Abstract
Objective: Inflammation plays a pivotal role in the pathogenesis of stroke. However, the proteins that initiate inflammatory responses remain unclear. In this study, we utilize proteomics to identify the core protein in acute ischemic stroke (AIS) patients and verify the relationship of the protein with NOD-like receptor protein 3 (NLRP3).
Methods: Peripheral blood from AIS patients and patients without AIS was collected and analyzed using a quantitative proteomic method (label-free) to screen for differential proteins. Subsequently, the differential protein was validated by ELISA. Additionally, a middle cerebral artery occlusion (MCAO) model was utilized to explore the relationship between mannose-binding lectin 2 (MBL2) and NLRP3. Co-immunoprecipitation (Co-IP) and western blot were also used to validate the interaction between the proteins and NLRP3.
Results: A total of ten AIS patients and controls were enrolled in the proteomics study. Compared with the control group, a total of 49 proteins were identified as potential proteins. Among these proteins, MBL2 was notably increased in the AIS group and selected for further analysis. Subsequent ELISA analysis confirmed a significant elevation of MBL2 levels in stroke patients (P < 0.001). Further, in animal studies, Co-IP assays showed an interaction between MBL2 and NLRP3 proteins in cerebral tissue after ischemic infarction. Western blot results demonstrated that the expression levels of NLRP3 and MBL2 were significantly increased in MCAO rats.
Conclusions: Our results suggest that MBL2 may be one of the promoters of inflammation by interacting with NLRP3 in AIS patients.
Introduction
Stroke, characterized as a neurological deficit of cerebrovascular cause, is one of the leading causes of death and neurological disorders worldwide. As a chronic non-communicable disease, stroke poses a substantial threat to public health. In addition to the heavy social and economic burden, the sequelae and complications of physical disorders caused by ischemic stroke seriously affect the quality of life of patients1, 2. Early intervention is crucial for stroke patients; however, effective targets for intervention remain elusive.
Inflammation plays a pivotal role in the pathogenesis of stroke, promoting neuronal death and inhibiting nerve tissue regeneration. Mediators of inflammation have long been investigated in stroke patients. The NOD-like receptor protein 3 (NLRP3) inflammasome is a multi-protein signaling complex integral to the chronic inflammatory response. It has been reported that the expression of the NLRP3 inflammasome (including constituent proteins NLRP3, ASC, and pro-Caspase-1), pro-IL-1β, and pro-IL-18 increases dramatically in neurons, astrocytes, and microglia in the ischemic core area of cerebral infarction3. The NLRP3 inflammasomes can cause the release of IL-1β and IL-18 after activating Caspase-1, triggering a series of inflammatory cascades and mediating the occurrence of ischemic stroke4. Nevertheless, the specific mechanism is still not quite clear.
Proteomics has been playing an important role in biomedical research in recent years. Through proteomics, the occurrence and development of diseases can be comprehensively explained5. By using proteomics, Lee et al. reported four coagulation cascade proteins showed higher expression levels in patients with stroke, and they speculate these blood coagulation proteins may help in diagnosing stroke more accurately and quickly6. Recently, the use of proteomics in human studies investigating stroke has been increasing. Plasma proteins that serve as biomarkers have been identified as changed in various diseases; however, the interpretation of the protein is challenging7, 8. In this study, we employed a quantitative proteomics approach to detect the differential expression of proteins in the peripheral blood of acute ischemic stroke (AIS) patients and identified the relationship of the target protein with the inflammation process during AIS.
Methods
Recruitment of Ischemic Stroke Patients
A total of 20 patients were enrolled in our study. Of them, 10 patients were diagnosed with AIS, and another 10 patients without AIS were enrolled as the control group. The average age of ischemic stroke patients was 62.7 ± 6.98 years, and that of the control patients was 59.7 ± 7.15 years. The peripheral blood (plasma) of patients was collected and analyzed using a quantitative proteomic method (Label-free). All patients signed informed consent forms, and this study was approved by the ethics committee of the Beijing Tiantan Hospital.
Experimental Animals
Male SD rats with an initial weight of 220 ± 10g were purchased from Beijing Weitonglihua Experimental Animal Technology Co., LTD. Experimental animal production license No.: SCXK (Beijing) 2021 - 0006. All rats were fed in separate cages at (22 ± 2) °C, with relative humidity at (45 ± 5) % and a 12-hour light cycle in the Laboratory Animal Center of Capital Medical University. All animal experiments were approved by the Ethics Committee of Capital Medical University.
Proteomic Detection
The proteins in the blood samples were extracted and quantified. A total of 50 μg of protein was taken for pre-processing. All samples were examined by Label-free protein mass spectrometry using Orbitrap Fusion Nanoscale reverse-phase chromatography (Thermo Fisher Scientific, model: Orbitrap Fusion). The original mass spectrometry files were processed by MaxQuant software. The differential proteins were analyzed using cluster volcano maps and heat map. Gene Ontology Resource (https://geneontology.org/) and KEGG database (Kyoto Encyclopedia of Genes and Genomes, https://www.genome.jp/kegg/) were used for protein function annotation and pathway analysis. Cytoscape was used to map the protein interaction network.
Detection of Mannan-binding Lectin-2 (MBL2) Level by ELISA
Blood samples were diluted 200 times prior to the experiment. Subsequently, the diluted samples were incubated overnight at 4°C on an ELISA Assay Plate (Invitrogen product No. EHMBL2, 96 tests). Following incubation, the plate was washed four times with 1X washing buffer. Next, 100 μl of biotin was added to each well and incubated at room temperature for 1 hour. The supernatant was then discarded, and the plate was washed four times with 300 μl of 1X washing buffer. Subsequently, a diluted HRP solution was added to each well and incubated at room temperature for 45 minutes. Following another four washes with 1X washing buffer, 100 μl of TMB substrate was added to each well and incubated at room temperature for 30 minutes. Finally, the reaction was terminated by adding 50 μl of termination solution to each well, and the absorbance was measured using a Thermo Fisher Scientific Multiskan FC plate reader.
Animal Model
The MCAO model was established as follows: rats were randomly divided into control group and model group. After anesthesia, the common carotid, external carotid, and internal carotid arteries were separated by a glass minute hand. The common carotid artery and external carotid artery were ligated; the internal carotid artery was temporarily closed using an artery clamp, and then the common carotid artery was cut at an oblique angle. The artery clamp was removed after the thread plug was placed into the neck, and the blood vessel was closed. The wound was sutured, and rats were placed on a heating blanket to recover. Upon awakening, successful establishment of the MCAO model was confirmed if the rats exhibited an unsteady gait, left limb paralysis, and circling behavior when the tail was lifted (N = 3). The rats in the control group only had their blood vessels exposed and their skin sutured without any treatment. After cerebral vascular occlusion for 24 hours, all rats were euthanized and the brains (with cerebral infarction) from each group were collected.
Co-immunoprecipitation (Co-IP) between MBL2 and NLRP3
A total of 15mg of brain tissue was extracted and homogenized on ice for 1 hour. After that, the samples were centrifuged at 12000 rpm at 4℃ for 10 minutes, and the supernatant was retained to obtain the total protein. The interaction between MBL2 and NLRP3 was tested using the Thermo Scientific Pierce™ Classic IP Kit (item 26146).
Western Blot Analysis
Approximately 0.5 g of rat brain tissues with cerebral infarction area were added to RIPA lysis buffer containing 1% protease inhibitor. The total protein content was measured by the BCA method. The proteins were initially separated using an SDS-PAGE gel and then transferred onto a PVDF membrane. The membrane was incubated with 5% skim milk at room temperature for 1 hour, then the membrane was subsequently exposed to the following antibodies: MBL2 (Invitrogen, NO.: PA5-106674) and NLRP3 antibody (Abcam Corporation, No.: ab263899). Incubate overnight in a shaker at 4℃. The next day, after washing the membrane with TBST three times, the corresponding secondary antibody was incubated for 1 hour at room temperature. Finally, chemiluminescence was performed using an ECL luminescent reagent.
Statistical Methods
Statistical analysis was performed using SPSS 22.0 software (IBM, Armonk, NY, USA). Group comparisons were assessed using Student’s T-test, with statistical significance defined as P < 0.05. Proteins identified through proteomics were considered significantly different if they exhibited a fold change ≥ 1.2 or ≤ 0.83, with P < 0.05 compared to the control group. Gene Ontology (GO) annotation was utilized to characterize molecular functions, cellular components, and biological processes associated with the identified proteins. Additionally, KEGG pathway analysis was employed to investigate the signaling pathways involving these differential proteins.
Results
Proteomic results
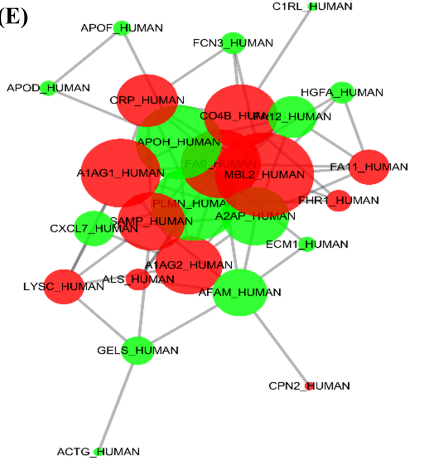
The samples were analyzed using a quantitative proteomic label-free method. Forty-nine proteins were found to have significant differences (fold change ≥1.2, P < 0.05) in patients with ischemic stroke. Of these differential proteins, 15 were up-regulated, and 34 were down-regulated (Figure 1 A). The differential proteins between AIS patients and controls were clustered using a heatmap (Figure 1 B). GO analysis results show that the most enriched GO terms mainly focused on acute-phase response and inflammatory response (Figure 1 C). These proteins are involved in multiple pathways such as complement system activation and cell defense (Figure 1 D). Additionally, PPI analysis of differential proteins identified MBL2 as a protein for further study (Figure 1 E).
The expression of MBL2 level detected by ELISA
To validate the findings from proteomics, we utilized ELISA to analyze Mannose-binding lectin 2 (MBL2) levels in peripheral blood samples from patients with acute ischemic stroke. There were 22 patients in each group. The ELISA results showed that the MBL2 protein level in the AIS group increased significantly (P < 0.01) (Figure 2).
MBL2 and NLRP3 Co-IP results
To further analyze the relationship between acute ischemic stroke and MBL2 elevation, we investigated the relationship between MBL2 and NLRP3 in the MCAO model using Co-IP. The Co-IP results showed some interaction between the MBL2 protein and the NLRP3 protein (Figure 3).
The Expression of NLRP3 and MBL2 in brain tissue detected by Western blot
Western blot results showed that the expressions of NLRP3 (p < 0.01) and MBL2 were significantly increased (p < 0.001) in the early stage of cerebral infarction (Figure 4).
Discussion
Ischemic stroke is characterized by the occlusion of brain tissue blood supply, leading to ischemia, hypoxic necrosis, and subsequent neurological deficits9. It is the most common type of stroke, accounting for about 70-80% of all stroke types, and is characterized by high mortality and disability10. In this study, we observed a significant increase in MBL2 levels in patients with acute ischemic stroke. Subsequently, we investigated the association between MBL2 and NLRP3 in an animal model. Based on these findings, we hypothesize that elevated MBL2 levels early in acute ischemic stroke may interact with NLRP3, initiating inflammation in the infarcted brain tissue and ultimately resulting in neuronal death. To our knowledge, this is the first study to address the relationship between MBL2 and NLRP3 in acute ischemic stroke.
The MBL2 gene encodes a 24 kDa polypeptide characterized by a 248-amino-acid sequence, first identified by Taylor and Sastry in 198911. Alvaro Cervera et al. highlighted significant associations between MBL2 polymorphisms and adverse outcomes at 3 months post-stroke. Their study found that patients with mutations in the MBL2 gene locus are linked to lower levels of C3, C4, CRP, and favorable outcomes12. Attila Szabo et al. conducted a study on 82 patients who underwent carotid endarterectomy and found that the incidence of restenosis after endarterectomy was closely related to the MBL2 genotype. The restenosis rate in wild-type homozygous MBL2 patients was significantly higher than in MBL2 mutant patients13. In this study, we also found that MBL2 levels are significantly increased both through proteomics and ELISA. The reasons are still not understood.
As a highly conserved acute-phase protein, MBL2 belongs to the C-type lectin superfamily. It is the most important first-line inflammatory immune molecule in host non-specific immunity, capable of selectively recognizing the sugar structure of various pathogens and activating the complement system14. Under stress, the concentration of MBL2 increases sharply in blood and local tissues, which can regulate the acquired immune response and local inflammatory response15. It has been confirmed that complement activation plays a key role in the pathogenesis of cerebrovascular diseases16. Shen et al. found that serum MBL and inflammation-related factor levels in the serum were significantly increased 3 days after surgery in patients with aneurysmal subarachnoid hemorrhage who underwent interventional embolization17. Additionally, Neglia demonstrated that MBL can exert a direct deleterious effect on ischemic brain endothelial cells18. Furthermore, a cohort involving 7588 patients with type 2 diabetes highlighted that serum MBL level was a major risk factor for cardiovascular disease19.
The lack of oxygen, glucose, and lipid supply in the brain tissue leads to the death of neurons around the infarction site. Effective strategies to mitigate early neuronal death following cerebral ischemia remain a major focus and challenge in clinical practice19. The complement system, primarily synthesized in the liver, can enter the brain through the disrupted blood-cerebrospinal fluid barrier. As the main effector of innate immunity, complement activation can lead to an inflammatory response and have harmful effects on the nervous system20, 21, 22. Studies by Liang et al. have demonstrated that the concentrations of C3 and CRP in acute ischemic stroke patients were significantly higher than those of healthy control groups up to 14 days after disease onset23. Complement activation can occur via various pathways, including the bypass activation pathway and the lectin pathway. The lectin pathway is initiated by MBL and MBL-associated serine protease (MASP) and may play a vital role in ischemia-reperfusion injury in patients with acute ischemic stroke14. By binding to sugar groups on the cell surface, MBL activates the lectin-complement pathway, promoting the agglutination and clearance of pathogens by phagocytes, thereby protecting the host from invasion for a short period. In the case of tissue injury, MBL is rapidly deposited on target cells and triggers downstream complement activation during the acute phase, thus enhancing C3 cleavage24.
Excessive activation of MBL2 can lead to an imbalanced pro-inflammatory response, highlighting its potential harmful effects. Although the clinical impact of MBL has been extensively studied, the mechanism by which it promotes inflammation is still not clear. In this study, we focused on the relationship between MBL2 and NLRP3 and found an interaction between these two proteins. NLRP3 is an intracellular sensor that can detect endogenous danger signals and environmental irritants, resulting in the formation and activation of the NLRP3 inflammasome. Assembly of the NLRP3 inflammasome leads to the activation of pro-inflammatory cytokines IL-1β and IL-18, which were also observed in the peripheral blood of stroke patients25, 26. We speculate that this may be one reason for the increased inflammation level at the onset of acute ischemic stroke.
Although we found increased MBL2 levels in the peripheral blood of AIS patients and identified the relationship between MBL2 and NLRP3, there are still some limitations that should not be ignored. Firstly, the samples for proteomics were from peripheral blood, which cannot reflect changes in MBL2 intracranially. Cerebrospinal fluid should be used for further analysis. Secondly, the sample for verifying the proteomic results is too small, especially for a stroke with high morbidity. The result needs to be further examined in a larger cohort. Thirdly, because the inflammation process is reported to be associated with many proteins and cytokines, we only observed the relationship between MBL2 and NLRP3. In addition, more studies have found the important role of sirtuins in neuronal cells following stroke27. However, the increase in MBL2 level with sirtuins is still not well understood and worth further exploration. Further in-depth screening with other inflammatory proteins and cytokines is needed.
Conclusions
In this study, using proteomics methods, we found that the MBL2 level increased dramatically in AIS patients. Our results may pave a path for identifying clinically relevant biomarkers specific to AIS and could further help to improve the therapeutic outcomes of AIS. Further analysis revealed that the increased MBL2 level may interact with NLRP3 to promote inflammation. Nevertheless, our study is a tentative exploration; whether MBL2 could be used as a prognostic biomarker for monitoring patients still needs to be verified in a large cohort study. The specific mechanism of MBL2 in neurodegeneration also warrants further exploration.
Abbreviations
AIS: Acute ischemic stroke, ASC: Apoptosis-associated speck-like protein containing a CARD, C-type lectin: Calcium-dependent carbohydrate-binding protein, C3: Complement component 3, C4: Complement component 4, Co-IP: Co-immunoprecipitation, CRP: C-reactive protein, ECL: Enhanced chemiluminescence, ELISA: Enzyme-linked immunosorbent assay, GO: Gene Ontology, HRP: Horseradish peroxidase, IL-1β: Interleukin 1 beta, IL-18: Interleukin 18, KEGG: Kyoto Encyclopedia of Genes and Genomes, MBL2: Mannose-binding lectin 2, MCAO: Middle cerebral artery occlusion, NLRP3: NOD-like receptor protein 3, PVDF: Polyvinylidene fluoride, RIPA: Radioimmunoprecipitation assay, SD rats: Sprague-Dawley rats, SPSS: Statistical Package for the Social Sciences, TBST: Tris-buffered saline with Tween 20, TMB: Tetramethylbenzidine
Acknowledgments
None.
Author’s contributions
BZ: Conceptualization, Methodology, Writing-Original draft preparation; XCL, YT, HY, MFW, SQW: Methodology, Data curation; BW, ZZG: Validation, Supervision,Writing-review & editing. All authors read and approved the final manuscript.
Funding
This study was supported by the Beijing Hospitals Authority Youth Programme, (Grant Number: QML20230512); the Training Fund for Open Projects at Clinical Institutes and Departments of Capital Medical University (Grant Number: CCMU2022ZKYXZ010).
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Ding
Q.,
Liu
S.,
Yao
Y.,
Liu
H.,
Cai
T.,
Han
L.,
Global, Regional, and National Burden of Ischemic Stroke, 1990-2019. Neurology.
2022;
98
(3)
:
e279-90
.
View Article PubMed Google Scholar -
Zhang
X.,
Lv
H.,
Chen
X.,
Li
M.,
Zhou
X.,
Jia
X.,
Analysis of ischemic stroke burden in Asia from 1990 to 2019: based on the global burden of disease 2019 data. Frontiers in Neurology.
2023;
14
:
1309931
.
View Article PubMed Google Scholar -
Poh
L.,
Kang
S.W.,
Baik
S.H.,
Ng
G.Y.,
She
D.T.,
Balaganapathy
P.,
Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain, Behavior, and Immunity.
2019;
75
:
34-47
.
View Article PubMed Google Scholar -
Coll
R.C.,
Schroder
K.,
Pelegrín
P.,
NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends in Pharmacological Sciences.
2022;
43
(8)
:
653-68
.
View Article PubMed Google Scholar -
Hochrainer
K.,
Yang
W.,
Stroke Proteomics: From Discovery to Diagnostic and Therapeutic Applications. Circulation Research.
2022;
130
(8)
:
1145-66
.
View Article PubMed Google Scholar -
Lee
J.,
Mun
S.,
Park
A.,
Kim
D.,
Lee
Y.J.,
Kim
H.J.,
Proteomics Reveals Plasma Biomarkers for Ischemic Stroke Related to the Coagulation Cascade. Journal of Molecular Neuroscience.
2020;
70
(9)
:
1321-31
.
View Article PubMed Google Scholar -
Okada
D.,
Plasma proteins as potential biomarkers of aging of single tissue and cell type. Biogerontology.
2024;
25
(1)
:
177-81
.
View Article PubMed Google Scholar -
Tsakanova
G.,
Stepanyan
A.,
Steffensen
R.,
Soghoyan
A.,
Jensenius
J.C.,
Arakelyan
A.,
Pattern Recognition Molecules of Lectin Complement Pathway in Ischemic Stroke. Pharmacogenomics and Personalized Medicine.
2021;
14
:
1347-68
.
View Article PubMed Google Scholar -
Lin
W.,
Zhao
X.Y.,
Cheng
J.W.,
Li
L.T.,
Jiang
Q.,
Zhang
Y.X.,
Signaling pathways in brain ischemia: mechanisms and therapeutic implications. Pharmacology & Therapeutics.
2023;
251
:
108541
.
View Article PubMed Google Scholar -
Martin
S.S.,
Aday
A.W.,
Almarzooq
Z.I.,
Anderson
C.A.,
Arora
P.,
Avery
C.L.,
American Heart Association Council on Epidemiology
Prevention Statistics Committee
Stroke Statistics Subcommittee
2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation.
2024;
149
(8)
:
e347-913
.
View Article PubMed Google Scholar -
Auriti
C.,
Prencipe
G.,
Moriondo
M.,
Bersani
I.,
Bertaina
C.,
Mond\`\i
V.,
Mannose-Binding Lectin: Biologic Characteristics and Role in the Susceptibility to Infections and Ischemia-Reperfusion Related Injury in Critically Ill Neonates. Journal of Immunology Research.
2017;
2017
:
7045630
.
View Article PubMed Google Scholar -
Cervera
A.,
Planas
A.M.,
Justicia
C.,
Urra
X.,
Jensenius
J.C.,
Torres
F.,
Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS One.
2010;
5
(2)
:
e8433
.
View Article PubMed Google Scholar -
Szabó
A.,
Laki
J.,
Madsen
H.O.,
Dósa
E.,
Prohászka
Z.,
Rugonfalvi-Kiss
S.,
Early rise in serum VEGF and PDGF levels predisposes patients with a normal MBL2 genotype to restenosis after eversion endarterectomy. Stroke.
2007;
38
(8)
:
2247-53
.
View Article PubMed Google Scholar -
Osthoff
M.,
Katan
M.,
Fluri
F.,
Schuetz
P.,
Bingisser
R.,
Kappos
L.,
Mannose-binding lectin deficiency is associated with smaller infarction size and favorable outcome in ischemic stroke patients. PLoS One.
2011;
6
(6)
:
e21338
.
View Article PubMed Google Scholar -
Yu
S.,
Liu
M.,
Gu
X.,
Ding
F.,
Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cellular and Molecular Neurobiology.
2008;
28
(8)
:
1067-78
.
View Article PubMed Google Scholar -
Széplaki
G.,
Szegedi
R.,
Hirschberg
K.,
Gombos
T.,
Varga
L.,
Karádi
I.,
Strong complement activation after acute ischemic stroke is associated with unfavorable outcomes. Atherosclerosis.
2009;
204
(1)
:
315-20
.
View Article PubMed Google Scholar -
Chen
S.,
Wei
S.,
Yuanjie
Z.,
Zhirong
Y.,
Huayi
C.,
Relationship between serum MBL,HRG,IL-23/IL-17 inflammatory axis and cerebral vasospasm and prognosis in patients with aneurysmal subarachnoid hemorrhage after interventional embolization. International Journal of Laboratory Medicine.
2024;
45
:
134-40
.
-
Neglia
L.,
Fumagalli
S.,
Orsini
F.,
Zanetti
A.,
Perego
C.,
De Simoni
M.G.,
Mannose-binding lectin has a direct deleterious effect on ischemic brain microvascular endothelial cells. Journal of Cerebral Blood Flow and Metabolism.
2020;
40
(8)
:
1608-20
.
View Article PubMed Google Scholar -
Gedebjerg
A.,
Bjerre
M.,
Kjaergaard
A.D.,
Steffensen
R.,
Nielsen
J.S.,
Rungby
J.,
Mannose-Binding Lectin and Risk of Cardiovascular Events and Mortality in Type 2 Diabetes: A Danish Cohort Study. Diabetes Care.
2020;
43
(9)
:
2190-8
.
View Article PubMed Google Scholar -
Fisher
M.,
Savitz
S.I.,
Pharmacological brain cytoprotection in acute ischaemic stroke - renewed hope in the reperfusion era. Nature Reviews. Neurology.
2022;
18
(4)
:
193-202
.
View Article PubMed Google Scholar -
Zhao
Y.,
Zhang
X.,
Chen
X.,
Wei
Y.,
Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (Review). International Journal of Molecular Medicine.
2022;
49
(2)
:
49
.
PubMed Google Scholar -
Mastellos
D.C.,
Hajishengallis
G.,
Lambris
J.D.,
A guide to complement biology, pathology and therapeutic opportunity. Nature Reviews. Immunology.
2024;
24
(2)
:
118-41
.
View Article PubMed Google Scholar -
Lizhen
L.,
Changqing
L.,
Chunli
W.,
The Relationship between the Activation of Complement and Acute Ischemic Stork. Prevention and Treatment of Cardio-Cerebral-Vascular Disease.
2005;
5
:
20-2
.
-
Auriti
C.,
Prencipe
G.,
Caravale
B.,
Coletti
M.F.,
Ronchetti
M.P.,
Piersigilli
F.,
MBL2 gene polymorphisms increase the risk of adverse neurological outcome in preterm infants: a preliminary prospective study. Pediatric Research.
2014;
76
(5)
:
464-9
.
View Article PubMed Google Scholar -
Sun
R.,
Peng
M.,
Xu
P.,
Huang
F.,
Xie
Y.,
Li
J.,
Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. Journal of Neuroinflammation.
2020;
17
(1)
:
330
.
View Article PubMed Google Scholar -
Swanson
K.V.,
Deng
M.,
Ting
J.P.,
The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Reviews. Immunology.
2019;
19
(8)
:
477-89
.
View Article PubMed Google Scholar -
Azedi
F.,
Tavakol
S.,
Ketabforoush
A.H.,
Khazaei
G.,
Bakhtazad
A.,
Mousavizadeh
K.,
Modulation of autophagy by melatonin via sirtuins in stroke: from mechanisms to therapies. Life Sciences.
2022;
307
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 11 (2024)
Page No.: 6883-6890
Published on: 2024-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 1409 times
- PDF downloaded - 495 times
- XML downloaded - 125 times
 Biomedpress
Biomedpress





