Abstract
Introduction: Shellfish allergy is prevalent in coastal countries across Asia. Despite crab being a popular dish, there is limited knowledge about the features of crab allergy in Viet Nam. This study aims to identify the IgE-binding allergens in the local crab species, Scylla paramamosain (S. paramamosain).
Methods: The study involved 12 patients with crab allergy and 5 subjects with crab tolerance. Each participant underwent a skin prick test (SPT) with crab extracts and was measured for specific IgE levels to crab, as well as house dust mite species Dermatophagoides farinae (DF) and Dermatophagoides pteronyssinus (DP). An immunoblot was used to identify IgE-binding proteins in S. paramamosain, followed by identification using nanoscale liquid chromatography coupled with tandem mass spectrometry analysis.
Results: Patients with crab allergy exhibited higher total IgE levels (2432 ± 772.26 vs 886.15 ± 3056.74), 100% positive SPT to crab, and higher specific IgE levels to crab (3.40 ± 6.19 vs 0.48 ± 8.86) compared to subjects with crab tolerance. The level of specific IgE to crab showed significant correlation with SPT results to DP and DF (r = 0.52, P = 0.033; r = 0.82, P < 0.001, respectively). The frequencies of IgE-binding proteins in crab allergy patients were higher than in tolerant subjects. Several putative allergens were identified, including hemocyanin as a major allergen, with arginine kinase, sarcoplasmic-calcium binding protein, and triosephosphate isomerase as minor allergens.
Conclusion: This study is the first to demonstrate potential molecular allergens in local mud crabs among Vietnamese individuals sensitized to house dust mites. These findings require further clinical evaluation in future studies for their application.
Introduction
Over the past few decades, there has been a global increase in the prevalence of food allergies1, 2. As food allergy depends on eating habits, each country’s burden and food allergens will differ3. In Asia, the population-based estimates of food allergy prevalence range from 1.1% to 7.7%2. The spectrum of food allergens in Asia shows different features compared to Western countries, with the predominance of shellfish and wheat3. In Viet Nam, where seafood plays a significant role in the daily diet, seafood allergy is most commonly observed among subjects with food allergies4, 5. Surveys have shown that IgE-mediated crustacean allergy is 2.6% in adults and 2.8% to 4.79% in children, representing the highest prevalence compared to other allergens in Viet Nam5.
Among crustaceans, crabs are highly consumed. According to the literature, the genus Scylla consists of four species of swimming crabs: Scylla serrata, Scylla olivacea, Scylla paramamosain, and Scylla tranquebarica6, 7. S. paramamosain is a type of crab frequently consumed in Southeast Asia. The increased consumption of crustacean products can be associated with a higher risk of allergy. Snow crab sensitization is common among workers and may contribute to occupational asthma8, 9. The prevalence of crab allergy is 15.2% in subjects with food allergies in Singapore [10] and 12.7% in Chinese asthmatic children10, 11. Until July 2022, Viet Nam was estimated to export 38 million U.S. dollars worth of crabs to the USA12. Therefore, evaluating the characteristics of crab allergens among local fishery products is necessary.
The mechanisms of crab allergy can be roughly divided into IgE- and non-IgE-mediated pathways13, 14. In cases where allergic reactions are triggered by an immune response mediated through IgE, patients can experience various symptoms ranging from mild to severe, including potentially fatal anaphylactic shock. It is worth noting that, among allergens within the same type of food, some tend to bind to IgE more frequently, potentially causing more severe reactions than others, and this can vary from person to person13, 15. Moreover, different types of food may contain similar allergens, leading to the possibility of cross-reactivity. Consequently, a comprehensive understanding of allergen components is crucial for diagnosing food allergies. Several key allergen components in seafood have been identified, such as tropomyosin (33-39 kDa), arginine kinase (AK) (38-45 kDa), and hemocyanin (HM) (77 kDa), which are prevalent in various shellfish species14, 16, 17, 18. However, research on the molecular allergen components of crabs in Viet Nam still needs to be expanded. In this study, we aimed to identify the molecular allergens in patients with an allergy to mud crab (Scylla paramamosain). Briefly, raw allergen extracts were obtained from local mud crabs. These allergens were incubated with the patient's serum to examine the components typically bound to the IgE antibodies. Subsequently, IgE-bound proteins were digested and identified using nanoscale liquid chromatography coupled with tandem mass spectrometry (nano-LC/MS).
Methods
Patient Recruitment
Patients with suspected crab allergy were enrolled from the Unit of Allergy and Clinical Immunology, University Medical Center (Ho Chi Minh City, Viet Nam). All subjects provided informed consent and underwent an allergy assessment. Participants were interviewed about their history of allergy and reactions after crab consumption. The diagnosis of crab allergy was confirmed if participants experienced either anaphylaxis after crab consumption or repetitive allergic events after crab consumption at home. Subsequently, participants were divided into two groups: crab allergic (CA) and crab tolerant (CT) groups. All participants underwent skin prick tests (SPT) with the following allergens: house dust mites, Dermatophagoides farinae (DF), Dermatophagoides pteronyssinus (DP), and extracts of raw crab S. paramamosain. The SPT was conducted according to the instructions of the European Academy of Allergy and Clinical Immunology19. Whole blood was collected from the participants in blood collection tubes without EDTA. The obtained blood samples were allowed to coagulate by being left undisturbed for 30 minutes at room temperature (RT) and then centrifuged at 3000 rpm for 10 minutes at 4°C. Patient sera were stored at –80°C for further use. The University of Medicine and Pharmacy Ethics Committee at Ho Chi Minh City approved this study.
Preparation of Crude Extracts
Mud crabs (Scylla paramamosain), approximately eight months old and female, were purchased from a local market before tissue samples were collected. Crude extracts were prepared as previously described20. Briefly, approximately 5 g of raw muscle tissues in the crab claws were homogenized in 20 mL of phosphate-buffered saline (PBS) extraction buffer and incubated overnight at 4°C on a constant temperature shaker. Subsequently, the extracts were centrifuged at 5000 × g for 20 minutes at 4°C. The supernatant was transferred to 100K Omega Microsep Advance Centrifugal Devices (Pall, USA - MCP100C41) for protein purification and centrifuged at 5000 × g for 20 minutes at 4°C. The filtered supernatant containing the retained bioactive compounds was preserved at -80°C. Protein concentrations were measured using Pierce™ Bradford Plus Protein Assay Kits (Thermo Fisher Scientific, Massachusetts, USA).
Visualization of Allergens Using Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Food allergens were subjected to 10-12% SDS-PAGE, followed by both Coomassie blue staining and electrotransfer into PVDF membranes. SDS-PAGE gels were then incubated in destain solution (10% methanol + 10% acetic acid). The SDS-PAGE gel containing the proteins was placed on a glass plate illuminated by a light box, and a razor blade was used to excise an individual band from the gel and place it into a microcentrifuge tube.
Detection of sIgE-Binding Epitopes Using Immunoblot
Patient sera (1:10 dilution) were used for immunoblotting, followed by the addition of HRP-conjugated anti-human IgG (ThermoFisher Scientific, USA). Immunocomplexes were visualized with 1-Step™ TMB-Blotting Substrate Solution (Thermo Fisher Scientific).
In-Gel Protein Digestion
The protein bands on the SDS-PAGE gel were sliced into small pieces and subjected to destain for 30 minutes until the gel particles became clear. Gel pieces were dehydrated by adding 100 µL of 100% acetonitrile (ACN). Then, ACN was removed and the gel pieces were left to air-dry for 5-10 minutes. The gel pieces were then washed with a 50:50 mixture of 100 mM NH4HCO3 and 50% ACN for 10 minutes, which was then discarded. The samples were resuspended in 10 mM DTT in 100 mM NH4HCO3 for 45 minutes for reduction, followed by incubation with 50 µL of 50 mM iodoacetamide in 100 mM NH4HCO3 and kept in the dark for 30 minutes. The gel pieces were washed as described above. Afterwards, gel pieces were rehydrated on ice with 10 ng/µL trypsin in 50 mM NH4HCO3 with 10% ACN at 4°C for 30 minutes. Fifty mM NH4HCO3 was added to cover the gel pieces, and the tubes were placed in a 30°C water bath overnight. To terminate the reaction, 30 µL of 1% formic acid was added and centrifuged at 13,000 rpm for 5 minutes to collect the peptide-rich first supernatant in a labeled Eppendorf tube. The gel pieces were incubated with 50% ACN and 5% formic acid for 45 minutes. The tubes were subjected to sonication, followed by centrifugation at 13,000 rpm for another 5 minutes, and the second supernatant was collected in a labeled Eppendorf tube. Later, gel pieces were incubated with 90% ACN with 5% formic acid for 5 minutes. The final supernatant was collected in a labeled Eppendorf tube. Finally, all supernatants were dried via SpeedVac and desalted with a Ziptip C18 column. The pipette was set to 10 µL and attached to the Ziptip column. To prepare the ZipTip for peptide binding, the column was conditioned with 3 washes of 100% acetonitrile and 3 washes of 0.1% formic acid. The sample was loaded onto the column by pipetting the protein digest up and down 10 times. The ZipTip was washed with 6 washes of 0.1% formic acid. The peptides were extracted from the ZipTip by pipetting the elution buffer 10 times in a 4 µL solution containing 60% acetonitrile/0.1% formic acid. The organic phase of the elution buffer eluted the peptides from the resin into the buffer. The sample was subsequently desalted and concentrated in a 4 µL solution.
NanoLC-MS/MS Analysis of Tryptic Peptides
Tryptic peptides were separated and sequenced by nanoscale liquid chromatography on a nanoAcquity system (Waters, Milford, MA, USA) and MS/MS on an Orbitrap Elite hybrid mass spectrometer (Thermo Electron, Bremen, Germany). For peptide separation, a 25 cm long and 75 μm ID- C18 BEH column (Waters, Milford, MA) packed with 1.7 μm particles with a pore size of 130 Å was used at a flow rate of 300 nL/min and a column temperature of 35°C. Peptides dissolved in Solvent A (0.1% formic acid in water) were loaded into the column and eluted by a 120-minute segmented gradient from 5 to 35% solvent B (acetonitrile with 0.1% formic acid). The peptides were sequenced by the tandem MS/MS method. Initially, the mass spectrometer scanned precursor ions in orbitrap mode (m/z 350−1600, 120,000 resolution at m/z 400, and an automatic gain control (AGC) target at 10^6). The most intense ions were then isolated for CID MS/MS fragmentation and detection in the linear ion trap (AGC target at 10,000). The mass spectrometer was operated using data-dependent scans of MS spectra, which excluded previously selected ions for 60 seconds. Single and unrecognized charge ions were also excluded for MS/MS fragmentation.
Protein Bioinformatic Analysis
Raw MS/MS data were first processed using PEAKS 6 software (Bioinformatics Solutions, Canada). The proteome database in PEAKS was taken from the UniProt Knowledgebase (UniProtKB), accessed in June 2023. Protein ID was identified by searching against the database with known parameters for protein modifications [variable oxidation (M) and fixed carbamidomethylation (C)]. Accepted peptides in protein ID were limited to two missed cleavages, and thresholds for parent mass inaccuracy and precursor mass tolerance were chosen at 10 ppm and 0.6 Da. The false detection rate (FDR) was set at 1%, and identified proteins must have had at least two unique peptides.
Statistical Analysis
The normality of the data was checked with the Shapiro–Wilk test. For continuous variables, data were presented as median ± standard error (SE), and comparisons between the two groups were performed using the Mann–Whitney U test. For categorical variables, data were displayed as N (%), and Fisher's exact test was used to compare the two groups. Correlation was calculated by Spearman's rank correlation. Data analysis was performed using the statistical software packages IBM SPSS 20.0 (Armonk, NY, USA), and JASP (Version 0.18.3) [Computer software]. Graphs were prepared using GraphPad Prism 8 (San Diego, CA, USA). A P-value of less than 0.05 was considered significant.
| Characteristics | All (N=17) | Crab allergic group (N=12) | Crab tolerant group (N=5) | P-values |
| Age (*) | 29 ± 1 (*) | 29 ± 1 | 27 ± 2 | 0.79 |
| Sex (female, %) | 11 (66.11%) | 7 (58.33%) | 4 (80%) | 0.24 |
| Comorbid fish/mollusk allergy | 0 | 0 | 0 | NA |
| Total IgE (*) | 2411 ± 869.65 | 2432 ± 772.26 | 886.15 ± 3056.74 | 0.48 |
| sIgE to seafood mix (*) | 0.35 ± 3.11 | 0 ± 4.02 | 0.56 ± 3.02 | 0.48 |
| sIgE to crab (*) | 2.05 ± 5.05 | 3.40 ± 6.19 | 0.48 ± 8.86 | 0.55 |
| Wheal diameter of Der P SPT (*) | 7 ± 1.3 | 8 ± 1.5 | 5 ± 2.6 | 0.35 |
| SPT to Der P (+) (*) | 16 (88.89%) | 12 (100%) | 4 (80%) | 1 |
| Wheal diameter of Der F SPT | 6 ± 1.7 | 7 ± 2 | 4 ± 3 | 0.20 |
| SPT to Der F (+) | 15 (83.33%) | 12 (100%) | 3 (60%) | 0.43 |
| Wheal diameter of crab SPT | 4 ± 0.5 | 4 ± 0.5 | 3.5 ± 1.1 | 0.79 |
| SPT to crab (+) | 15 (83.33%) | 12 (100%) | 3 (60%) | 0.43 |
| Co-sensitization to Der P and crab | 15 (83.33%) | 12 (100%) | 3 (60%) | 0.43 |
| Co-sensitization to Der F and crab | 15 (83.33%) | 12 (100%) | 3 (60%) | 0.43 |
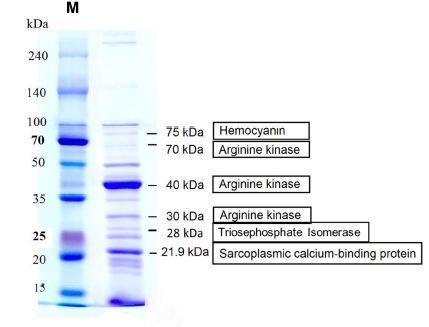
| Band No. | MW (kDa) observed | MW (kDa) Predicted | Protein identification | Organism | Accession No. | Coverage of protein sequence |
| 1 | 20-25 | 21.9 | Sarcoplasmic calcium-binding protein | Scylla paramamosain | I2DDG2|SCP_SCYPA | 59 |
| 2 | 25-35 | 26.7 | Triosephosphate isomerase | Macaca mulatta | P15426|TPIS_MACMU | 14 |
| 3 | 35-50 | 40 | Arginine kinase | Scylla paramamosain | H6VGI3|KARG0_SCYPA | 86 |
| 4 | 50-70 | 40 | Arginine kinase | Scylla paramamosain | C9EIP1|KARG_SCYSE | 32 |
| 5 | 70-100 | 75 | Hemocyanin | Scylla serrata | C9EIP1|KARG_SCYSE | 12 |
Results
Characteristics of subjects with mud crab allergy
In Table 1, 17 subjects were enrolled in this study, including 12 subjects with mud crab allergy (CA) and 5 with mud crab tolerance (CT). The median age for all participants was 29 years old, while the CT participants were relatively younger (27 years old). There was a predominance of females among the participants. Regarding allergic assessment, CA participants were more atopic than CT subjects, as indicated by the higher level of total IgE (2432 ± 772.26 vs. 886.15 ± 3056.74) and a higher percentage of positive SPT to DP (100% vs. 80%) and to DF (100% vs. 60%). However, no significant differences were observed between the groups.
Sensitization to mud crab and clinical symptoms after crab consumption
Sensitization to mud crab was assessed by SPT using the crude extracts and levels of sIgE to crab. All CA subjects had a positive crab SPT (100%) compared to CT subjects (60%) (Table 1). Moreover, the levels of specific IgE to crab among CA subjects were higher compared to the CT group, 3.40 ± 6.19 vs. 0.48 ± 8.86, despite no significant differences. These data advocate that the CA group was more sensitized to the crude crab allergens than the CT group.
In terms of clinical symptoms, non-specific rashes were frequently reported with the highest prevalence (76.47%), followed by urticaria in the majority (70.59%), upper airway reactions such as sore throat/itchy throat/coughing (58.82%), sneezing/itchy nose (41.18%), and itchy eyes/watery eyes/eye redness (35.29%). The spectrum of allergic symptoms for each subject was described in the Supplementary Table.
Correlation between SPT and specific IgE
There was no significant correlation between the level of specific IgE and the wheal size of SPT to crab extracts. Specific IgE to crab correlated with both specific IgE to house dust mites DP (r = 0.52, P = 0.033) and DF (r = 0.82, P < 0.001).
Immunoblotting analysis of IgE-binding proteins among subjects with mud crab allergy
We analyzed the major and minor proteins of raw mud crab S. paramamosain. SDS-PAGE of raw mud crab S. paramamosain extract revealed protein profiles with molecular masses ranging from 15 to 240 kDa (Figure 2A). Immunoblotting using 17 sera from CA patients and CT subjects demonstrated several IgE-binding proteins at various molecular masses between 15 and 240 kDa. Generally, more IgE-binding was detected in the immunoblotting of the CA group compared to CT groups (Figure 2B-C). Proteins at five different MW ranges: 20-25 kDa, 25-35 kDa, 35-50 kDa, 50-70 kDa, 70-100 kDa, and 100-140 kDa exhibited the highest frequency of IgE-binding protein bands in the tested sera of the crab-allergic patient group. Specifically, proteins with MW ranges from 70-100 kDa and 50-70 kDa were identified as the major allergens of S. paramamosain with frequencies of 50% and 41.67%, respectively, in the CA group. Besides, more than 10% of the tested sera in the CT group retained IgE-binding to the protein bands from 20-25 kDa and 35-50 kDa MW ranges in the S. paramamosain raw extract.
Allergen identification
Table 2 summarizes the major allergens of S. paramamosain. The band at 20-25 kDa correlated with sarcoplasmic calcium-binding protein (SCP) from S. paramamosain, with peptide sequence coverage of 59%. The second band at 25-35 kDa was identical to triosephosphate isomerase (TIM). Meanwhile, the bands at 35-50 kDa and 50-70 kDa major allergens were similar to arginine kinase (AK), corresponding to 86% and 32% sequence coverages, respectively. Next, the band at 70-100 kDa minor allergen was found to be identical to hemocyanin from Scylla serrata, which corresponded to 12% sequence coverage. The identified proteins were illustrated in Figure 2D. We observed another band at the highest molecular weight > 100 kDa, which was identified as arginine kinase.
Association of IgE-binding proteins with clinical relevance
Patients A3, A5, A6, and A9 showed strong reactivity to the proteins at 70-100 kDa by immunoblotting. We found that these patients had higher levels of total IgE, and specific IgE levels to crab compared to the remaining subjects (4807.80 ± 1276.52 vs. 2421.50 ± 1039.98; 12.44 ± 18.02 vs. 2.72 ± 4.09, respectively). However, no significant differences were observed between groups. Regarding clinical manifestations, there were no specific clinical symptoms (Supplementary Table), yet two patients, A5 and A6, displayed various symptoms.
Discussion
Vietnam has an extensive coastline and abundant seafood resources, making it one of the leading seafood exporters in Asia21. However, little is known about the characteristics of shellfish allergens among local fishery products. This study identified the major mud allergens of the crab S. paramamosain among subjects with crab allergies, including HM, AK, TIM, and SCP. These allergens may play a role in clinical reactions and sensitization to arthropod allergens in Vietnamese crab-allergic subjects.
Patients with CA exhibited various symptoms after crab consumption, with rash and urticaria predominating. This finding was similar to a survey conducted among those with Vietnamese crustacean allergies, which showed that cutaneous symptoms were the most common. Cross-reactivity between house dust mites and crab was noted, as a portion of CT subjects did not have any reactions after crab consumption, yet their SPT remained positive for crab extracts. Cross-reactivity between arthropod allergens and crustacean allergens is well demonstrated. Tropomyosin (~38 kDa) is a pan-allergen in house dust mites and cockroaches, which have a high sequence homology to shellfish tropomyosin22. Tropomyosin is identified in shrimp and coral swimmer crab Charybdis feriatus, blue swimming crab Portunus pelagicus, and mangrove crab Scylla tranquebarica23, 24, 25. In countries with hot and humid conditions, where house dust mite sensitization is prominent, exposure to HDM tropomyosin can be the primary sensitizer for shellfish allergy22. Regarding shrimp allergy, tropomyosin is no longer an accurate marker allergen due to the fact that there are additional shrimp allergens, such as AK, myosin light chain, SCP, HM, etc.26. However, the diagnostic role of tropomyosin remains vague for crab allergy.
Depending on IgE-binding frequency, an allergen can be classified as major (>50% IgE-binding) or minor (<50% IgE-binding)27. In this study, sera from CA patients mostly reacted to protein bands from 70-100 kDa, suggesting another major allergen rather than tropomyosin, which is identical to HM from S. serrata. HM (60-80 kDa) is a sizeable copper-containing glycoprotein in the hemolymph of various arthropods and mollusks28. HM was considered a heat-stable, highly conserved protein. However, its physicochemical properties remain unclear28, 29, 30. Evidence has accumulated on the critical role of HM as a crustacean allergen, especially for crabs. HM exerts antimicrobial properties and is a necessary respiratory protein for invertebrates29. Interestingly, HM was considered a minor allergen in the Chinese mitten crab Eriocheir sinensis28 and a major non-cross-reactive allergen in the giant freshwater prawn Macrobrachium rosenbergii30, 31. A study from Malaysia by Jasim et al. also discovered the presence of HM in Scylla tranquebarica as an allergenic protein among crab-allergic subjects23. Sensitization to HM contributed to occupational crab allergy among crab processing workers32. HM can be a potential allergenic protein for crab allergy in Viet Nam.
Other minor allergens of S. paramamosain were revealed, including AK, SCP, and TIM. These proteins were found in various shrimps and crabs17, 23, 24, 25, 31, 32, 33. AK is a minor allergen detected in raw shrimp but a major allergen in the blue swimming crab Portunus pelagicus24. Its function is to regulate the ATP level in the muscles of invertebrates34. Besides tropomyosin, AK is a cross-reactive allergen in crustaceans and arthropods24, 26, 31.
Notably, AK was detected in bands of higher molecular weights, indicating the binding of IgE to oligomers. A tetrameric AK of 150 kDa was found exceptionally in some marine worms35. SCP is an EF-hand-type Ca2+ binding protein, previously identified as an allergen in Penaeus monodon (Pen m 4), Crangon crangon (Cra c 4), Litopenaeus vannamei (Lit v 4) and also in mud crab S. paramamosain36, 37, 38. Lastly, current evidence showed that TIM is a novel allergen of crab and exerts a Th2 skewed reaction in Balb/c mice39. TIM is discovered in the octopus Octopus fangsiao, which may contribute to cross-reactions between crustaceans and mollusks40. While AK was suggestive of occupational crab allergy among workers, the clinical role of SCP and TIM remains limited and needs further evaluation.
Compared to commercial IgE tests, such as specific IgE and SPT, commercial allergens may contain cross-reactive allergens or exclude some components depending on the purification method. In our study, the specific IgE to crab strongly correlated with SPT to house dust mites (DP, DF), while there was no correlation between SPT to crab and specific IgE to crab. These findings reinforced the role of molecular allergen and component-resolved diagnosis in the precise diagnosis of crab allergy. Thus, understanding the allergen components of local crab species is mandatory for component-resolved diagnosis.
A major limitation of this study was the small sample size. Therefore, the findings would be applicable to patients in our center and cannot be generalized to the Vietnamese population. Secondly, several phenotypes in food allergy have been described41. In this study, we investigated the allergens involved in IgE-mediated seafood allergy with immediate reactions after food exposure. With regard to the mixed IgE- and non-IgE mediated seafood allergy, further studies are necessary to evaluate the profile of associated allergens.
Conclusions
In conclusion, we illustrated the Vietnamese local crab's major (HM) and minor allergens (AK, SCP, TIM) among Vietnamese individuals sensitized to house dust mites. These findings contribute to the characterization of molecular allergens in seafood in Viet Nam, creating a platform for component-resolved diagnosis and precision medicine. Future studies are necessary to evaluate these proteins’ clinical reactivity and diagnostic applications.
Abbreviations
ACN: Acetonitrile, AK: Arginine Kinase, ATP: Adenosine Triphosphate, CA: Crab Allergic, CT: Crab Tolerant, DF: Dermatophagoides farinae, DP: Dermatophagoides pteronyssinus, HM: Hemocyanin, HRP: Horseradish Peroxidase, IgE: Immunoglobulin, MS: Mass Spectrometry, MS/MS: Tandem Mass Spectrometry, MW: Molecular Weight, nano-LC/MS: Nanoscale Liquid Chromatography/Tandem Mass Spectrometry, SCP: Sarcoplasmic Calcium-Binding Protein, SE: Standard Error, SPT: Skin Prick Test, TIM: Triosephosphate Isomerase
Acknowledgments
None.
Author’s contributions
Hoang Kim Tu Trinh drafted the study protocol, contributed to writing and supervised the whole process. Kieu-Minh Le performed experiments, data analysis and drafted the manuscript. Thanh Niem Vo Van performed the experiment and contributed to writing. Duy Le Pham, and Hieu Thao Nguyen recruited patients and contributed to writing. Minh Nguyet Tran Thi, Bao Yen Pham, Dinh Minh Pham conducted experiments for protein identification and contributed to writing. All authors read and approved the final manuscript.
Funding
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 108.02-2020.17.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The University of Medicine and Pharmacy Ethics Committee at Ho Chi Minh City approved this study (no: 1105/HĐĐĐ-ĐHYD, on 9th November 2023).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Tang
M.L.,
Mullins
R.J.,
Food allergy: is prevalence increasing?. Internal Medicine Journal.
2017;
47
(3)
:
256-61
.
View Article PubMed Google Scholar -
Warren
C.M.,
Jiang
J.,
Gupta
R.S.,
Epidemiology and burden of food allergy. Current Allergy and Asthma Reports.
2020;
20
(2)
:
6
.
View Article PubMed Google Scholar -
Tham
E.H.,
Leung
D.Y.,
How different parts of the world provide new insights into food allergy. Allergy, Asthma & Immunology Research.
2018;
10
(4)
:
290-9
.
View Article PubMed Google Scholar -
Le
T.T.,
Nguyen
D.H.,
Vu
A.T.,
Ruethers
T.,
Taki
A.C.,
Lopata
A.L.,
A cross-sectional, population-based study on the prevalence of food allergies among children in two different socio-economic regions of Vietnam. Pediatric Allergy and Immunology.
2019;
30
(3)
:
348-55
.
View Article PubMed Google Scholar -
Le
T.T.,
Tran
T.T.,
Ho
H.T.,
Vu
A.T.,
Lopata
A.L.,
Prevalence of food allergy in Vietnam: comparison of web-based with traditional paper-based survey. The World Allergy Organization Journal.
2018;
11
(1)
:
16
.
View Article PubMed Google Scholar -
Le Vay
L.,
Ecology and management of mud crab Scylla spp. Asian Fisheries Science.
2001;
14
(2)
:
101-12
.
View Article Google Scholar -
Ng
P.K.,
Guinot
D.,
Davie
P.J.,
Systema Brachyurorum: part I. An annotated checklist of extant brachyuran crabs of the world. Raffles Bulletin of Zoology.
2008;
17
(1)
:
1-286
.
-
Cartier
A.,
Lehrer
S.B.,
Horth-Susin
L.,
Swanson
M.,
Neis
B.,
Howse
D.,
Prevalence of crab asthma in crab plant workers in Newfoundland and Labrador. International journal of circumpolar health.
2004;
63
(sup2)
:
333-6
.
View Article Google Scholar -
Gautrin
D.,
Cartier
A.,
Howse
D.,
Horth-Susin
L.,
Jong
M.,
Swanson
M.,
Occupational asthma and allergy in snow crab processing in Newfoundland and Labrador. Occupational and Environmental Medicine.
2010;
67
(1)
:
17-23
.
View Article PubMed Google Scholar -
Guo
R.,
Wang
L.,
Yuan
X.P.,
Sun
P.,
Skin prick testing to identify food allergens in 8393 children and adolescents with asthma in Chongqing, Southwest China. Medical Science Monitor.
2019;
25
:
8221-9
.
View Article PubMed Google Scholar -
Thong
B.Y.,
Arulanandam
S.,
Tan
S.C.,
Tan
T.C.,
Chan
G.Y.,
Tan
J.W.,
Shellfish/crustacean oral allergy syndrome among national service pre-enlistees in Singapore. Asia Pacific Allergy.
2018;
8
(2)
:
e18
.
View Article PubMed Google Scholar -
Statista Research Department. Crab export value Vietnam 2022, by trade partner 2023. Available from: https://www.statista.com/statistics/1331068/vietnam-crab-export-value-by-trade-partner/..
.
-
Stone
K.D.,
Prussin
C.,
Metcalfe
D.D.,
IgE, mast cells, basophils, and eosinophils. The Journal of Allergy and Clinical Immunology.
2010;
125
(2)
:
73-80
.
View Article PubMed Google Scholar -
Ruethers
T.,
Taki
A.C.,
Johnston
E.B.,
Nugraha
R.,
Le
T.T.,
Kalic
T.,
Seafood allergy: A comprehensive review of fish and shellfish allergens. Molecular Immunology.
2018;
100
:
28-57
.
View Article PubMed Google Scholar -
Chapman
M.D.,
Allergen nomenclature. Allergens and allergen immunotherapy.
2008;
2008
:
65-76
.
-
Kobayashi
A.,
Tanaka
H.,
Hamada
Y.,
Ishizaki
S.,
Nagashima
Y.,
Shiomi
K.,
Comparison of allergenicity and allergens between fish white and dark muscles. Allergy.
2006;
61
(3)
:
357-63
.
View Article PubMed Google Scholar -
Kuehn
A.,
Hilger
C.,
Lehners-Weber
C.,
Codreanu-Morel
F.,
Morisset
M.,
Metz-Favre
C.,
Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: component resolved diagnosis using parvalbumin and the new allergens. Clinical and Experimental Allergy.
2013;
43
(7)
:
811-22
.
View Article PubMed Google Scholar -
Hemmer-Hansen
J.,
Therkildsen
N.O.,
Pujolar
J.M.,
Population genomics of marine fishes: next-generation prospects and challenges. The Biological Bulletin.
2014;
227
(2)
:
117-32
.
View Article PubMed Google Scholar -
van Kampen
V.,
de Blay
F.,
Folletti
I.,
Kobierski
P.,
Moscato
G.,
Olivieri
M.,
EAACI position paper: skin prick testing in the diagnosis of occupational type I allergies. Allergy.
2013;
68
(5)
:
580-4
.
View Article PubMed Google Scholar -
N.Q.N. Nguyen,
Le
K.M.,
H.T. Nguyen,
B.T. Duong,
Pham
L.D.,
H.K.T. Trinh,
The Application of Basophil Activation Test in Seafood Allergy Diagnosis. The Application of Basophil Activation Test in Seafood Allergy Diagnosis: The Preliminary Results. International Conference on the Development of Biomedical Engineering in Vietnam; 2022: Springer.
2022
.
View Article Google Scholar -
Agriculture Organization of the United Nations. Fisheries Department. The State of World Fisheries and Aquaculture, 2000. Food & Agriculture Org.; 2000.
.
-
Wong
L.,
Huang
C.H.,
Lee
B.W.,
Shellfish and House Dust Mite Allergies: Is the Link Tropomyosin?. Allergy, Asthma & Immunology Research.
2016;
8
(2)
:
101-6
.
View Article PubMed Google Scholar -
Jasim
H.A.,
Misnan
R.,
Yadzir
Z.H.,
Abdullah
N.,
Bakhtiar
F.,
Arip
M.,
Identification of common and novel major crab allergens in Scylla tranquebarica and the allergen stability in untreated and vinegar-treated crab. Iranian Journal of Allergy, Asthma, and Immunology.
2021;
20
(1)
:
76-87
.
View Article PubMed Google Scholar -
Rosmilah
M.,
Shahnaz
M.,
Zailatul
H.M.,
Noormalin
A.,
Normilah
I.,
Identification of tropomyosin and arginine kinase as major allergens of Portunus pelagicus (blue swimming crab). Tropical Biomedicine.
2012;
29
(3)
:
467-78
.
PubMed Google Scholar -
Leung
P.S.,
Chen
Y.C.,
Gershwin
M.E.,
Wong
S.H.,
Kwan
H.S.,
Chu
K.H.,
Identification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergen. The Journal of Allergy and Clinical Immunology.
1998;
102
(5)
:
847-52
.
View Article PubMed Google Scholar -
Grilo
J.,
Vollmann
U.,
Aumayr
M.,
Sturm
G.J.,
Bohle
B.,
Tropomyosin is no accurate marker allergen for diagnosis of shrimp allergy in Central Europe. Allergy.
2022;
77
(6)
:
1921-3
.
View Article PubMed Google Scholar -
Lowenstein
H.,
Quantitative immunoelectrophoretic methods as a tool for the analysis and isolation of allergens. Progress in Allergy.
1978;
25
:
1-62
.
View Article Google Scholar -
Lu
J.,
Luan
H.,
Wang
C.,
Zhang
L.,
Shi
W.,
Xu
S.,
Molecular and allergenic properties of natural hemocyanin from Chinese mitten crab (Eriocheir sinensis). Food Chemistry.
2023;
424
:
136422
.
View Article PubMed Google Scholar -
Zhang
Y.L.,
Peng
B.,
Li
H.,
Yan
F.,
Wu
H.K.,
Zhao
X.L.,
C-terminal domain of hemocyanin, a major antimicrobial protein from Litopenaeus vannamei: structural homology with immunoglobulins and molecular diversity. Frontiers in Immunology.
2017;
8
:
611
.
View Article PubMed Google Scholar -
Piboonpocanun
S.,
Jirapongsananuruk
O.,
Tipayanon
T.,
Boonchoo
S.,
Goodman
R.E.,
Identification of hemocyanin as a novel non-cross-reactive allergen from the giant freshwater shrimp Macrobrachium rosenbergii. Molecular Nutrition {&}amp; Food Research.
2011;
55
(10)
:
1492-8
.
View Article PubMed Google Scholar -
Srinroch
C.,
Srisomsap
C.,
Chokchaichamnankit
D.,
Punyarit
P.,
Phiriyangkul
P.,
Identification of novel allergen in edible insect, Gryllus bimaculatus and its cross-reactivity with Macrobrachium spp. allergens. Food Chemistry.
2015;
184
:
160-6
.
View Article PubMed Google Scholar -
Thomassen
M.R.,
Kamath
S.D.,
Bang
B.E.,
Nugraha
R.,
Nie
S.,
Williamson
N.A.,
Occupational allergic sensitization among workers processing king crab (Paralithodes camtschaticus) and edible crab (Cancer pagurus) in Norway and identification of novel putative allergenic proteins. Frontiers in Allergy.
2021;
2
:
718824
.
View Article PubMed Google Scholar -
Abramovitch
J.B.,
Kamath
S.,
Varese
N.,
Zubrinich
C.,
Lopata
A.L.,
O'Hehir
R.E.,
IgE reactivity of blue swimmer crab (Portunus pelagicus) Tropomyosin, Por p 1, and other allergens; cross-reactivity with black tiger prawn and effects of heating. PLoS One.
2013;
8
(6)
:
e67487
.
View Article PubMed Google Scholar -
Yao
C.L.,
Ji
P.F.,
Kong
P.,
Wang
Z.Y.,
Xiang
J.H.,
Arginine kinase from Litopenaeus vannamei: cloning, expression and catalytic properties. Fish & Shellfish Immunology.
2009;
26
(3)
:
553-8
.
View Article PubMed Google Scholar -
Robin
Y.,
Phosphagens and molecular evolution in worms. Bio Systems.
1974;
6
(1)
:
49-56
.
View Article PubMed Google Scholar -
Shiomi
K.,
Sato
Y.,
Hamamoto
S.,
Mita
H.,
Shimakura
K.,
Sarcoplasmic calcium-binding protein: identification as a new allergen of the black tiger shrimp Penaeus monodon. International Archives of Allergy and Immunology.
2008;
146
(2)
:
91-8
.
View Article PubMed Google Scholar -
Ayuso
R.,
Grishina
G.,
Ibáñez
M.D.,
Blanco
C.,
Carrillo
T.,
Bencharitiwong
R.,
Sarcoplasmic calcium-binding protein is an EF-hand-type protein identified as a new shrimp allergen. The Journal of Allergy and Clinical Immunology.
2009;
124
(1)
:
114-20
.
View Article PubMed Google Scholar -
Bauermeister
K.,
Wangorsch
A.,
Garoffo
L.P.,
Reuter
A.,
Conti
A.,
Taylor
S.L.,
Generation of a comprehensive panel of crustacean allergens from the North Sea Shrimp Crangon crangon. Molecular Immunology.
2011;
48
(15-16)
:
1983-92
.
View Article PubMed Google Scholar -
Yang
Y.,
He
X.R.,
He
S.G.,
Liu
M.,
Zhang
Y.X.,
Xia
F.,
Two allergens from Scylla paramamosain share common epitopes showed different allergenic potential in Balb/c mice. Food Chemistry.
2022;
371
:
131132
.
View Article PubMed Google Scholar -
Yang
Y.,
Chen
Z.W.,
Hurlburt
B.K.,
Li
G.L.,
Zhang
Y.X.,
Fei
D.X.,
Identification of triosephosphate isomerase as a novel allergen in Octopus fangsiao. Molecular Immunology.
2017;
85
:
35-46
.
View Article PubMed Google Scholar -
Eapen
A.A.,
Kim
H.,
The phenotype of the food-allergic patient. Immunology and Allergy Clinics of North America.
2021;
41
(2)
:
165-75
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 11 No 11 (2024)
Page No.: 6891-6899
Published on: 2024-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 2917 times
- PDF downloaded - 585 times
- XML downloaded - 72 times
- Supplement downloaded - 428 times
 Biomedpress
Biomedpress




