Abstract
Melanoma is a highly aggressive and deadly form of skin cancer, with its incidence and mortality rates increasing significantly worldwide. Recent research suggests that miRNA-based therapies could help improve outcomes for melanoma patients by controlling gene expression at the posttranscriptional level, which affects how the tumor grows and spreads. This review aims to examine the role of microRNAs (miRNAs) in melanoma progression, highlighting their potential as therapeutic targets and exploring how they may be utilized in diagnostic and prognostic processes.
Introduction
Melanoma is a type of skin cancer resulting from the malignant transformation of melanocytes in the top layer of the skin (Figure 1)1, 2. It is characterized by a combination of genetic changes leading to neoplastic transformation, an aggressive course, and the potential for metastasis, and it has a high mortality rate. In 2020, researchers diagnosed more than 1.5 million cases of skin cancer worldwide and reported more than 120,000 deaths related to the disease3. Molecular biomarkers, such as microRNAs (miRNAs), play an essential role in several biological and pathological processes, including cell differentiation, lipid metabolism, inflammation, and cancers such as melanoma4. Researchers have shown that miRNAs play an important role in controlling genes that contribute to the growth and spread of melanoma. Moreover, recent studies have highlighted the diagnostic and prognostic potential of miRNAs in melanoma. Circulating miRNAs such as miR-21, miR-146a, miR-205-5p, miR-137, and miR-221 have been shown to be linked to melanoma progression and metastatic potential. The ability to detect these miRNAs in patient serum makes them valuable biomarkers for early diagnosis and monitoring of disease stages5, 6. These findings highlight the role of miRNAs not only as molecular markers but also as therapeutic targets, opening new avenues for personalized treatment strategies.
MiRNAs negatively regulate the expression of genes at the mRNA level by binding to their target mRNAs and causing the repression of translation or the degradation of the mRNA. A complex system of DNA-binding proteins and enzymes synthesizes and processes miRNAs. MiRNAs follow a pathway from transcription to post-transcriptional regulation and play a critical role in gene regulation7, 8. Our understanding of the functions and effects of miRNAs has expanded considerably in recent years, largely due to the discovery of their interactions with additional regulatory mechanisms, including epigenetic changes and transcription factors. This article aims to provide information on the potential use of miRNAs in melanoma for diagnostic, prognostic, and therapeutic purposes.
miRNA and Their Functions
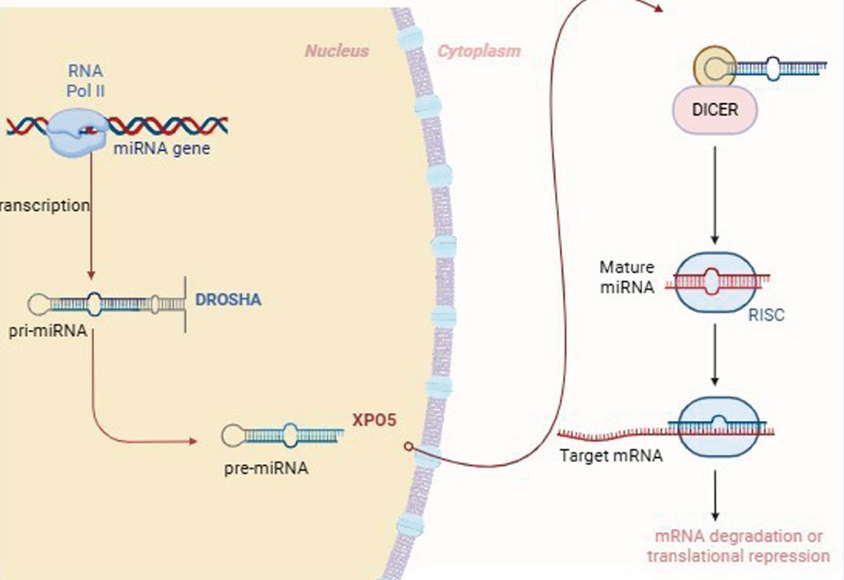
Small non-coding RNA molecules, 20–22 nucleotides (nt) long, called miRNAs, have been shown to modulate the activity of genes since their discovery in 1993. Research into the role of miRNAs intensified after the discovery that the miRNAs lin-4 and let-7 control when stem cells in the nematode Caenorhabditis divide and differentiate into different types7, 9. In addition to being present in all eukaryotic cells, scientists estimate that miRNAs control the activity of about 30% of protein-coding genes in mammals. Approximately 2200 miRNAs have been identified in the mammalian genome, and more than 1000 have been reported in the human genome10. The biogenesis of miRNAs follows a pathway from transcription to post-transcriptional regulation and plays a critical role in gene regulation (Figure 2). Additionally, miRNAs regulate processes that contribute to tumor cell proliferation, metastasis, and immune system evasion, influencing key cancer-related pathways such as resistance to apoptosis, suppression of tumor suppressor genes, unlimited cell proliferation, and angiogenesis (Figure 3 )11, 12.
Previously, studies have demonstrated that inhibiting the miR-200b/a/429 lncRNA promotes increased cell growth, migration, and invasion in individuals with melanoma13. ILF3-AS1 (an oncogenic long non-coding RNA involved in the regulation of gene expression) has also been implicated in these processes14.
Another study found higher levels of miR-7 (involved in cell proliferation and migration), miR-19b-3p (associated with cancer progression), miR-21 (a well-known oncogenic miRNA that promotes tumor growth), miR-126 (associated with angiogenesis and tumor development), and miR-149 (which may regulate apoptosis and metastasis) in people with metastatic melanoma (Figure 4). In addition, studies have shown that the miRNA Let-7b targets parts of the cell cycle and stops the growth of malignant melanoma cells that are not anchored15. The process of forming metastatic melanoma from melanocytes to primary cancer cells and then from primary cells has been determined to upregulate miR-133a, miR-199b, miR-453, miR-520f, MIR-521, and miR-551b. Moreover, the expression of miR-126, miR-29c, miR-506, miR-507, and miR-520d was shown to be increased in the early stages of melanoma and decreased in the later metastatic stages. Furthermore, a panel of miR-150-5p, miR-15b-5p, and miR-16-5p miRNAs, together with miR-374b-3p miRNAs, suggested that we could predict the expression levels of these miRNAs and the likelihood of melanoma tumors metastasizing to the brain16, 17. In this context, these findings provide evidence for the role of miRNAs in the development and progression of melanoma. On the other hand, miR-181a/b was found to be associated with drug resistance in melanoma and may act as a biostimulant to predict responses to targeted therapy, demonstrating it as a potential target for melanoma treatment. Melanoma miRNA profiling can reveal additional molecular pathways, new bio-enhancers, and therapeutic targets, enabling the development of treatments that specifically target these pathways18. However, further research is needed to fully understand the mechanisms of miRNAs in melanoma and their therapeutic applications.
| miRNA | Role in Melanoma | Mechanism of Action | In Vivo/In Vitro | Reference |
|---|---|---|---|---|
| miR-137 | Tumor suppressor; downregulates MITF and CDK6 | Targets c-MET, YB1, EZH2; induces G1 cell cycle arrest, decreases cell growth | Both | 19 |
| miR-148 | Negatively regulates MITF | Binds to MITF's 3′UTR; influences melanoma cell behavior | Both | 20 |
| miR-182 | Promotes migration and survival | Downregulates MITF and FOXO3; enhances proliferation and invasion in melanoma cells | In Vitro | 21 |
| miR-211 | Inhibits migration; regulates NUAK1 and POU3F2 (BRN2) | Lower levels in invasive melanoma; influences cell adhesion and metastatic potential | In Vitro | 22 |
| miR-221/222 | Targets cell cycle regulators like P27 | Downregulates c-Kit, affecting MITF and tyrosinase expression; involved in melanoma progression | Both | 23 |
| miR-205 | Reduces proliferation and invasion | Targets E2F1, influencing cell cycle regulation; affects AKT signaling pathways | Both | 24 |
| miR-625 | Associated with metastasis | Targets SOX-2, stimulating proliferation and invasion when downregulated | Both | 25 |
| miR-514a | Enhances melanoma growth; regulates NF1 | Modulates sensitivity to BRAF-targeted therapy | In Vitro | 26 |
| miR-200c | Correlates with resistance to BRAFi | Low levels associated with drug resistance | In Vitro | 27 |
| miR-155 | Downregulated in melanoma; potential therapeutic target | Induces apoptosis when overexpressed in melanoma cell lines | In Vitro | 28 |
Biomarkers and the Role of miRNAs in Melanoma Diagnosis and Prognosis
miRNAs are important biomarkers for the diagnosis and prognosis of melanoma. Their expression in melanoma cells, serum, and tissues provides insight into the molecular mechanisms of melanoma progression. These miRNAs help in the early detection of cancer and act as indicators of the prognosis of the disease and the response to treatment5.
Diagnostic Biomarkers
Certain miRNAs are reliable diagnostic markers because of the role they play in transforming melanocytes and growing tumors. Some of these miRNAs, as outlined below, are highly expressed in melanoma tissue and play crucial roles in tumor progression.
miR-21, miR-221, and miR-222: Overexpressed in melanoma tissue and detectable in the bloodstream, these miRNAs correlate with melanoma aggressiveness, making them valuable for early diagnosis29, 30. miR-21 functions as an oncogenic miRNA in several cancer types, including melanoma. This miRNA promotes cell proliferation, invasion, and metastasis by suppressing the expression of the tumor suppressor gene Phosphatase and Tensin Homolog (PTEN). Therefore, the relationship between miR-21 and PTEN is emerging as an important therapeutic target in melanoma treatment. Therapies such as High-Intensity Focused Ultrasound (HIFU) can reduce miR-21 expression, increase PTEN levels, and subsequently inhibit the migration and metastatic potential of melanoma cells31. The promyelocytic leukemia zinc finger (PLZF)-miRNA-221/-222 pathway significantly influences melanoma progression by regulating various oncogenic mechanisms. miR-221 and miR-222 promote cell proliferation, migration, and invasion by targeting tumor suppressors like p27Kip1 and PTEN 32. This interaction promotes melanoma cell survival and metastasis by activating key signaling pathways such as PI3K/AKT and MAPK. The PLZF-miR-221/-222 axis is therefore a critical target for potential therapeutic and diagnostic intervention33.
Prognostic Biomarkers
miR-137: Low levels of miR-137 are associated with poor prognosis, particularly in advanced melanoma, as it targets genes involved in cell proliferation and metastasis. miR-137 acts as a tumor suppressor in prolactinomas by targeting MITF, a key gene involved in cell proliferation. It also modulates the Wnt signaling pathway, reducing tumor growth and invasiveness. These effects highlight the potential of miR-137 as a prognostic target for the treatment of prolactinoma34.
miR-182: Overexpression of miR-182 promotes tumor growth and metastasis, indicating melanoma aggressiveness and guiding personalized treatment plans35.
miR-150-5p: Predicts brain metastasis, assisting in early intervention decisions15. Recent studies have identified miR-205-5p, miR-145-5p, and miR-203-3p as important biomarkers for the prognosis of melanoma. Lower levels of miR-205-5p are associated with distant metastases, while reduced expression of miR-145-5p and miR-203-3p is associated with increased tumor aggressiveness. These findings suggest their potential utility in assessing melanoma progression and metastatic risk36.
Circulating miRNAs as Non-Invasive Biomarkers
Circulating miRNAs, as discussed below, provide a non-invasive method for monitoring melanoma, aiding in early detection and progression tracking.
miR-34a, miR-100, and miR-125b: Elevated expression levels of these miRNAs in resistant cells and patient blood samples indicate drug resistance and help clinicians tailor treatment37.
miR-99b and let-7e: These miRNAs associated with myeloid-derived suppressor cells (MDSCs) suggest a more aggressive disease phenotype and a worse prognosis, providing guidance for immunotherapy strategies15.
miRNA-Based Treatment Methods
Synthetic miRNA Mimics
Synthetic miRNA mimics are designed to restore the function of downregulated tumor suppressor miRNAs. In melanoma, these mimics are introduced to simulate the function of natural miRNAs by targeting genes that regulate proliferation and metastasis38. The key advantage of using miRNA mimics is their ability to specifically target multiple genes involved in oncogenic pathways, thus providing a multi-target approach. However, challenges remain, such as their stability in vivo, as miRNAs are susceptible to degradation by nucleases, limiting their therapeutic efficacy if not protected (e.g., with chemical modifications or carriers)39. A study showed that chemically modified synthetic miRNA-205 significantly inhibited melanoma growth by targeting genes such as E2F1 and VEGF, which are critical for cell proliferation and angiogenesis, as well as suppressing the anti-apoptotic gene BCL2, which promotes cell apoptosis40.
Viral Vectors
Viral vectors such as lentivirus, adenovirus, and adeno-associated virus (AAV) are increasingly being used to deliver microRNAs (miRNAs) in the treatment of melanoma due to their efficiency in gene delivery. These vectors can be engineered to deliver miRNA mimics or antagonists directly to melanoma cells, ensuring high levels of gene expression41.
Lentiviral Vectors
Lentiviral vectors are effective because they are able to integrate the miRNA sequences into the host genome and thus ensure the long-term expression of miRNAs. Studies have shown that the use of lentiviral vectors encoding miR-21 significantly improves expression levels in various cell types, demonstrating its therapeutic potential in the treatment of melanoma42.
Adenoviral Vectors
Helper-dependent adenoviral vectors (HD AdVs) have been modified to delete all viral protein-coding sequences, minimizing immunogenicity while increasing transgene efficiency. Research has exhibited that HD AdVs are effective in the delivery of miRNA mimics, leading to significant therapeutic effects in a variety of cancer models43.
AAV Vectors
Adeno-associated viruses (AAVs) are known for their low immunogenicity and ability to provide long-term expression without integrating into the host genome. Recent studies have focused on optimizing AAVs for targeted delivery of miRNAs specifically to tumor cells, thereby increasing therapeutic efficacy while reducing off-target effects. Research on miR-21 indicates that its overexpression inhibits melanoma cell growth and metastasis by specifically targeting MKK3, a kinase involved in oncogenic signaling pathways. By downregulating MKK3, miR-21 interferes with melanoma progression, suggesting its role as a potential therapeutic target for controlling tumor growth and metastasis in melanoma44.
Risks Associated with Viral Vectors
Despite their advantages, viral vectors carry risks such as insertional mutagenesis and potential toxicity. Integration of viral DNA can disrupt essential genes, leading to oncogenesis, while immune responses can limit the effectiveness of repeat administrations45.
Non-Viral Delivery Methods
Non-viral methods such as lipid nanoparticles, polymers, and exosomes are increasingly being explored due to the limitations of viral vectors. Exosomes have emerged as a promising delivery vehicle for miRNAs, offering a natural, less immunogenic vehicle that can effectively cross biological barriers. The aim of these systems is to deliver miRNA mimics directly to melanoma cells in order to improve therapeutic outcomes while minimizing off-target effects42.
Targets of miRNAs in melanoma
Research in melanoma shows that miRNAs influence various biological processes, including cell cycle control, epigenetics, proliferation, invasion, immune response, and carcinogenic pathways (Figure 4 )46. Specifically, cell cycle molecules such as CYLD (a tumor suppressor regulating cell growth and apoptosis), ITCH (an E3 ubiquitin-protein ligase involved in cell signaling), FOXM1 (a transcription factor that promotes cell proliferation), HOX-B7 (a gene regulating cell differentiation), BMP4 (a signaling molecule that influences cell growth), and MITF (a transcription factor linked to microphthalmia) are targeted by these miRNAs47. MITF, which is critical for melanoma growth, proliferation, survival, formation, and heterogeneity, is regulated by transcriptional, post-transcriptional, and post-translational mechanisms.
Additionally, miRNAs like miR-137, miR-148, miR-182, miR-26a, miR-211, miR-542-3p, miR-340, miR-101, and miR-218 are involved in these regulatory processes. Researchers have found that miR-137 expression is associated with poor prognosis in stage IV melanoma patients, highlighting its importance as a potential biomarker and therapeutic target48. It also plays a role in the downregulation of several oncogenic target mRNAs, including c-MET, YB1, EZH2, and PIK3R349. Furthermore, studies suggest that miR-182 is overexpressed in both diseased tissues and melanoma cells. Targeting the aberrant expression of miR-182 has been shown to contribute to melanoma progression by suppressing FOXO3 and MITF. This is accompanied by a reduction in apoptosis and cell cycle arrest at the S-phase50. Additionally, another study showed that miRNA-211 expression was lower in highly invasive melanoma cell lines compared to those with less invasive potential. miRNA-603 was also reported to promote cutaneous melanoma progression by regulating the expression of T-box transcription factor 5 (TBX5)51. Recent studies have shown that miRNAs exhibit promising results in various functions, such as controlling the cell cycle in melanoma, supporting tumor growth, and facilitating metastasis and invasion. The establishment of molecular and therapeutic targets for miRNAs in melanoma promises hope for treatment and facilitates the development of innovative treatment approaches. Innovations in targeting ERK include dual inhibitors that block MEK-catalyzed ERK phosphorylation, thereby limiting the extent of ERK reactivation after feedback52. In melanoma, miRNAs have been shown to regulate key signaling pathways such as the MAPK/ERK pathway, and these regulations have a direct impact on cancer progression. Therefore, RAF and MEK play important roles in the MAPK/ERK signaling pathway, and alterations to these proteins can render melanoma resistant to targeted therapies.
The chemokine monocyte chemoattractant protein-1 (MCP-1/CCL2) is one of the key chemokines regulating the migration and infiltration of monocytes and macrophages and has been detected in most solid tumor microenvironments and in circulation. It appears that CCL2 levels are associated with tumor growth in cells that are not responding to treatment. This suggests that CCL2 and miRNAs may be useful for determining the severity of a patient's condition and for developing new ways to treat metastatic melanoma53. Certain miRNAs in circulation, such as let-7e, miR-99b, miR-100, miR-125a, miR-125b, and miR-146a, influence the activity of myeloid-derived suppressor cells (MDSCs) in individuals with melanoma54. Research has demonstrated that miR-514a, a factor important in initiating melanocyte transformation and supporting melanoma development, modulates the tumor suppressor gene NF1 to regulate the sensitivity of BRAF-targeted therapy55. These studies have shown that miRNAs affect BRAFI in melanoma and are critical for the development of anti-resistance therapies. This may provide further evidence for miRNA-based melanoma research, representing a promising area of study.
MiRNAs as Immunotherapy Targets in Melanoma
Immunotherapy is the fourth safest and most successful treatment after surgery, radiation, and chemotherapy. Cancer immunotherapy stimulates active or passive anti-tumor immune responses to reduce cancer cell proliferation and invasion. To treat melanoma, anti-CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4) drugs, such as ipilimumab, are used to block a receptor on T cells that normally inhibits immune activation, thereby boosting the body’s immune response against tumor cells. Similarly, PD-1 (Programmed Cell Death Protein 1) inhibitors, such as pembrolizumab or nivolumab, target another receptor that reduces immune response. By blocking PD-1, these drugs enhance the immune system’s ability to effectively recognize and destroy melanoma cells. However, 50 to 60 percent of patients do not respond to these drugs56. Recent studies have indicated that miRNAs can be used alongside immunotherapies to target immune evasion mechanisms in cancer cells. In particular, miRNAs such as miR-21 and miR-34a have been shown to enhance the response to immunotherapy. Previous studies indicate that clinical trials of many immunotherapeutic drugs, particularly immune checkpoint blockers (ICBs) such as anti-CTLA-4 and anti-PD-1/PD-L1 inhibitors, have demonstrated success in treating metastatic melanoma. In Phase II-IV clinical trials, these ICBs significantly improved both progression-free survival (the length of time during and after treatment when the cancer does not get worse) and overall survival rates for patients. These results underscore the effectiveness of ICBs in enhancing the immune system's ability to target and destroy melanoma cells, offering promising results for patients with advanced stages of the disease57. In recent years, miRNA research in the field of immunotherapy has been very active, achieving many remarkable results16. MiRNAs, like oncogenes or anti-oncogenes, regulate target genes for carcinogenesis and melanoma and are vital in immunotherapy. MiRNAs play a crucial role in regulating both the innate and adaptive immune systems. Therefore, it is important to investigate miRNA-based biostimulants for immunotherapy responses.
Cancer immunosurveillance consists of immunoediting, which has three stages that occur in a certain order: elimination, equilibrium, and escape. These stages are driven by the constant interaction of the tumor microenvironment, tumor cells, and immune cells. The immune system recognizes and eliminates cancer cells through a multi-step process, but some cells can evade this surveillance. Immunotherapies re-activate the immune system against this escape58. Studies have also shown that miR-155 is involved in the escape of melanoma cells from immune surveillance. MiR-155 regulates the downregulation of endogenous MITF-M expression in melanoma cells triggered by IL-1β59.
Future Research Needs and New Technologies in miRNA Therapy for Melanoma
Despite the significant progress in miRNA research for melanoma treatment, many areas still require in-depth exploration. New technologies may also play a crucial role in enhancing the potential of miRNA-based therapies for melanoma treatment. Although targeting apoptotic pathways, such as Bcl-2, shows promise in cancer therapy, treatments may still not be fully successful. Therefore, further investigation of miRNA-based strategies may provide complementary approaches to improve therapeutic outcomes60. For instance, nanoparticle-mediated miRNA inhibitors show great promise in improving stability, increasing bioavailability, and minimizing side effects. CRISPR/Cas9 gene editing technology aims to improve treatment accuracy by precisely editing specific miRNAs. Artificial intelligence (AI) and machine learning have enabled the discovery of new signature molecules in miRNA profiling, too. AI-supported analyses can facilitate the development of new therapeutic approaches by offering a deeper understanding of the molecular structure and pathways involved in this aggressive cancer type, thereby enabling the identification of novel targets for treatment. In this context, prospective studies should aim to better understand the molecular interactions between miRNA target pathways and use this information to develop new therapeutic strategies. A clearer understanding of the complex role of miRNA in melanoma biology will lead to a wider availability of these therapies in clinical applications.
Conclusions
The latest research on melanoma has demonstrated that miRNAs enhance metastasis, invasion, and proliferation. In addition, some miRNAs in melanoma have the potential to serve as biomarkers for early diagnosis. Recently, researchers have discovered the efficacy of miRNAs in drug resistance and immunotherapy, and miRNAs have become therapeutic targets and tools. A comprehensive understanding of the genetic structure and molecular processes underlying melanoma is essential for the advancement of correct diagnostic techniques and effective therapeutic interventions. Targeted treatments, such as the combination of drugs with miRNAs, have the potential to enhance outcomes. Further research in these areas could lead to more accurate diagnoses and improved treatments for melanoma. In conclusion, miRNA-based approaches have the potential to improve diagnosing, treating, and prognosticating melanoma. However, more research is needed to fully understand how miRNAs work in melanoma and how to use them for therapeutic purposes.
Abbreviations
AAV - Adeno-associated virus, AI - Artificial intelligence, ATP - Adenosine triphosphate, BCL2 - B-cell lymphoma 2, BRAFi - BRAF inhibitor, BMP4 - Bone morphogenetic protein 4, CDK6 - Cyclin-dependent kinase 6, CCL2 - C-C motif chemokine ligand 2, CRISPR/Cas9 - Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR associated protein 9, CTLA-4 - Cytotoxic T-Lymphocyte Associated Antigen 4, c-MET - Hepatocyte growth factor receptor, CYLD - Cylindromatosis, ERK - Extracellular signal-regulated kinase, EZH2 - Enhancer of zeste homolog 2, FOXM1 - Forkhead box M1, FOXO3 - Forkhead box O3, HIFU - High-Intensity Focused Ultrasound, HD AdVs - Helper-dependent adenoviral vectors, HOX-B7 - Homeobox B7, ICBs - Immune checkpoint blockers, IL-1β - Interleukin 1 beta, ITCH - Itchy E3 ubiquitin protein ligase, MAPK - Mitogen-activated protein kinase, MCP-1/CCL2 - Monocyte chemoattractant protein-1/Chemokine (C-C motif) ligand 2, MDSCs - Myeloid-derived suppressor cells, MITF - Microphthalmia-associated transcription factorm, iRNA - microRNA, NUAK1 - NUAK family SNF1-like kinase 1, PD-1 - Programmed cell death protein 1, PD-L1 - Programmed death-ligand 1, PI3K/AKT - Phosphatidylinositol 3-kinase/protein kinase B, PIK3R3 - Phosphoinositide-3-kinase regulatory subunit 3, PLZF - Promyelocytic leukemia zinc finger, POU3F2 - POU class 3 homeobox 2, PTEN - Phosphatase and tensin homolog, TBX5 - T-box transcription factor 5, VEGF - Vascular endothelial growth factor, YB1 - Y-box binding protein 1
Acknowledgments
None.
Author’s contributions
Berna OZDEM, Gulsah EVYAPAN: Design & Conceptualization, Investigation, Writing - Original draft, table & figure, Review & Editing. Gulsevinc AKSOY: Writing - Original Draft. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Puckett
Y.,
Wilson
A.M.,
Farci
F.,
Thevenin
C.,
Melanoma Pathology. StatPearlsStatPearls Publishing 2024.
Google Scholar -
Evyapan
G.,
Luleyap
U.,
Comertpay
G.,
Aksoy
G.,
Kaplan
H.M.,
Oksuz
H.,
Combination treatment with ornidazole and dacarbazine inhibits proliferation, cell migration and induces DNA damage in melanoma cells: ornidazole and dacarbazine therapy for melanoma cells. Indian Journal of Experimental Biology.
2024;
62
(09)
:
722-9
.
-
Arnold
M.,
Singh
D.,
Laversanne
M.,
Vignat
J.,
Vaccarella
S.,
Meheus
F.,
Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatology.
2022;
158
(5)
:
495-503
.
View Article PubMed Google Scholar -
Philippidou
D.,
Schmitt
M.,
Moser
D.,
Margue
C.,
Nazarov
P.V.,
Muller
A.,
Signatures of microRNAs and selected microRNA target genes in human melanoma. Cancer Research.
2010;
70
(10)
:
4163-73
.
View Article PubMed Google Scholar -
Mumford
S.L.,
Towler
B.P.,
Pashler
A.L.,
Gilleard
O.,
Martin
Y.,
Newbury
S.F.,
Circulating microRNA biomarkers in melanoma: tools and challenges in personalised medicine. Biomolecules.
2018;
8
(2)
:
21
.
View Article PubMed Google Scholar -
Carpi
S.,
Polini
B.,
Fogli
S.,
Podestà
A.,
Ylösmäki
E.,
Cerullo
V.,
Circulating microRNAs as biomarkers for early diagnosis of cutaneous melanoma. Expert Review of Molecular Diagnostics.
2020;
20
(1)
:
19-30
.
View Article PubMed Google Scholar -
O'Brien
J.,
Hayder
H.,
Zayed
Y.,
Peng
C.,
Overview of microRNA biogenesis, mechanisms of actions, and circulation. Frontiers in Endocrinology (Lausanne).
2018;
9
:
402
.
View Article PubMed Google Scholar -
Gurtan
A.M.,
Sharp
P.A.,
The role of miRNAs in regulating gene expression networks. Journal of Molecular Biology.
2013;
425
(19)
:
3582-600
.
View Article PubMed Google Scholar -
Saliminejad
K.,
Khorram Khorshid
H.R.,
Soleymani Fard
S.,
Ghaffari
S.H.,
An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. Journal of Cellular Physiology.
2019;
234
(5)
:
5451-65
.
View Article PubMed Google Scholar -
Ardekani
A.M.,
Naeini
M.M.,
The role of microRNAs in human diseases. Avicenna Journal of Medical Biotechnology.
2010;
2
(4)
:
161-79
.
PubMed Google Scholar -
Peng
Y.,
Croce
C.M.,
The role of MicroRNAs in human cancer. Signal Transduction and Targeted Therapy.
2016;
1
(1)
:
15004
.
View Article PubMed Google Scholar -
Manap
A.S. Abdul,
Wisham
A.A.,
Wong
F.W.,
Najmi
H.R. Ahmad,
Ng
Z.F.,
Diba
R.S.,
Mapping the function of MicroRNAs as a critical regulator of tumor-immune cell communication in breast cancer and potential treatment strategies. Frontiers in Cell and Developmental Biology.
2024;
12
:
1390704
.
View Article PubMed Google Scholar -
Liu
H.,
Lei
C.,
He
Q.,
Pan
Z.,
Xiao
D.,
Tao
Y.,
Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Molecular Cancer.
2018;
17
(1)
:
64
.
View Article PubMed Google Scholar -
Han
S.,
Song
L.,
Chen
Y.,
Hou
M.,
Wei
X.,
Fan
D.,
The long non-coding RNA ILF3-AS1 increases the proliferation and invasion of retinoblastoma through the miR-132-3p/SMAD2 axis. Experimental Cell Research.
2020;
393
(2)
:
112087
.
View Article PubMed Google Scholar -
Schultz
J.,
Lorenz
P.,
Gross
G.,
Ibrahim
S.,
Kunz
M.,
MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Research.
2008;
18
(5)
:
549-57
.
View Article PubMed Google Scholar -
Ghafouri-Fard
S.,
Gholipour
M.,
Taheri
M.,
MicroRNA signature in melanoma: biomarkers and therapeutic targets. Frontiers in Oncology.
2021;
11
:
608987
.
View Article PubMed Google Scholar -
Poniewierska-Baran
A.,
S\luczanowska-G\l\kabowska
S.,
Ma\lkowska
P.,
Sierawska
O.,
Zadroga
\L.,
Pawlik
A.,
Role of miRNA in melanoma development and progression. International Journal of Molecular Sciences.
2022;
24
(1)
:
201
.
View Article PubMed Google Scholar -
Barbato
A.,
Iuliano
A.,
Volpe
M.,
D'Alterio
R.,
Brillante
S.,
Massa
F.,
Integrated genomics identifies miR-181/TFAM pathway as a critical driver of drug resistance in melanoma. International Journal of Molecular Sciences.
2021;
22
(4)
:
1801
.
View Article PubMed Google Scholar -
Hartman
M. L.,
Czyz
M.,
MITF in melanoma: mechanisms behind its expression and activity. Cellular and Molecular Life Sciences.
2015;
72
:
1249-1260
.
View Article Google Scholar -
N.Afrang
Imani
M.,
Honardoost
M.,
Melanoma and Associated MicroRNAs. Journal of Skin and Stem Cell.
2016;
3
(4)
:
e63854
.
View Article Google Scholar -
Dar
A.A.,
Majid
S.,
Semir
D. de,
Nosrati
M.,
Bezrookove
V.,
Kashani-Sabet
M.,
miRNA-205 suppresses melanoma cell proliferation and induces senescence via regulation of E2F1 protein. Journal of Biological Chemistry.
2011;
286
(19)
:
16606-16614
.
View Article Google Scholar -
Gajos-Michniewicz
A.,
Czyz
M.,
Role of miRNAs in melanoma metastasis. Cancers.
2019;
11
(3)
:
326
.
View Article Google Scholar -
Lv
N.,
Hao
S.,
Luo
C.,
Abukiwan
A.,
Hao
Y.,
Gai
F.,
Huang
W.,
Huang
L.,
Xiao
X.,
Eichmüller
S. B.,
miR-137 inhibits melanoma cell proliferation through downregulation of GLO1. Science China Life Sciences.
2018;
61
:
541-549
.
View Article Google Scholar -
Bell
R.E.,
Khaled
M.,
Netanely
D.,
Schubert
S.,
Golan
T.,
Buxbaum
A.,
Janas
M.M.,
Postolsky
B.,
Goldberg
M. S.,
Shamir
R.,
Transcription factor/microRNA axis blocks melanoma invasion program by miR-211 targeting NUAK1. Journal of Investigative Dermatology.
2014;
134
(2)
:
441-451
.
View Article Google Scholar -
Ferracin
M.,
Broseghini
E.,
Dika
E.,
Pathophysiology roles and translational opportunities of miRNAs in cutaneous melanoma. MicroRNA in Human Malignancies.
2022;
2022
:
339-384
.
View Article Google Scholar -
Li
H.,
Song
J.B.,
Chen
H.X.,
Meng
L.X.,
Li
Y.,
MiR-155 inhibits proliferation, invasion and migration of melanoma via targeting CBL.. European Review for Medical & Pharmacological Sciences.
2019;
23
(21)
:
9525-9534
.
View Article Google Scholar -
Motti
M. L.,
Minopoli
M.,
Carluccio
G. D.,
Ascierto
P. A.,
Carriero
M. V.,
MicroRNAs as key players in melanoma cell resistance to MAPK and immune checkpoint inhibitors. International journal of molecular sciences.
2020;
21
(12)
:
4544
.
View Article Google Scholar -
Lorusso
C.,
Summa
S. De,
Pinto
R.,
Danza
K.,
Tommasi
S.,
miRNAs as key players in the management of cutaneous melanoma. Cells.
2020;
9
(2)
:
415
.
View Article Google Scholar -
Mirzaei
H.,
Gholamin
S.,
Shahidsales
S.,
Sahebkar
A.,
Jaafari
M.R.,
Mirzaei
H.R.,
MicroRNAs as potential diagnostic and prognostic biomarkers in melanoma. European Journal of Cancer (Oxford, England).
2016;
53
:
25-32
.
View Article PubMed Google Scholar -
Di Martino
M.T.,
Arbitrio
M.,
Caracciolo
D.,
Cordua
A.,
Cuomo
O.,
Grillone
K.,
miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: A systematic review. Molecular Therapy. Nucleic Acids.
2022;
27
:
1191-224
.
View Article PubMed Google Scholar -
Li
H.,
Yuan
S.M.,
Yang
M.,
Zha
H.,
Li
X.R.,
Sun
H.,
High intensity focused ultrasound inhibits melanoma cell migration and metastasis through attenuating microRNA-21-mediated PTEN suppression. Oncotarget.
2016;
7
(31)
:
50450-60
.
View Article PubMed Google Scholar -
Felicetti
F.,
Errico
M.C.,
Segnalini
P.,
Mattia
G.,
Carè
A.,
MicroRNA-221 and -222 pathway controls melanoma progression. Expert Review of Anticancer Therapy.
2008;
8
(11)
:
1759-65
.
View Article PubMed Google Scholar -
Felicetti
F.,
Errico
M.C.,
Bottero
L.,
Segnalini
P.,
Stoppacciaro
A.,
Biffoni
M.,
The promyelocytic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Research.
2008;
68
(8)
:
2745-54
.
View Article PubMed Google Scholar -
Lei
C.,
Jing
G.,
Jichao
W.,
Xiaohui
L.,
Fang
Q.,
Hua
G.,
MiR-137 - Tumor Suppression on Prolactinomas by Targeting MITF and Modulating Wnt Signaling Pathway. The Journal of Clinical Endocrinology and Metabolism.
2019;
104
(12)
:
6391-402
.
View Article PubMed Google Scholar -
Sameti
P.,
Tohidast
M.,
Amini
M.,
Bahojb Mahdavi
S.Z.,
Najafi
S.,
Mokhtarzadeh
A.,
The emerging role of MicroRNA-182 in tumorigenesis; a promising therapeutic target. Cancer Cell International.
2023;
23
(1)
:
134
.
View Article PubMed Google Scholar -
Valentini
V.,
Zelli
V.,
Gaggiano
E.,
Silvestri
V.,
Rizzolo
P.,
Bucalo
A.,
MiRNAs as Potential Prognostic Biomarkers for Metastasis in Thin and Thick Primary Cutaneous Melanomas. Anticancer Research.
2019;
39
(8)
:
4085-93
.
View Article PubMed Google Scholar -
Gajos-Michniewicz
A.,
Czyz
M.,
Role of miRNAs in melanoma metastasis. Cancers (Basel).
2019;
11
(3)
:
326
.
View Article PubMed Google Scholar -
Kim
T.,
Croce
C.M.,
MicroRNA: trends in clinical trials of cancer diagnosis and therapy strategies. Experimental & Molecular Medicine.
2023;
55
(7)
:
1314-21
.
View Article PubMed Google Scholar -
Felicetti
F.,
Errico
M.C.,
Bottero
L.,
Segnalini
P.,
Stoppacciaro
A.,
Biffoni
M.,
The promyelocytic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Research.
2008;
68
(8)
:
2745-54
.
View Article PubMed Google Scholar -
Noguchi
S.,
Iwasaki
J.,
Kumazaki
M.,
Mori
T.,
Maruo
K.,
Sakai
H.,
Chemically Modified Synthetic microRNA-205 Inhibits the Growth of Melanoma Cells In Vitro and In Vivo. Molecular therapy : the journal of the American Society of Gene Therapy.
2013;
21
(6)
:
1204-11
.
View Article PubMed Google Scholar -
Geisler
A.,
Fechner
H.,
MicroRNA-regulated viral vectors for gene therapy. World Journal of Experimental Medicine.
2016;
6
(2)
:
37-54
.
View Article PubMed Google Scholar -
Melnik
B.C.,
MiR-21: an environmental driver of malignant melanoma?. Journal of Translational Medicine.
2015;
13
(1)
:
202
.
View Article PubMed Google Scholar -
A. Thyagarajan,
K.Y. Tsai,
R.P. Sahu,
MicroRNA heterogeneity in melanoma progression. Seminars in cancer biology.
2019;
59
:
208-220
.
View Article Google Scholar -
Zhou
M.,
Yu
X.,
Jing
Z.,
Wu
W.,
Lu
C.,
Overexpression of microRNA21 inhibits the growth and metastasis of melanoma cells by targeting MKK3. Molecular Medicine Reports.
2019;
20
(2)
:
1797-807
.
View Article PubMed Google Scholar -
Hamilton
B.A.,
Wright
J.F.,
Challenges posed by immune responses to AAV vectors: addressing root causes. Frontiers in Immunology.
2021;
12
:
675897
.
View Article PubMed Google Scholar -
Noack
F.,
Calegari
F.,
Micro-RNAs meet epigenetics to make for better brains. EMBO Reports.
2014;
15
(12)
:
1224-5
.
View Article PubMed Google Scholar -
Segura
M.F.,
Hanniford
D.,
Menendez
S.,
Reavie
L.,
Zou
X.,
Alvarez-Diaz
S.,
Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proceedings of the National Academy of Sciences of the United States of America.
2009;
106
(6)
:
1814-9
.
View Article PubMed Google Scholar -
Li
N.,
Low expression of Mir-137 predicts poor prognosis in cutaneous melanoma patients. Medical Science Monitor.
2016;
22
:
140-4
.
View Article PubMed Google Scholar -
Luo
C.,
Tetteh
P.W.,
Merz
P.R.,
Dickes
E.,
Abukiwan
A.,
Hotz-Wagenblatt
A.,
miR-137 inhibits the invasion of melanoma cells through downregulation of multiple oncogenic target genes. The Journal of Investigative Dermatology.
2013;
133
(3)
:
768-75
.
View Article PubMed Google Scholar -
Segura
M.F.,
Hanniford
D.,
Menendez
S.,
Reavie
L.,
Zou
X.,
Alvarez-Diaz
S.,
Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proceedings of the National Academy of Sciences of the United States of America.
2009;
106
(6)
:
1814-9
.
View Article PubMed Google Scholar -
Dong
X.,
Wang
Y.,
Qu
Y.,
Liu
J.,
Feng
X.,
Xu
X.,
New perspectives on targeting RAF, MEK and ERK in melanoma. Current opinion in oncology.
2021;
33
(2)
:
120-6
.
View Article Google Scholar -
Dumaz
N.,
Lebbé
C.,
New perspectives on targeting RAF, MEK and ERK in melanoma. Current Opinion in Oncology.
2021;
33
(2)
:
120-6
.
View Article PubMed Google Scholar -
Vergani
E.,
Di Guardo
L.,
Dugo
M.,
Rigoletto
S.,
Tragni
G.,
Ruggeri
R.,
Overcoming melanoma resistance to vemurafenib by targeting CCL2-induced miR-34a, miR-100 and miR-125b. Oncotarget.
2016;
7
(4)
:
4428-41
.
View Article PubMed Google Scholar -
Huber
V.,
Vallacchi
V.,
Fleming
V.,
Hu
X.,
Cova
A.,
Dugo
M.,
Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. The Journal of Clinical Investigation.
2018;
128
(12)
:
5505-16
.
View Article PubMed Google Scholar -
Stark
M.S.,
Klein
K.,
Weide
B.,
Haydu
L.E.,
Pflugfelder
A.,
Tang
Y.H.,
The prognostic and predictive value of melanoma-related microRNAs using tissue and serum: a microRNA expression analysis. EBioMedicine.
2015;
2
(7)
:
671-80
.
View Article PubMed Google Scholar -
Postow
M.A.,
Chesney
J.,
Pavlick
A.C.,
Robert
C.,
Grossmann
K.,
McDermott
D.,
Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England Journal of Medicine.
2015;
372
(21)
:
2006-17
.
View Article PubMed Google Scholar -
Ribas
A.,
Wolchok
J.D.,
Cancer immunotherapy using checkpoint blockade. Science.
2018;
359
(6382)
:
1350-5
.
View Article PubMed Google Scholar -
Verma
N.,
Thapa
K.,
Kanojia
N.,
Chandel
P.,
Immunobiology of Cancer. In: Bhatt, S., Dureja, H., Gunvantbhai Patel, S., Samir Patel, A., Dua, K. (eds) Biosimilars for Cancer Treatment. Springer, Singapore. 2024;
:
15-34
.
View Article Google Scholar -
Varrone
F.,
Caputo
E.,
The miRNAs role in melanoma and in its resistance to therapy. International Journal of Molecular Sciences.
2020;
21
(3)
:
878
.
View Article PubMed Google Scholar -
B. ÖZDEM,
I. YILDIRIM,
A. KILINÇLI ÇETİN,
İ. TEKEDERELİ,
Cynarine Exhibits Antiproliferative Activity and Bcl-2-Mediated Apoptotic Cell Death in Breast Cancer Cells. Pharmaceutical Chemistry Journal.
2024;
58
:
963-967
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 11 (2024)
Page No.: 6912-6922
Published on: 2024-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 1438 times
- PDF downloaded - 549 times
- XML downloaded - 75 times
 Biomedpress
Biomedpress






