) and neutropenia (38.89%). Mild liver function abnormalities were observed in 33.33-38.89% of patients, and 1.85% experienced Grade II cardiac toxicity.
| Adverse Effect | n = 108 | Ratio (%) |
| Haematological | ||
| Mild Anemia (Hb 100-80g/dL) | 18 | 16.67 |
| Severe Anemia (Hb <80g/dL) | 2 | 1.85 |
| Leukopenia | 28 | 25.93 |
| Neutropenia | 42 | 38.89 |
| Liver Function | ||
| Mild GOT Elevation | 36 | 33.33 |
| Mild GPT Elevation | 42 | 38.89 |
| Renal Function | ||
| Increased Creatinine | 2 | 1.85 |
| Increased Ure | 2 | 1.85 |
| Other Adverse Effect | ||
| Nausea and Vomiting | 49 | 45.37 |
| Fatigue | 88 | 81.48 |
| Hair Loss | 96 | 88.89 |
| Cardiac Toxicity | 2 | 1.85 |
Discussion
Study Population Characteristics
The average age of our study population was 45.16 ± 9.98 years (median 45 years, range 29 – 66), with only 33.3% over the age of 50. This is consistent with the findings of Le T.D. et al. (2023)5, but the average age in our study is lower than that reported in more recent studies by Nguyen T.P.T. (2021) and Nguyen T.M.P. (2022), which involved high-dose 4AC-4T regimens11, 12. Despite these differences, the age distribution in our study aligns with global data, showing peak breast cancer incidence in women aged 40–50 in Southeast Asia1.
In terms of tumor staging, the majority of our patients had T4 tumors (53.70%) and significant nodal involvement, particularly N2 (70.37%). This high rate of advanced tumors reflects the trend in Viet Nam13, where patients are often diagnosed at later stages. This trend is also observed in Southeast Asia, with studies from Thailand and Malaysia similarly reporting high rates of T3 and T4 tumors at diagnosis. Additionally, 15% of patients had N3 nodal metastasis, consistent with global patterns of advanced breast cancer where nodal involvement is common14, 15.
In terms of histopathological characteristics, the majority of tumors in our study (90.74%) were invasive ductal carcinoma of no special type (NST), with Grade II accounting for 48.15%. This result is higher than the 70–80% typically reported by the World Health Organization (WHO)16. It also exceeds the findings of Le T. Duc et al. (2023), where NST accounted for 76.1% of 126 stage III breast cancer patients treated at a hospital between 2009 - 20125. However, it is lower than more recent reports, such as those by Nguyen T.P.T. (2021) and Nguyen T.M.P. (2022), where NST accounted for 96.7% and 95.4%, respectively11, 12. Phung T.H. (2021) even reported a rate of 100% (17). These findings suggest that NST is the predominant subtype in most stage III breast cancers.
Response after Neoadjuvant Chemotherapy
The effectiveness of NAC in tumor downstaging is well-established (Figure 1), and our study reinforces these findings. The overall clinical response rate was 94.44% after four cycles and 97.22% after eight. These rates are consistent with other regional studies, such as one conducted at the 108 Military Central Hospital in Viet Nam, which reported a similar clinical response rate of 97.7%12. The slight increase in response after eight cycles suggests that extending chemotherapy regimens may further benefit tumor reduction. The reduction in tumor size and lymph node size, measuring 38.1 mm and 13.94 mm, respectively, after eight treatment cycles, demonstrates the response efficacy of the 4AC-4T regimen. These findings align with those from studies in China, which reported similar reductions in tumor size following dose-dense NAC17, 18.
The pathological complete response (pCR) rate in our study was 29.63%, consistent with other studies of dose-dense NAC regimens. For example, Nguyen T.M.P. (2022) reported a pCR rate of 31.8% at the same institution. Globally, pCR rates typically range from 20% to 30% for patients treated with anthracycline- and taxane-based NAC regimens. In our study, 71.29% of patients had residual disease (RD), including both local and regional RD, which aligns with similar studies in the region reporting RD rates of 65–70% after NAC. Although achieving pCR is considered a favorable prognostic factor, the presence of RD does not necessarily indicate poor outcomes, as some patients with RD can still achieve long-term disease control with appropriate adjuvant therapies19.
Using the St. Gallen consensus for molecular classification, Luminal B was the most common subtype (53.70%), followed by HER2-positive (42.59%) and triple-negative breast cancer (TNBC) (18.52%). The pCR rate for HER2-positive tumors (42.59%) aligns with previous studies showing high pCR rates in HER2-positive breast cancer treated with trastuzumab-based regimens. In contrast, the lower pCR rate in the TNBC group (18.52%) highlights the aggressive nature of these tumors and their relatively poor response to conventional chemotherapy compared to HER2-positive and Luminal B subtypes. These findings underscore the need for tailored treatment strategies, particularly for TNBC, which remains a significant clinical challenge. Our findings are consistent with other studies in the Asia-Pacific region. For instance, a study in Malaysia reported a similar pCR rate of 25% for patients treated with NAC for stage III breast cancer20. In comparison, a multicenter study in Japan found a slightly higher pCR rate of 35%, potentially reflecting differences in infrastructure, earlier access to treatment, and genetic variations in tumor biology21. The clinical response rates observed in our study are also comparable to those reported in China, where a large follow-up study found high response rates with dose-dense NAC regimens. However, variations in treatment protocols, such as the addition of targeted therapies like pertuzumab for HER2-positive cases, may explain the differences in pCR rates between countries in the region22.
The side effects observed during treatment were mostly mild to moderate (Grade I-II), with the most common being fatigue (88/108 patients), hair loss (96/108), and digestive disturbances (24/108). Notably, two patients (1.85%) experienced Grade II cardiac toxicity, but this did not necessitate changes in treatment. The overall side effect profile is consistent with other studies of dose-dense NAC regimens, emphasizing the manageable nature of these side effects when properly monitored.
Our study provides important insights into the response of stage III breast cancer to NAC in Vietnamese patients, but several limitations remain. First, the sample size of 108 patients, while sufficient for analysis, may not be representative of the broader population. A multicenter study with a larger sample size would yield more comprehensive results. Second, as a cross-sectional study, we were unable to assess long-term survival data and recurrence rates. Future research should focus on these outcomes to better understand the long-term efficacy of NAC.
Conclusion
The study population had an average age of 45.16 years, with the majority in stage III disease, primarily stages IIIA and IIIB. NAC treatment showed a clinical response rate of 94.44% after four cycles and 97.22% after eight cycles. The pCR rate was 29.63%, similar to regional studies, while 71.29% had residual disease. Side effects were generally manageable with appropriate monitoring.
Abbreviations
AJCC - American Joint Committee on Cancer, ANOVA - Analysis of Variance, BC - Breast Carcinoma, CR - Complete Response, CTCAE - Common Terminology Criteria for Adverse Events, ER - Estrogen Receptor, G-CSF - Granulocyte Colony-Stimulating Factor, GLOBOCAN - Global Cancer Observatory, HER2 - Human Epidermal growth factor Receptor 2, NAC - Neoadjuvant Chemotherapy, NST - No Special Typep, CR - Pathological Complete Response, PD - Progressive Disease, PR - Progesterone Receptor, RD - Residual Disease, RECIST - Response Evaluation Criteria in Solid Tumors, SD - Stable Disease, SPSS - Statistical Package for the Social Sciences, TNBC - Triple-Negative Breast Cancer, TNM - Tumor, Node, Metastasis, WHO - World Health Organization
Acknowledgments
We want to thank the Internal Oncology Department 6 - Vietnam National Cancer Hospital (Tan Trieu campus) team, especially Associate Professor Dr. Phung Thi Huyen, for their dedicated guidance and assistance in collecting research data and completing this article.
Author’s contributions
The authors collaboratively conducted this study and made distinct contributions: Nguyen Truong Thien was responsible for data processing and drafting the manuscript. Le Hong Quang provided direct guidance and orientation throughout the research process, while Nguyen Ngoc Trung reviewed and critically revised the manuscript to enhance its academic quality. All authors have read and approved the final version of the manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This research is part of a medical doctorate thesis and was approved by the Ethics Council in Biological Research at K Hospital (Vietnam Cancer Hospital). The approval code is IRB-VN 01034, and the certificate number is 1951/BVK-HDĐĐ, signed in Hanoi on August 1, 2022.
Out of 250 women over the age of 18 diagnosed with stage III breast cancer, based on the 2017 8th edition of the American Joint Committee on Cancer (AJCC) TNM classification, we selected 108 patients for a treatment regimen of eight cycles of dose-dense chemotherapy: four cycles of Doxorubicin and Cyclophosphamide (4AC), followed by four cycles of Paclitaxel (4T). These patients were indicated for neoadjuvant chemotherapy (NAC) and subsequent surgical intervention, mainly total mastectomy (with or without breast reconstruction), at the Vietnam National Cancer Hospital between August 2021 and August 2022. All patients had complete medical records and consented to participate in the study. Exclusion criteria included non-compliance with the full NAC regimen, severe underlying medical conditions (heart failure, chronic kidney or liver failure) that could interfere with chemotherapy, surgery, or anaesthesia, as well as pregnancy, breastfeeding, or having second cancer.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Sung
H.,
Ferlay
J.,
Siegel
R.L.,
Laversanne
M.,
Soerjomataram
I.,
Jemal
A.,
Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer Journal for Clinicians.
2021;
71
(3)
:
209-49
.
View Article PubMed Google Scholar -
Campbell
J.I.,
Yau
C.,
Krass
P.,
Moore
D.,
Carey
L.A.,
Au
A.,
Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Research and Treatment.
2017;
165
(1)
:
181-91
.
View Article PubMed Google Scholar -
Wang
X.,
Xu
Y.,
Guo
S.,
Zhang
J.,
Abe
M.,
Tan
H.,
T1-2N1M0 triple-negative breast cancer patients from the SEER database showed potential benefit from post-mastectomy radiotherapy. Oncology Letters.
2020;
19
(1)
:
735-44
.
PubMed Google Scholar -
Phung
H.T.,
Nguyen
H.T.,
Nguyen
T.V.,
Nguyen
T.V.,
Dinh
L.A.,
Nguyen
C.V.,
Pathological Complete Response with Neoadjuvant Trastuzumab Combined with Chemotherapy in HER2 Positive Breast Cancer: A Single Institution Retrospective Analysis from Vietnam. Breast Cancer (Dove Medical Press).
2020;
12
:
117-22
.
View Article PubMed Google Scholar -
Le
D.T.,
Bui
L.T.,
Nguyen
C.V.,
Do
K.H.,
Tran
G.L.,
Do
T.A.,
Neoadjuvant Doxorubicin-Paclitaxel Combined Chemotherapy in Patients with Inoperable Stage III Breast Cancer: A Retrospective Cohort Study with 10 Years of Follow-Up in Vietnam. Oncology and Therapy.
2023;
11
(3)
:
327-41
.
View Article PubMed Google Scholar -
Kantor
O.,
Laws
A.,
Pastorello
R.G.,
King
C.,
Wong
S.,
Dey
T.,
Comparison of Breast Cancer Staging Systems After Neoadjuvant Chemotherapy. Annals of Surgical Oncology.
2021;
28
(12)
:
7347-55
.
View Article PubMed Google Scholar -
Gnant
M.,
Thomssen
C.,
Harbeck
N.,
St. Gallen/Vienna 2015: A Brief Summary of the Consensus Discussion. Breast Care (Basel, Switzerland).
2015;
10
(2)
:
124-30
.
View Article PubMed Google Scholar -
Miller
T.P.,
Fisher
B.T.,
Getz
K.D.,
Sack
L.,
Razzaghi
H.,
Seif
A.E.,
Unintended consequences of the evolution of the common terminology criteria for adverse events. Pediatric Blood & Cancer.
2019;
66
(7)
:
e27747
.
View Article PubMed Google Scholar -
Therasse
P.,
Arbuck
S.G.,
Eisenhauer
E.A.,
Wanders
J.,
Kaplan
R.S.,
Rubinstein
L.,
New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute.
2000;
92
(3)
:
205-16
.
View Article PubMed Google Scholar -
Chevallier
B.,
Roche
H.,
Olivier
J.P.,
Chollet
P.,
Hurteloup
P.,
Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. American Journal of Clinical Oncology.
1993;
16
(3)
:
223-8
.
View Article PubMed Google Scholar -
Nguyen
T.P.T.,
Nguyen
D.L.,
Pham
C.P.,
Efficacy of neoadjuvant 4AC-4T regiment in locally advanced Breast cancer. Vietnam Journal of Medicine.
2021;
503
(2)
:
205-9
.
-
Nguyen
T.M.P.,
Pham
D.P.,
Nguyen
N.M.T.,
Treatment outcomes of dose-dense 4AC-4T regimen in neoadjuvant chemotherapy for patients with stage II, III breast cancer. Journal of 108 - Clinical Medicine and Pharmacy.
2022;
17
(TA)
:
46-51
.
View Article Google Scholar -
Thuan
T. Van,
Anh
P.T.,
Tu
D. Van,
Cancer control in Vietnam: where are we now. Cancer control.
2016;
99
:
99-104
.
-
Thaineua
V.,
Ansusinha
T.,
Auamkul
N.,
Taneepanichskul
S.,
Urairoekkun
C.,
Jongvanich
J.,
Impact of regular Breast Self-Examination on breast cancer size, stage, and mortality in Thailand. The Breast Journal.
2020;
26
(4)
:
822-4
.
View Article PubMed Google Scholar -
Tan
K.F.,
Adam
F.,
Hussin
H.,
Mujar
N.M. Mohd,
A comparison of breast cancer survival across different age groups: a multicentric database study in Penang, Malaysia. Epidemiology and Health.
2021;
43
:
e2021038
.
View Article PubMed Google Scholar -
Tan
P.H.,
Ellis
I.,
Allison
K.,
Brogi
E.,
Fox
S.B.,
Lakhani
S.,
Classification of Tumours Editorial Board
WHO,
The 2019 World Health Organization classification of tumours of the breast. Histopathology.
2020;
77
(2)
:
181-5
.
View Article PubMed Google Scholar -
Phung
T.H.,
Efficacy of neoadjuvant therapy with chemotherapy combined with trastuzumab and pertuzumab in patients with her2-neu positive breast cancer. Vietnam Journal of Medicine.
2021;
509
(1)
:
310-6
.
-
Qiu
A.F.,
Miao
Z.L.,
Ge
G.K.,
Wang
C.B.,
Bian
J.,
Ma
H.Y.,
[Response and prognosis of neoadjuvant dose-dense or standard schedule chemotherapy with anthracyclines and taxanes for Luminal B breast cancer]. Zhonghua Yi Xue Za Zhi.
2017;
97
(44)
:
3466-70
.
PubMed Google Scholar -
Winder
A.A.,
Dijkstra
B.,
Is pathological complete response predictable after neoadjuvant chemotherapy in breast cancer? A single institution's retrospective experience. ANZ Journal of Surgery.
2021;
91
(9)
:
1779-83
.
View Article PubMed Google Scholar -
Sung
H.,
Devi
B.C.,
Tang
T.S.,
Rosenberg
P.S.,
Anderson
W.F.,
Yang
X.R.,
Divergent breast cancer incidence trends by hormone receptor status in the state of Sarawak, Malaysia. International Journal of Cancer.
2020;
147
(3)
:
829-37
.
View Article PubMed Google Scholar -
Takahashi
M.,
Cortés
J.,
Dent
R.,
Pusztai
L.,
McArthur
H.,
Kümmel
S.,
Pembrolizumab Plus Chemotherapy Followed by Pembrolizumab in Patients With Early Triple-Negative Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Network Open.
2023;
6
(11)
:
e2342107
.
View Article PubMed Google Scholar -
Cheng
Y.,
Xiang
H.,
Xin
L.,
Duan
X.,
Liu
Y.,
Chinese Society of Breast Surgery CSoSoCMA. Neoadjuvant therapy for early human epidermal growth factor receptor two positive breast cancer in China: A multicenter real-world study (CSBrS-015). Chinese Medical Journal.
2022;
135
(19)
:
2311-8
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 11 (2024)
Page No.: 6923-6931
Published on: 2024-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 1890 times
- PDF downloaded - 564 times
- XML downloaded - 82 times
 Biomedpress
Biomedpress



