Abstract
A common eye condition known as dry eye syndrome is characterized by insufficient tear production or rapid tear evaporation, causing dryness, irritation, and pain. A common etiological cause for dry eye disease (DED) that affects both the composition and production of tears is meibomian gland dysfunction (MGD). Meibum, an oily substance produced by the meibomian glands, is essential for preventing tear evaporation. The temporary alleviation offered by standard dry eye therapies has prompted researchers to investigate new therapeutic modalities. Platelet-rich plasma (PRP) therapy has been identified as a viable approach for stimulating meibomian gland activity and improving tear production in dry eye patients. Highly concentrated platelets and growth factors found in PRP are essential for promoting tissue regeneration and accelerating the healing process. By the application or injection of PRP in the affected region, the growth factors could stimulate the production and release of meibum, thereby improving the functionality of the meibomian glands and reinstating the stability of the tear film. The objective of this review article is to conduct a comprehensive analysis of clinical evidence supporting the utilization of PRP therapy in managing MGD and dry eye syndrome. Several studies have demonstrated the efficacy of PRP therapy in enhancing meibomian gland function, mitigating ocular surface inflammation, and alleviating symptoms associated with dry eye. Based on the research outcomes, it has been determined that PRP therapy exhibits potential efficacy as a novel intervention for treating dry eye syndrome. This therapeutic approach demonstrates the ability to enhance the health of the ocular surface and target the fundamental etiological factors associated with this condition. Additional research and investigation are required to ascertain the optimal protocols and long-term efficacy of PRP therapy in treating dry eye and MGD. In conclusion, PRP therapy represents a promising and effective treatment option for patients suffering from dry eye syndrome and MGD, offering significant improvements in ocular surface health and patient quality of life.
Introduction
The number and quality of tears that lubricate and protect the eyes are affected by the common eye ailment known as dry eye. If the eyes do not generate enough tears or if they evaporate too quickly, the eyes become dry, irritated, and uncomfortable. Dryness, burning, itching, redness, and impaired vision are typical signs and symptoms of dry eye. Anyone can get dry eye syndrome regardless of age or gender, but older persons, particularly women, are more likely to do so1. Certain medications, such as antihistamines and antidepressants, medical conditions such as diabetes and rheumatoid arthritis, environmental factors such as low humidity and wind exposure, and prolonged use of digital devices such as computers can increase the risk of developing dry eye2. Depending on the research population and the diagnostic methods used, the prevalence of dry eye illness is thought to vary from 5% to 50% worldwide3. The global prevalence of dry eye disease is estimated to be 11.59 percent4.
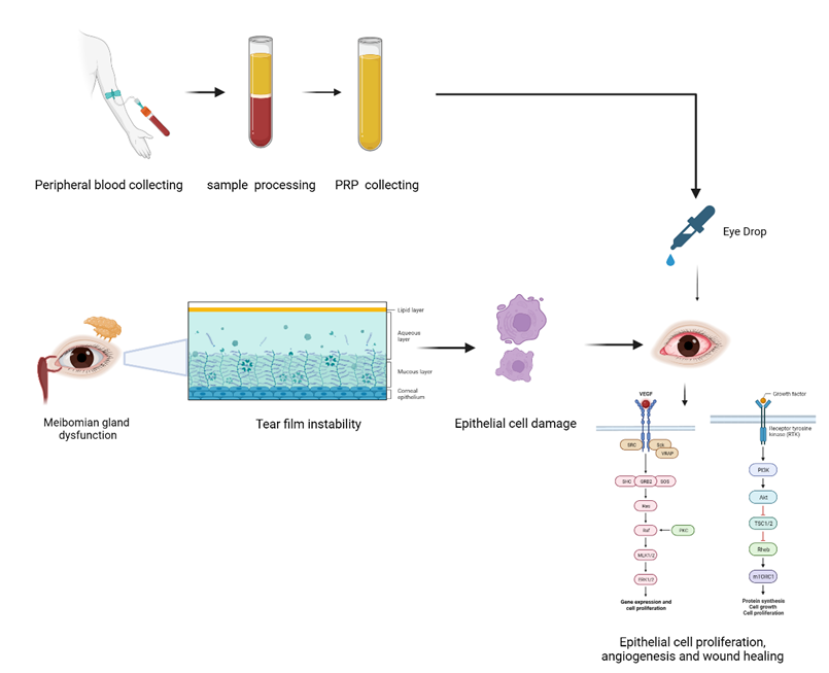
PRP therapy is utilized in the context of dry eye conditions with the objective of stimulating the meibomian glands and augmenting tear production. The meibomian glands play a crucial role in the synthesis of the lipid component of the tear film, which functions to reduce excessive evaporation of tears5. Through the topical application or injection of PRP in the affected region, the concentrated platelets and growth factors have the potential to induce the production and secretion of meibum6. Consequently, this process can enhance the functionality of the meibomian glands and reinstate stability to the tear film.
The therapeutic benefits of PRP extend beyond the stimulation of tear production. PRP is composed of multiple growth factors that possess the ability to mitigate inflammation and facilitate the process of tissue regeneration, thereby playing a significant role in enhancing the overall well-being of the ocular surface7. Moreover, it has been demonstrated in previous studies that PRP therapy can effectively improve the structural integrity of the meibomian glands8. This enhancement may have the potential to mitigate the development of complications that arise from untreated meibomian gland dysfunction, including corneal ulcers and infections.
With the increasing incidence of dry eye3, there is an increasing imperative to explore innovative treatment modalities, such as PRP therapy, that present promising strategies for the effective management of this particular condition. PRP therapy presents a viable and potentially advantageous method for addressing the root causes of dry eye and enhancing the health of the ocular surface by harnessing the regenerative capabilities inherent in the patient's own blood.
In the subsequent sections of this review article, an in-depth analysis will be conducted on the clinical evidence that substantiates the utilization of PRP therapy for the treatment of meibomian gland dysfunction and dry eyes. This examination will encompass an evaluation of the outcomes derived from pertinent studies, as well as a comprehensive discussion of the potential advantages and considerations associated with this emerging therapeutic approach.
The Pathology of Meibomian Gland Dysfunction
MGD is a leading cause of evaporative dry eye disease. The Meibomian glands, located within the tarsal plates of the upper and lower eyelids, are responsible for producing a lipid-rich secretion called meibum, which slows the evaporation of the tear film. Despite its prevalence, the pathogenesis of MGD is not yet fully understood.
Androgen deficiency is a key factor in the pathophysiology of MGD. Androgens are crucial for the normal functioning of the Meibomian glands as they influence lipid biosynthesis, which is essential for maintaining the integrity of the tear film9. The presence of androgen receptors in Meibomian gland tissues indicates that androgens directly modulate gland activity and lipid secretion10. Androgen deficiency leads to significant changes in the lipid composition of Meibomian gland secretions, resulting in altered neutral and polar lipid patterns that contribute to evaporative dry eye11. These lipid profile changes are particularly evident in conditions associated with low androgen levels, such as aging and Sjogren's syndrome12.
Androgenic decline in individuals with MGD is primarily linked to hormonal deficiencies and aging processes that affect the Meibomian glands' functionality. Aging is associated with reduced testosterone production, primarily due to changes in Leydig cells, which are responsible for steroidogenesis10, 13. This reduction in testosterone can exacerbate MGD symptoms, as lower androgen levels correlate with decreased Meibomian gland function. Myotonic dystrophy patients exhibit lower adrenal androgens, which may contribute to MGD14.
Keratinization plays a critical role in the pathophysiology of MGD. Hyperkeratinization of the ductal epithelium obstructs the Meibomian orifice, halting meibum secretion and causing acinar atrophy15. Staphylococcus aureus can induce hyperkeratinization and pro-inflammatory cytokine expression, exacerbating gland dysfunction16. Aging and environmental stressors can lead to gland atrophy and epithelial plugging, complicating the role of keratinization in MGD17. The increased expression of proline-rich proteins, such as small proline-rich protein 1B (SPRR1B), is implicated in the keratinization process of epithelial cells, which can be a response to inflammatory conditions like DED18. These proteins contribute to the structural integrity of the ocular surface, acting as protective agents against proteolytic damage.
Conditions such as ichthyoses and psoriasis can cause keratinization abnormalities, affecting the ocular surface and leading to complications like keratitis and conjunctivitis19. Desiccating stress can exacerbate hyperkeratinization, as seen in mouse models where exposure leads to ductal dilation and increased lipid synthesis17.
PPARγ, a nuclear receptor, regulates various physiological processes, including lipid metabolism and inflammation, which are relevant to MGD. The detection of PPARγ signals in the eye involves complex molecular mechanisms that include ligand binding, transcriptional regulation, and interactions with various signaling pathways. Understanding these mechanisms is crucial for elucidating their roles in ocular health and disease. PPARγ is constitutively expressed in the retinal pigment epithelium and neuroretina, with increased expression observed in models of age-related macular degeneration20. Ligands for PPARγ, such as rosiglitazone, enhance its activity, and novel methods like surface plasmon resonance have been developed to improve the detection of ligand-PPARγ interactions21. The modulation of PPARγ signaling pathways is linked to oxidative stress responses and the regulation of vascular endothelial growth factor (VEGF), which are critical in the context of retinal degeneration20. While the focus has been on PPARγ's protective roles in retinal health, the potential for adverse effects or dysregulation in pathological conditions warrants further investigation.
The PPARγ pathway modulates gene expression in MGD cells primarily through its influence on adipogenesis and lipid metabolism. PPARγ, a member of the nuclear hormone receptor superfamily, regulates numerous target genes by binding to specific response elements, thereby affecting cellular functions. PPARγ can directly activate genes associated with adipogenesis, such as the adipocyte fatty acid binding protein (aP2), through ligand binding, which enhances transcriptional activity22. Agonists like thiazolidinediones induce significant changes in gene expression profiles, with studies showing over 579 genes being regulated during adipocyte differentiation22. PPARγ forms heterodimers with retinoid X receptors, which is essential for the transcriptional regulation of genes involved in fat metabolism23. PPARγ influences other pathways, such as β-catenin signaling, which is downregulated in adipocytes upon PPARγ activation, indicating a complex regulatory network in adipogenesis24.
S100 Calcium-Binding Proteins
The S100 protein family, characterized by their calcium-binding properties, has a rich history intertwined with various biological functions and disease associations. Initially identified due to their solubility in saturated ammonium sulfate, S100 proteins have been linked to critical cellular processes such as apoptosis, differentiation, and inflammation25. Over 25 types have been identified, with significant roles in skin homeostasis and pathologies, as well as in cancer progression26.
S100 proteins, particularly S100A8 and S100A9, are involved in regulating inflammation and immune responses in ocular surface diseases27. The elevated levels of S100 protein in inflammatory conditions suggest a role in mediating cellular responses to stress and injury, thereby influencing Meibomian gland function and meibum. The S100A8 and S100A9 proteins play critical roles in regulating inflammation and immune responses in ocular surface diseases. These calcium-binding proteins are involved in various inflammatory processes, particularly through their interaction with Toll-like receptors, which are pivotal in innate immunity. S100A8 and S100A9 are upregulated in ocular surface diseases, enhancing neutrophil migration and inflammation27. S100A9 acts as a Damage Associated Molecular Pattern (DAMP) that activates TLR4, leading to inflammatory signaling pathways, including MyD88 and NF-κB28, 29. S100A9 contributes to DED pathogenesis by blocking autophagy and promoting chronic inflammation30.
The interaction between S100 Calcium-Binding Proteins (S100) and PPARγ involves complex molecular mechanisms that are influenced by calcium binding and conformational changes. S100 proteins, particularly S100B, undergo significant structural alterations upon calcium binding, which exposes hydrophobic residues essential for interactions with target proteins, including PPARγ31, 32. The binding of calcium enhances the affinity of S100B for target peptides, which may include regulatory domains of PPARγ32. The S100 protein family exhibits functional redundancy, with multiple members capable of interacting with PPARγ, suggesting a complex network of interactions that can compensate for each other33.
Decreased expressions of claudin 3 and cell adhesion molecules significantly contribute to the altered phenotype in Meibomian glands, impacting their function and integrity. This alteration is primarily linked to the disruption of tight junctions and intercellular cohesion, leading to gland dysfunction. In fact, the absence of claudin 3 resulted in structural defects in epithelial tissues, indicating its critical role in maintaining cellular architecture and function34. Cell adhesion molecules, such as E-cadherin, are demonstrated to be vital for intercellular cohesion. Their decreased expression can lead to reduced cell-to-cell adhesion, resulting in altered gland morphology and function35. Inflammatory conditions, such as diabetes, have been shown to increase the expression of inflammatory cytokines and adhesion molecules, further exacerbating MGD36. While decreased expressions of claudin 3 and adhesion molecules are detrimental, some studies suggest that targeted therapies may restore function and improve gland health, indicating potential avenues for treatment in affected individuals.
The structure of tear film
The tear film is a complex structure essential for ocular health, composed of three primary layers: the inner mucin layer, the middle aqueous layer, and the outer lipid layer. Each layer plays a critical role in maintaining the integrity and function of the ocular surface, with the lipid layer being particularly vital for preventing evaporation and ensuring tear film stability. The inner mucin layer provides lubrication and helps in the adhesion of the tear film to the ocular surface. The middle aqueous layer, secreted mainly by the lacrimal glands, contains water, electrolytes, and proteins such as lactoferrin and lysozyme, which protect against pathogens37. The outer lipid layer, composed of nonpolar and polar lipids synthesized in the meibomian glands, reduces surface tension and evaporation38. The tear film lipid layer (TFLL) consists of wax esters and O-acyl-ω-hydroxy fatty acids, which self-assemble into monolayers that resist evaporation39. A model TFLL with specific lipid ratios (40% wax esters, 40% cholesteryl esters, 20% polar lipids) demonstrated an 11% reduction in water evaporation rates at optimal surface pressures40. The TFLL's structure increases film compactness at the air-water interface, which is essential for minimizing water loss40. The tear film also acts as a barrier against environmental irritants and pathogens41 and ensures smooth movement of the eyelids over the cornea, preventing friction. It also provides essential nutrients to the avascular cornea and conjunctiva37. Despite its protective functions, dysfunctions in the tear film can lead to conditions like dry eye syndrome. Alterations in the structure of the tear film significantly impact dry eye disease (DED) and other ocular disorders, primarily through the disruption of its three essential layers: lipid, aqueous, and mucin. These changes can lead to increased evaporation, hyperosmolarity, and inflammation, creating a vicious cycle of discomfort and potential damage to the ocular surface.
Relationship Between Meibomian Gland Dysfunction and Dry Eye
The glands are responsible for the secretion of a lipid-based substance known as meibum. Meibum plays a crucial role in preserving the stability of the tear film and mitigating the occurrence of DED42, 43. The etiology of MGD encompasses a wide range of factors, including, but not limited to, the natural process of aging, hormonal fluctuations, various environmental influences, and underlying medical conditions43. In the absence of appropriate medical intervention, MGD culminates in complications including corneal ulcers, infections, and visual impairment44.
At the molecular level, various biological mechanisms and enzymes are involved in the pathophysiology of MGD. Lipase and esterase enzymes, produced by bacteria on the eyelids, degrade meibum into free fatty acids, which are irritating to the ocular surface and trigger inflammation. Inflammatory cytokines, such as interleukin-1 and tumor necrosis factor-alpha, are released in response to chronic inflammation, worsening gland dysfunction. Matrix metalloproteinases play a role in the degradation of the extracellular matrix, leading to structural damage to the meibomian glands and surrounding tissues.
The progression of MGD can be classified into stages based on the severity of symptoms and gland dysfunction. In the early stage, patients may experience mild symptoms such as occasional dryness and irritation, with slightly turbid gland secretions. In the moderate stage, symptoms become more persistent, including increased dryness, burning, and discomfort, accompanied by more viscous and opaque gland secretions. In advanced stages, patients suffer from severe dryness, significant discomfort, and visual disturbances. At this point, gland secretions become thick and waxy, and complete obstruction may occur, leading to gland atrophy.
The lipid layer, which is secreted by the meibomian glands, functions to inhibit the evaporation of tears from the ocular surface45. In individuals with MGD, there is a deficiency in the production of meibum or a deterioration in its quality. This results in heightened evaporation of tears and reduced stability of the tear film46, 47. MGD is the predominant etiology of evaporative dry eye, responsible for approximately 86% of reported cases6. Furthermore, it is worth noting that MGD frequently occurs alongside other forms of dry eye, including aqueous deficient dry eye, which is characterized by insufficient tear production44.
Inflammation of the meibomian glands can lead to changes in the production of meibum, both in terms of its quality and quantity. The condition may give rise to ocular manifestations characterized by sensations of burning, stinging, and discomfort. Furthermore, apart from the manifestation of dry eye symptoms, MGD can also result in ocular redness, edema, and discomfort in the eyelids43. If these complications are not treated, they have the potential to result in significant damage to the cornea and potentially lead to a loss of vision43. Hence, it is imperative to preserve the functionality of the meibomian glands to uphold the general well-being of the eye and mitigate potential complications associated with ocular surface disorders.
Importance of Diagnosing and Treating Meibomian Gland Dysfunction for Dry Eye Management
Effective management of dry eye necessitates the diagnosis and treatment of MGD48. MGD is widely recognized as the primary cause of evaporative dry eye, constituting the predominant underlying factor in most cases of dry eye. Thus, the recognition and resolution of MGD in individuals with dry eye are imperative for restoring meibomian gland functionality and enhancing the general well-being of the ocular surface49. A range of diagnostic tools can be employed in the assessment of ocular health. For instance, meibography utilizes advanced imaging technology to provide a visual representation of the meibomian glands. Additionally, non-invasive tear film break-up time (NIBUT) is used to evaluate the stability of the tear film. The tear film has been extensively studied42.
The current standard of care for treating MGD primarily includes warm compress therapy, eyelid hygiene, and various advanced treatments such as intense pulsed light (IPL) therapy and thermal pulsation. While these methods can provide symptom relief and improve ocular surface health, they also have notable disadvantages. Warm compresses are primarily palliative, offering temporary relief rather than a cure50. There is no consensus on the optimal application method or duration, leading to inconsistent patient outcomes50. Other procedures like meibomian gland probing and thermal pulsation can be invasive and expensive, limiting accessibility for some patients51. While combined therapies (such as intense pulsed light combined with meibomian gland probing) show promise, not all combinations yield significant improvements52. Regarding pharmacological treatments, some patients may develop allergies to ingredients in eyelid hygiene products, complicating treatment51. Topical steroids, while effective, carry risks of glaucoma and cataracts with prolonged use51.
Despite these challenges, ongoing research into tailored approaches and emerging therapies may enhance treatment efficacy and patient satisfaction in managing MGD. Recent research has indicated that PRP could potentially serve as a viable therapeutic approach for restoring meibomian gland function in individuals suffering from MGD8, 53, 54. PRP is composed of growth factors and various healing agents, which have the potential to facilitate tissue regeneration and repair, resulting in enhanced meibomian gland structure and function54.
Healthcare providers could enhance dry eye management and alleviate symptoms in patients with dry eye by accurately diagnosing and effectively treating MGD. Ultimately, the implementation of this intervention has the potential to enhance patients' quality of life and mitigate the risk of complications that may arise from untreated MGD, including corneal ulcers, infections, and visual impairment53.
PRP and Its Therapeutic Potential for Restoring Meibomian Gland Function
Using the patient's own blood, the regenerative medicine procedure known as PRP therapy promotes tissue regeneration and healing54, 55. A small sample of the patient's blood is drawn for this procedure, which is subsequently processed to concentrate platelets and other growth factors important for tissue repair and regeneration55. The obtained blood is first centrifuged to separate the various components before being made into PRP. Platelets and plasma, which contain growth factors and other healing agents, are separated from red and white blood cells. After that, platelets and plasma are further treated to concentrate them and boost their potency56, 57.
Platelets, plasma, and growth factors are concentrated to create the PRP solution, which can be injected or applied topically to the affected area58. PRP's high platelet and growth factor concentration encourages tissue regeneration, reduces inflammation, and accelerates the healing process59. PRP therapy has been applied in a number of medical specialties, including dermatology55 and orthopedics60, 61. In ophthalmology, PRP therapy has been demonstrated to be useful in treating meibomian gland dysfunction and other ocular surface conditions8, 62. It is believed that the growth factors in PRP encourage cell regeneration, reduce inflammation, and increase meibum production and secretion, which may aid in restoring meibomian gland function and alleviating the symptoms of dry eyes8. With these encouraging findings, several studies have investigated the use of PRP for meibomian gland dysfunction and dry eyes.
Clinical Evidence of PRP Therapy for MGD
Clinical research regarding the efficacy of PRP therapy in managing DED and MGD has revealed significant enhancements in both symptomatology and tear film metrics, particularly in individuals who exhibit resistance to conventional therapeutic modalities. Investigations concerning the application of PRP eye drops for evaporative dry eye disease have indicated marked alleviation of symptoms and improvements in tear film stability, as evidenced by a Canadian Dry Eye Assessment score enhancement of -5.45 (p < 0.001) and superior non-invasive break-up times8. Comparative studies have evaluated the efficacy of PRP injections into the lacrimal gland, revealing significant improvements in non-invasive tear break-up time and Schirmer test scores when compared to control treatments using preservative-free eye drops63. In randomized clinical trials, PRP injections have demonstrated superior efficacy in enhancing corneal staining and tear parameters compared to hyaluronic acid treatments in patients with severe dry eye syndrome64. In assessments comparing autologous platelet-rich plasma (aPRP) to artificial tears, aPRP consistently resulted in more pronounced symptom relief, including a significant reduction in the Ocular Surface Disease Index (OSDI) by -22.7 (p < 0.001) and improved tear stability65. This efficacy is attributed to the elevated concentration of growth factors in PRP, such as fibroblast growth factor (FGF) and epidermal growth factor (EGF), which are crucial for ocular surface healing and the maintenance of tear film stability62. Notably, 93.3% of patients reported symptom improvement with PRP drops, in contrast to traditional artificial tears, which do not possess the complex, preservative-free formulation that PRP provides66. Patients with severe DED who received PRP injections and PRP eye drops experienced significant improvements in best-corrected visual acuity, Schirmer test scores, TBUT, and corneal staining. These treatments were well-tolerated, with no adverse reactions reported, further supporting the efficacy and safety profile of PRP66. A retrospective case series of patients treated with PRP for ocular surface disease demonstrated a statistically significant reduction in OSDI scores (from 39.5 to 30.8, p = 0.02). Additionally, there was a borderline increase in Schirmer test scores, and 14% of eyes showed improvement in conjunctival staining67.
Comparative studies involving autologous serum (AS) eye drops in patients with primary Sjögren’s syndrome have shown that PRP exhibits similar efficacy in alleviating the signs and symptoms of DED. However, PRP has the advantage of a quicker preparation time compared to AS, making it a more convenient and preferable alternative for treating dry eye in primary Sjögren’s syndrome68. A multicentric study on plasma rich in growth factors (PRGF) demonstrated significant reductions in corneal epithelial erosions and improvements in SANDE scores. Additionally, 74.3% of patients exhibited decreased corneal staining69.
Randomized studies comparing PRP to artificial tears containing sodium hyaluronate have shown that PRP yields superior outcomes in visual acuity and reduction of hyperemia, particularly in cases of moderate to severe DED70. PRP therapy has also been shown to be effective in alleviating chronic ocular surface syndrome following LASIK surgery, resulting in improvements in dry eye symptoms in 85% of cases and significant reductions in conjunctival hyperemia71. Furthermore, the application of immunologically safe plasma rich in growth factors (PRGF) for treating dry eye in patients with Sjögren’s syndrome has resulted in substantial symptom improvement, suggesting that PRGF is a safe and effective option for managing immune-mediated dry eye conditions72. These clinical findings highlight the potential of PRP as a therapeutic option for dry eye, especially in cases resistant to conventional treatments. Future research involving larger patient cohorts is necessary to refine treatment protocols and assess the long-term benefits of PRP across diverse DED populations.
| Participants | Treatment | Duration | Key Findings | Reference |
| Patients with evaporative dry eye disease | PRP eye drops | 1 months | Significant improvement in Canadian Dry Eye Assessment score (-5.45, p<0.001) and better tear film stability | 8 |
| Severe DED patients | PRP lacrimal gland injections | 3 months | Increased non-invasive tear break-up time and Schirmer test scores compared to preservative-free eye drops | 63 |
| Patients with severe dry eye | PRP injections vs. hyaluronic acid | 3 months | PRP improved corneal staining and tear parameters more effectively than hyaluronic acid | 64 |
| Patients with DED | Autologous PRP vs. artificial tears | 3 weeks | aPRP is secure and more efficacious than AT in managing individuals with moderate to severe symptomatic DED | 65 |
| Patients with severe DED and ocular surface disease | PRP eye drops | 15 months | 93.3% of patients reported symptom improvement, significant increase in best-corrected visual acuity, and reduction in corneal staining | 66 |
| Patients with primary Sjögren’s syndrome | PRP vs . AS eye drops | 3 months | PRP demonstrated similar efficacy to AS eye drops; quicker preparation time, making it a viable alternative for SS-related DED | 68 |
| Patients with dry eyes disease | PRP eye drops | 6 months | Marked reduction in corneal epithelial erosions and improved SANDE scores, with 74.3% of patients showing decreased corneal staining | 69 |
| Patients with hyposecretory dry eye | PRP and artificial tears of sodium hyaluronate | 1 month | PRP treatment in hyposecretory dry eye induces a more significant positive effect over symptomatology and different dry eye signs than sodium hyaluronate | 70 |
| Patients affected by post-LASIK chronic ocular surface syndrome | PRP eye drops | 6 weeks | Improvement in dry eye symptoms in 85% of cases, significant reduction in conjunctival hyperemia | 71 |
| Sjögren’s syndrome dry eye patients | PRGF | N/D | Significant symptom improvement, suggesting PRGF as a safe and effective option for immune-mediated dry eye conditions | 72 |
Mechanisms of PRP Treatment for MGD-Induced Evaporative Dry Eye (EDE)
DED, which arises from an imbalance in tear production or quality, manifests through symptoms such as irritation, visual disturbances, and damage to the ocular surface. MGD, a primary cause of evaporative DED, worsens this condition by disrupting lipid secretion, leading to faster tear evaporation and increased exposure of the ocular surface to environmental stressors. This interplay fosters a cycle of inflammation and cellular damage, highlighting the necessity for effective therapeutic interventions. One promising approach to interrupt this cycle is the use of PRP, an autologous blood derivative enriched with growth factors and anti-inflammatory cytokines. PRP has emerged as a potential treatment for ocular surface disorders due to its capacity to promote healing and reduce inflammation. This therapeutic strategy is particularly beneficial for patients with moderate to severe DED or MGD who do not respond to conventional treatments.
Numerous studies highlight the effectiveness of PRP in alleviating symptoms and promoting ocular surface health. For example, research involving patients with moderate DED unresponsive to other treatments revealed significant improvements following PRP therapy. Specifically, 80% of participants reported substantial symptom relief, as indicated by lower scores on the Ocular Surface Disease Index (OSDI). Additionally, objective measures such as tear break-up time (TBUT) and ocular staining scores (OSS) showed marked enhancement, with 85% of eyes exhibiting prolonged TBUT and 90% demonstrating reduced OSS scores. In hyperosmotic models, PRP was found to decrease the expression of pro-inflammatory mediators, including interleukin-1, tumor necrosis factor-alpha, and matrix metalloproteinases (MMP-1 and MMP-3), underscoring its anti-inflammatory capabilities at the cellular level73.
Inflammation is a pivotal factor in both DED and MGD. In MGD patients, meibomitis frequently causes conjunctival hyperemia, superficial punctate keratopathy (SPK), and neovascularization of the ocular surface. If the inflammatory response remains untreated, it can severely disrupt tear stability and ocular surface integrity, leading to tissue damage and worsening DED symptoms74. The anti-inflammatory properties of PRP, which include reductions in interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and matrix metalloproteinases, make it a promising approach to mitigating these inflammatory processes. This positions PRP as a comprehensive treatment option for MGD and associated DED. PRP has demonstrated the ability to promote the differentiation of meibomian gland epithelial cells and enhance their proliferative capacity. For instance, PRP has been shown to enhance the levels of EGF, TGF-β, VEGF, and PDGF in the tear film of individuals diagnosed with DED resulting from MGD. The growth factors have the potential to initiate multiple signaling pathways, including the mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways. These pathways play a crucial role in regulating various aspects of epithelial cell behavior, such as cell cycle progression, survival, migration, and differentiation75, 76. These modifications have the potential to facilitate the reestablishment of ocular surface homeostasis and integrity following PRP treatment.
By improving the function of the meibomian glands, PRP directly enhances the quality of the tear film and reduces ocular inflammation, significantly improving the condition of patients with MGD and DED77. PRP has been shown to reduce the level of apoptosis (programmed cell death) in meibomian gland epithelial cells. In research models, this reduction in apoptosis helps maintain the structure and function of the glands, thereby improving tear film stability and alleviating chronic inflammatory symptoms on the ocular surface78.
PRP also holds promise in the broader context of wound healing and angiogenesis. Research on PRP-derived exosomes has shown that sphingosine-1-phosphate (S1P), a lipid signaling molecule present in PRP, plays a crucial role in promoting angiogenesis and tissue repair through the S1PR1/AKT/FN1 signaling pathway. S1P and its associated signaling pathways have significant implications for enhancing blood flow and maintaining vascular homeostasis on the ocular surface. This can potentially alleviate tissue ischemia and promote healing in cases of severe DED with neovascularization79. Furthermore, research indicates that the stimulation of platelets by PRP through PAR-1 and PAR-4 receptors enhances the release of VEGF. This process promotes angiogenesis, which may help counteract the ocular surface damage observed in chronic DED and MGD80.
PRP shows significant promise as a treatment for moderate to severe DED and MGD. Its multifaceted approach—encompassing anti-inflammatory effects, support for cellular repair, and stimulation of angiogenesis—addresses the complex pathology of DED and MGD at various levels. As a blood-derived treatment, PRP is both practical and effective, offering patients an alternative when conventional therapies fail. Its potential for use in various storage conditions, ease of preparation, and notable efficacy in studies suggest that PRP could become a staple treatment for ocular surface diseases, significantly improving the quality of life for patients suffering from chronic DED and MGD.
Conclusion
The dysfunction of meibomian glands is a significant factor in the development of dry eye syndrome, which results in the impairment of tear film and the overall health of the ocular surface. Although conventional treatments provide relief for symptoms, there is ongoing research to find more efficient and durable solutions. The utilization of PRP therapy has emerged as a potentially effective therapeutic strategy for targeting the root causes of dry eye syndrome and reinstating the proper functioning of the meibomian glands.
Numerous clinical investigations have been conducted to assess the effectiveness of PRP therapy in individuals diagnosed with MGD and dry eye. The implementation of standardized techniques for the preparation of PRP and the establishment of consistent treatment protocols would significantly enhance the reproducibility and reliability of treatment outcomes.
Furthermore, it is necessary to conduct comparative studies that assess the efficacy of PRP therapy in comparison to currently available treatments and combination therapies. Moreover, additional investigation is required to delve into the fundamental mechanisms of PRP therapy in addressing meibomian gland dysfunction and dry eye, potentially paving the way for the advancement of novel and more comprehensively understood therapeutic approaches. As scientific inquiry advances and our comprehension of PRP therapy deepens, there arises the possibility of a paradigm shift in the management of dry eye syndrome, leading to substantial improvements in the well-being and overall quality of life for affected individuals.
Abbreviations
aPRP: Autologous Platelet-Rich Plasma, AS: Autologous Serum, DED: Dry Eye Disease, EDE: Evaporative Dry Eye, EDED: Evaporative Dry Eye Disease, EGF: Epidermal Growth Factor, FGF: Fibroblast Growth Factor, IL-1: Interleukin-1, IL-10: Interleukin-10, JAK/STAT: Janus Kinase/Signal Transducer and Activator of Transcription, LASIK: Laser-Assisted in Situ Keratomileusis, MGD: Meibomian Gland Dysfunction, MAPK: Mitogen-Activated Protein Kinase, MMPs: Matrix Metalloproteinases, NIBUT: Non-Invasive Tear Film Break-Up Time, OSDI: Ocular Surface Disease Index, PDGF: Platelet-Derived Growth Factor, PI3K: Phosphatidylinositol 3-Kinase, PRGF: Plasma Rich in Growth Factors, PRP: Platelet-Rich Plasma, SANDE: Symptom Assessment in Dry Eye, TBUT: Tear Break-Up Time, TGF-β: Transforming Growth Factor-beta, TFLL: Tear Film Lipid Layer, TMH: Tear Meniscus Height, TNF-α: Tumor Necrosis Factor-alpha, VEGF: Vascular Endothelial Growth Factor
Acknowledgments
None.
Author’s contributions
All authors equally contributed to this work, read and approve the final proof for publication.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Rouen
P.A.,
White
M.L.,
Dry Eye Disease: Prevalence, Assessment, and Management. Home Healthcare Now.
2018;
36
(2)
:
74-83
.
View Article PubMed Google Scholar -
Talens-Estarelles
C.,
García-Marqués
J.V.,
Cerviño
A.,
García-Lázaro
S.,
Dry Eye-Related Risk Factors for Digital Eye Strain. Eye {&}amp; Contact Lens.
2022;
48
(10)
:
410-5
.
View Article PubMed Google Scholar -
Papas
E.B.,
The global prevalence of dry eye disease: A Bayesian view. Ophthalmic & Physiological Optics.
2021;
41
(6)
:
1254-66
.
View Article PubMed Google Scholar -
Farrand
K.F.,
Fridman
M.,
Stillman
I.O.,
Schaumberg
D.A.,
Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. American Journal of Ophthalmology.
2017;
182
:
90-8
.
View Article PubMed Google Scholar -
Arita
R.,
Fukuoka
S.,
Morishige
N.,
New Insights Into the Lipid Layer of the Tear Film and Meibomian Glands. Eye & Contact Lens.
2017;
43
(6)
:
335-9
.
View Article PubMed Google Scholar -
Butovich
I.A.,
Meibomian glands, meibum, and meibogenesis. Experimental Eye Research.
2017;
163
:
2-16
.
View Article PubMed Google Scholar -
Sánchez-Avila
R.M.,
Merayo-Lloves
J.,
Fernández
M.L.,
Rodríguez-Gutiérrez
L.A.,
Rodríguez-Calvo
P.P.,
Fernández-Vega Cueto
A.,
Plasma rich in growth factors eye drops to treat secondary ocular surface disorders in patients with glaucoma. International Medical Case Reports Journal.
2018;
11
:
97-103
.
View Article PubMed Google Scholar -
Murtaza
F.,
Toameh
D.,
Chiu
H.H.,
Tam
E.S.,
Somani
S.,
Autologous Platelet-Rich Plasma Drops for Evaporative Dry Eye Disease from Meibomian Gland Dysfunction: A Pilot Study. Clinical Ophthalmology (Auckland, N.Z.).
2022;
16
:
2199-208
.
View Article PubMed Google Scholar -
Wang
L.X.,
Deng
Y.P.,
Androgen and meibomian gland dysfunction: from basic molecular biology to clinical applications. International Journal of Ophthalmology.
2021;
14
(6)
:
915-22
.
View Article PubMed Google Scholar -
Sullivan
D.A.,
Sullivan
B.D.,
Evans
J.E.,
Schirra
F.,
Yamagami
H.,
Liu
M.,
Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Annals of the New York Academy of Sciences.
2002;
966
(1)
:
211-22
.
View Article PubMed Google Scholar -
Sullivan
B.D.,
Evans
J.E.,
Dana
M.R.,
Sullivan
D.A.,
Impact of androgen deficiency on the lipid profiles in human meibomian gland secretions. Lacrimal Gland, Tear Film, and Dry Eye Syndromes . Basic Science and Clinical Relevance Part A and B.
2002;
2002
:
449-58
.
View Article Google Scholar -
Nanavaty
M.A.,
Long
M.,
Malhotra
R.,
Transdermal androgen patches in evaporative dry eye syndrome with androgen deficiency: a pilot study. The British Journal of Ophthalmology.
2014;
98
(4)
:
567-9
.
View Article PubMed Google Scholar -
Chen
H.,
Midzak
A.,
Luo
Ld,
Zirkin
B.R.,
Aging and the Decline of Androgen Production. 2007;
:
117-31
.
View Article Google Scholar -
Carter
J.N.,
Steinbeck
K.S.,
Reduced adrenal androgens in patients with myotonic dystrophy. The Journal of Clinical Endocrinology and Metabolism.
1985;
60
(3)
:
611-4
.
View Article PubMed Google Scholar -
Du
Y.L.,
Peng
X.,
Liu
Y.,
Wang
J.S.,
Ye
Y.F.,
Xu
K.K.,
Ductal Hyperkeratinization and Acinar Renewal Abnormality: New Concepts on Pathogenesis of Meibomian Gland Dysfunction. Current Issues in Molecular Biology.
2023;
45
(3)
:
1889-901
.
View Article PubMed Google Scholar -
Chen
H.,
Gao
H.,
Xie
H.T.,
Liu
S.T.,
Huang
Y.K.,
Zhang
M.C.,
Hyperkeratinization and Proinflammatory Cytokine Expression in Meibomian Glands Induced by Staphylococcus aureus. Investigative Ophthalmology & Visual Science.
2021;
62
(13)
:
11
.
View Article PubMed Google Scholar -
Jester
J.V.,
Parfitt
G.J.,
Brown
D.J.,
Meibomian gland dysfunction: hyperkeratinization or atrophy. BMC ophthalmology.
2015;
15
:
3-11
.
View Article Google Scholar -
Li
S.,
Nikulina
K.,
DeVoss
J.,
Wu
A.J.,
Strauss
E.C.,
Anderson
M.S.,
Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Investigative Ophthalmology & Visual Science.
2008;
49
(1)
:
34-41
.
View Article PubMed Google Scholar -
Zdebik
A.,
Zdebik
N.,
Fischer
M.,
Ocular manifestations of skin diseases with pathological keratinization abnormalities. Postepy Dermatologii i Alergologii.
2021;
38
(2)
:
14-20
.
View Article PubMed Google Scholar -
Herzlich
A.A.,
Ding
X.,
Shen
D.,
Ross
R.J.,
Tuo
J.,
Chan
C.C.,
Peroxisome Proliferator-Activated Receptor Expression in Murine Models and Humans with Age-related Macular Degeneration. The Open Biology Journal.
2009;
2
:
141-8
.
View Article PubMed Google Scholar -
Wang
Y.,
Luo
M.,
Che
L.,
Wu
Q.,
Li
J.,
Ma
Y.,
Enhanced detection of ligand-PPARγ binding based on surface plasmon resonance through complexation with SRC1- or NCOR2-related polypeptide. International Journal of Biological Macromolecules.
2024;
268
(Pt 1)
:
131865
.
View Article PubMed Google Scholar -
Burris
T.P.,
Pelton
P.D.,
Zhou
L.,
Osborne
M.C.,
Cryan
E.,
Demarest
K.T.,
A novel method for analysis of nuclear receptor function at natural promoters: peroxisome proliferator-activated receptor gamma agonist actions on aP2 gene expression detected using branched DNA messenger RNA quantitation. Molecular Endocrinology (Baltimore, Md.).
1999;
13
(3)
:
410-7
.
PubMed Google Scholar -
Brun
R.P.,
Kim
J.B.,
Hu
E.,
Spiegelman
B.M.,
Peroxisome proliferator-activated receptor gamma and the control of adipogenesis. Current Opinion in Lipidology.
1997;
8
(4)
:
212-8
.
View Article PubMed Google Scholar -
Gerhold
D.L.,
Liu
F.,
Jiang
G.,
Li
Z.,
Xu
J.,
Lu
M.,
Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferator-activated receptor-gamma agonists. Endocrinology.
2002;
143
(6)
:
2106-18
.
View Article PubMed Google Scholar -
Cerón
J.J.,
Ortín-Bustillo
A.,
López-Martínez
M.J.,
Martínez-Subiela
S.,
Eckersall
P.D.,
Tecles
F.,
S-100 Proteins: Basics and Applications as Biomarkers in Animals with Special Focus on Calgranulins (S100A8, A9, and A12). Biology (Basel).
2023;
12
(6)
:
881
.
View Article PubMed Google Scholar -
Abdi
W.,
Romasco
A.,
Alkurdi
D.,
Santacruz
E.,
Okinedo
I.,
Zhang
Y.,
An overview of S100 proteins and their functions in skin homeostasis, interface dermatitis conditions and other skin pathologies. Experimental Dermatology.
2024;
33
(8)
:
e15158
.
View Article PubMed Google Scholar -
Tong
L.,
Lan
W.,
Lim
R.R.,
Chaurasia
S.S.,
S100A proteins as molecular targets in the ocular surface inflammatory diseases. The Ocular Surface.
2014;
12
(1)
:
23-31
.
View Article PubMed Google Scholar -
Chisholm
L.O.,
Jaeger
N.M.,
Murawsky
H.E.,
Harms
M.J.,
S100A9 interacts with a dynamic region on CD14 to activate Toll-like receptor 4. bioRxiv.
2024;
2024
:
2024-05
.
View Article Google Scholar -
Le
Y.,
Zhang
J.,
Lin
Y.,
Ren
J.,
Xiang
L.,
Zhang
C.,
S100A9 Exacerbates the Inflammation in Rosacea through Toll-Like Receptor 4/MyD88/NF-κB Signaling Pathway. The Journal of Investigative Dermatology.
2024;
144
(9)
:
1985-1993.e1
.
View Article PubMed Google Scholar -
Liang
L.,
Yang
X.,
Zeng
H.,
Liao
K.,
Zhang
R.,
Wang
B.,
S100A9-TLR4 axis aggravates dry eye through the blockage of autophagy. Experimental Eye Research.
2024;
247
.
View Article PubMed Google Scholar -
Marlatt
N.M.,
Shaw
G.S.,
Amide exchange shows calcium-induced conformational changes are transmitted to the dimer interface of S100B. Biochemistry.
2007;
46
(25)
:
7478-87
.
View Article PubMed Google Scholar -
Markowitz
J.,
Rustandi
R.R.,
Varney
K.M.,
Wilder
P.T.,
Udan
R.,
Wu
S.L.,
Calcium-binding properties of wild-type and EF-hand mutants of S100B in the presence and absence of a peptide derived from the C-terminal negative regulatory domain of p53. Biochemistry.
2005;
44
(19)
:
7305-14
.
View Article PubMed Google Scholar -
Simon
M.A.,
Ecsédi
P.,
Kovács
G.M.,
Póti
Á.L.,
Reményi
A.,
Kardos
J.,
High-throughput competitive fluorescence polarization assay reveals functional redundancy in the S100 protein family. The FEBS Journal.
2020;
287
(13)
:
2834-46
.
View Article PubMed Google Scholar -
Bardet
C.,
Ribes
S.,
Wu
Y.,
Diallo
M.T.,
Salmon
B.,
Breiderhoff
T.,
Claudin Loss-of-Function Disrupts Tight Junctions and Impairs Amelogenesis. Frontiers in Physiology.
2017;
8
:
326
.
View Article PubMed Google Scholar -
Rötzer
V.,
Melega
F.,
Garreis
F.,
Paulsen
F.,
Waschke
J.,
E-Cadherin Is Important for Meibomian Gland Function as Revealed by a New Human ex Vivo Slice Culture Model. American Journal of Pathology.
2019;
189
(8)
:
1559-68
.
View Article PubMed Google Scholar -
Guo
W.,
Song
Y.,
Sun
Y.,
Du
H.,
Cai
Y.,
You
Q.,
Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: evidence from NHANES 2011-2018. Frontiers in Endocrinology (Lausanne).
2022;
13
:
1071465
.
View Article PubMed Google Scholar -
Chang
A.Y.,
Purt
B.,
Biochemistry, Tear Film, in StatPearls. 2024: Treasure Island (FL).
.
-
Keramatnejad
M.,
DeWolf
C.,
A biophysical study of tear film lipid layer model membranes. Biochimica et Biophysica Acta. Biomembranes.
2023;
1865
(3)
:
184102
.
View Article PubMed Google Scholar -
Viitaja
T.,
Moilanen
J.,
Svedström
K.J.,
Ekholm
F.S.,
Paananen
R.O.,
Tear Film Lipid Layer Structure: Self-Assembly of O-Acyl-ω-hydroxy Fatty Acids and Wax Esters into Evaporation-Resistant Monolayers. Nano Letters.
2021;
21
(18)
:
7676-83
.
View Article PubMed Google Scholar -
Xu
X.,
Li
G.,
Zuo
Y.Y.,
Effect of Model Tear Film Lipid Layer on Water Evaporation. Investigative Ophthalmology & Visual Science.
2023;
64
(1)
:
13
.
View Article PubMed Google Scholar -
Mondal
M.,
Vohra
M.,
Sangwan
J.,
Verma
S.,
Coulson-Thomas
V.J.,
Chandru
A.,
In vitro Assay to Examine the Function of Tears on Corneal Epithelial Cells. Bio-Protocol.
2024;
14
(1)
:
e4910
.
View Article PubMed Google Scholar -
Geerling
G.,
Baudouin
C.,
Aragona
P.,
Rolando
M.,
Boboridis
K.G.,
Benítez-Del-Castillo
J.M.,
Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: proceedings of the OCEAN group meeting. The Ocular Surface.
2017;
15
(2)
:
179-92
.
View Article PubMed Google Scholar -
Sabeti
S.,
Kheirkhah
A.,
Yin
J.,
Dana
R.,
Management of meibomian gland dysfunction: a review. Survey of Ophthalmology.
2020;
65
(2)
:
205-17
.
View Article PubMed Google Scholar -
Chhadva
P.,
Goldhardt
R.,
Galor
A.,
Meibomian Gland Disease: The Role of Gland Dysfunction in Dry Eye Disease. Ophthalmology.
2017;
124
(11)
:
20-6
.
View Article PubMed Google Scholar -
Pflugfelder
S.C.,
Stern
M.E.,
Biological functions of tear film. Experimental Eye Research.
2020;
197
:
108115
.
View Article PubMed Google Scholar -
Sweeney
D.F.,
Millar
T.J.,
Raju
S.R.,
Tear film stability: a review. Experimental Eye Research.
2013;
117
:
28-38
.
View Article PubMed Google Scholar -
Georgiev
G.A.,
Eftimov
P.,
Yokoi
N.,
Structure-function relationship of tear film lipid layer: A contemporary perspective. Experimental Eye Research.
2017;
163
:
17-28
.
View Article PubMed Google Scholar -
Gurnani
B.,
Kaur
K.,
Meibomian Gland Disease, in StatPearls. 2023: Treasure Island (FL).
.
-
Villani
E.,
Marelli
L.,
Dellavalle
A.,
Serafino
M.,
Nucci
P.,
Latest evidences on meibomian gland dysfunction diagnosis and management. The Ocular Surface.
2020;
18
(4)
:
871-92
.
View Article PubMed Google Scholar -
Lee
G.,
Evidence-Based Strategies for Warm Compress Therapy in Meibomian Gland Dysfunction. Ophthalmology and Therapy.
2024;
13
(9)
:
2481-93
.
View Article PubMed Google Scholar -
Thode
A.R.,
Latkany
R.A.,
Current and Emerging Therapeutic Strategies for the Treatment of Meibomian Gland Dysfunction (MGD). Drugs.
2015;
75
(11)
:
1177-85
.
View Article PubMed Google Scholar -
Weng
T.H.,
Chang
Y.M.,
Chien
K.H.,
Chen
Y.H.,
Tai
M.C.,
Feng
K.M.,
Efficacy and Clinical Outcome of Single and Combined Therapies for Refractory Meibomian Gland Dysfunction: Probing, Intense Pulsed Light, and Thermal Pulsation Treatments. Yixue Yanjiu Zazhi.
2024;
44
(6)
:
243-9
.
View Article Google Scholar -
Lam
P.Y.,
Shih
K.C.,
Fong
P.Y.,
Chan
T.C.,
Ng
A.L.,
Jhanji
V.,
A Review on Evidence-Based Treatments for Meibomian Gland Dysfunction. Eye & Contact Lens.
2020;
46
(1)
:
3-16
.
View Article PubMed Google Scholar -
Le
A.D.,
Enweze
L.,
DeBaun
M.R.,
Dragoo
J.L.,
Platelet-Rich Plasma. Clinics in Sports Medicine.
2019;
38
(1)
:
17-44
.
View Article PubMed Google Scholar -
Emer
J.,
Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin Therapy Letter.
2019;
24
(5)
:
1-6
.
PubMed Google Scholar -
Melo
B.A.,
Luzo
Â.C.,
Lana
J.F.,
Santana
M.H.,
Centrifugation Conditions in the L-PRP Preparation Affect Soluble Factors Release and Mesenchymal Stem Cell Proliferation in Fibrin Nanofibers. Molecules (Basel, Switzerland).
2019;
24
(15)
:
2729
.
View Article PubMed Google Scholar -
Lei
X.,
Yang
Y.,
Shan
G.,
Pan
Y.,
Cheng
B.,
Preparation of ADM/PRP freeze-dried dressing and effect of mice full-thickness skin defect model. Biomedical Materials (Bristol, England).
2019;
14
(3)
:
035004
.
View Article PubMed Google Scholar -
Qu
W.,
Wang
Z.,
Hunt
C.,
Morrow
A.S.,
Urtecho
M.,
Amin
M.,
The Effectiveness and Safety of Platelet-Rich Plasma for Chronic Wounds: A Systematic Review and Meta-analysis. Mayo Clinic Proceedings.
2021;
96
(9)
:
2407-17
.
View Article PubMed Google Scholar -
Dhurat
R.,
Sukesh
M.,
Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author's Perspective. Journal of Cutaneous and Aesthetic Surgery.
2014;
7
(4)
:
189-97
.
View Article PubMed Google Scholar -
Sung
K.,
Zheng
K.,
Williams
C.,
Cunningham
D.,
Sussman
W.I.,
Pseudoseptic reaction to an intra-articular platelet-rich plasma injection into the knee: a case report. Regenerative Medicine.
2023;
18
(6)
:
455-9
.
View Article PubMed Google Scholar -
Peng
J.,
Wang
Q.,
Xu
Y.,
He
H.,
Platelet-rich plasma treatment for talar cartilage repair: a systematic review and meta-analysis. BMC Musculoskeletal Disorders.
2023;
24
(1)
:
366
.
View Article PubMed Google Scholar -
Wróbel-Dudzińska
D.,
Przekora
A.,
Kazimierczak
P.,
Ćwiklińska-Haszcz
A.,
Kosior-Jarecka
E.,
Żarnowski
T.,
The Comparison between the Composition of 100% Autologous Serum and 100% Platelet-Rich Plasma Eye Drops and Their Impact on the Treatment Effectiveness of Dry Eye Disease in Primary Sjogren Syndrome. Journal of Clinical Medicine.
2023;
12
(9)
:
3126
.
View Article PubMed Google Scholar -
Mohammed
M.A.,
Allam
I.Y.,
Shaheen
M.S.,
Lazreg
S.,
Doheim
M.F.,
Lacrimal gland injection of platelet rich plasma for treatment of severe dry eye: a comparative clinical study. BMC Ophthalmology.
2022;
22
(1)
:
343
.
View Article PubMed Google Scholar -
Avila
M.Y.,
Igua
A.M.,
Mora
A.M.,
British Journal of Ophthalmology.
Randomised, prospective clinical trial of platelet-rich plasma injection in the management of severe dry eye. The British Journal of Ophthalmology.
2018;
103
(5)
:
648-53
.
View Article PubMed Google Scholar -
Rawat
P.,
Agrawal
R.,
Bhaisare
V.,
Walia
S.,
Kori
N.,
Gupta
R.,
Autologous platelet-rich plasma eye drop versus artificial tear eye drop for symptomatic dry eye disease: A prospective comparative interventional study. Indian Journal of Ophthalmology.
2022;
70
(5)
:
1549-53
.
View Article PubMed Google Scholar -
Hassan
A.,
Telandro
A.,
Barguigua
A.,
Baba
M.,
Körber
N.,
Evaluation of the Use of Highly Concentrated Autologous Platelet-Rich Plasma and Platelet-Rich Fibrin Membrane to Improve the Outcome in the Management of Severe Dry Eye Disease, Corneal Neurotrophic Ulcer and Corneal Burn. Cureus.
2024;
16
(1)
:
e51794
.
View Article PubMed Google Scholar -
Nadelmann
J.B.,
Bunya
V.Y.,
Ying
G.S.,
Hua
P.,
Massaro-Giordano
M.,
Effect of Autologous Platelet-Rich Plasma Drops in the Treatment of Ocular Surface Disease. Clinical Ophthalmology (Auckland, N.Z.).
2022;
16
:
4207-13
.
View Article PubMed Google Scholar -
Kang
M.J.,
Lee
J.H.,
Hwang
J.,
Chung
S.H.,
Efficacy and safety of platelet-rich plasma and autologous-serum eye drops for dry eye in primary Sjögren's syndrome: a randomized trial. Scientific Reports.
2023;
13
(1)
:
19279
.
View Article PubMed Google Scholar -
Soifer
M.,
Tovar
A.,
Wang
M.,
Mousa
H.M.,
Yennam
S.,
Sabater
A.L.,
A multicenter report of the use of plasma rich in growth factors (PRGF) for the treatment of patients with ocular surface diseases in North America. The Ocular Surface.
2022;
25
:
40-8
.
View Article PubMed Google Scholar -
García-Conca
V.,
Abad-Collado
M.,
Hueso-Abancens
J.R.,
Mengual-Verdú
E.,
Piñero
D.P.,
Aguirre-Balsalobre
F.,
Efficacy and safety of treatment of hyposecretory dry eye with platelet-rich plasma. Acta Ophthalmologica.
2019;
97
(2)
:
e170-8
.
View Article PubMed Google Scholar -
Alio
J.L.,
Rodriguez
A.E.,
Abdelghany
A.A.,
Oliveira
R.F.,
Autologous Platelet-Rich Plasma Eye Drops for the Treatment of Post-LASIK Chronic Ocular Surface Syndrome. Journal of Ophthalmology.
2017;
2017
:
2457620
.
View Article PubMed Google Scholar -
Sanchez-Avila
R.M.,
Merayo-Lloves
J.,
Riestra
A.C.,
Anitua
E.,
Muruzabal
F.,
Orive
G.,
The Effect of Immunologically Safe Plasma Rich in Growth Factor Eye Drops in Patients with Sjögren Syndrome. Journal of Ocular Pharmacology and Therapeutics.
2017;
33
(5)
:
391-9
.
View Article PubMed Google Scholar -
Jung
J.U.,
Lee
S.H.,
Kim
H.K.,
Effects of platelet-rich plasma on ocular surface in patients with dry eye syndrome: clinico-experimental analysis. Journal of the Korean Ophthalmological Society.
2019;
60
(12)
:
1169-75
.
View Article Google Scholar -
Suzuki
T.,
Inflamed Obstructive Meibomian Gland Dysfunction Causes Ocular Surface Inflammation. Investigative Ophthalmology & Visual Science.
2018;
59
(14)
:
94-101
.
View Article PubMed Google Scholar -
Hussain
N.,
Johal
H.,
Bhandari
M.,
An evidence-based evaluation on the use of platelet rich plasma in orthopedics - a review of the literature. SICOT-J.
2017;
3
:
57
.
View Article PubMed Google Scholar -
Pavlovic
V.,
Ciric
M.,
Jovanovic
V.,
Stojanovic
P.,
Platelet Rich Plasma: a short overview of certain bioactive components. Open Medicine (Warsaw).
2016;
11
(1)
:
242-7
.
View Article PubMed Google Scholar -
Maldonado
B.A.,
Furcht
L.T.,
Epidermal growth factor stimulates integrin-mediated cell migration of cultured human corneal epithelial cells on fibronectin and arginine-glycine-aspartic acid peptide. Investigative Ophthalmology & Visual Science.
1995;
36
(10)
:
2120-6
.
PubMed Google Scholar -
Kenchegowda
S.,
Bazan
N.G.,
Bazan
H.E.,
EGF stimulates lipoxin A4 synthesis and modulates repair in corneal epithelial cells through ERK and p38 activation. Investigative Ophthalmology & Visual Science.
2011;
52
(5)
:
2240-9
.
View Article PubMed Google Scholar -
Chen
T.,
Song
P.,
He
M.,
Rui
S.,
Duan
X.,
Ma
Y.,
Sphingosine-1-phosphate derived from PRP-Exos promotes angiogenesis in diabetic wound healing via the S1PR1/AKT/FN1 signalling pathway. Burns and Trauma.
2023;
11
:
tkad003
.
View Article PubMed Google Scholar -
Etulain
J.,
Mena
H.A.,
Negrotto
S.,
Schattner
M.,
Stimulation of PAR-1 or PAR-4 promotes similar pattern of VEGF and endostatin release and pro-angiogenic responses mediated by human platelets. Platelets.
2015;
26
(8)
:
799-804
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 11 (2024)
Page No.: 6900-6911
Published on: 2024-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 1948 times
- PDF downloaded - 571 times
- XML downloaded - 107 times
 Biomedpress
Biomedpress



