Abstract
Introduction: Severe hemolytic disease of the fetus and newborn (HDFN) due to red cell alloimmunization is a frequent cause of recurrent fetal loss. This condition becomes critical when standard treatments like intrauterine transfusion (IUT) or immune-modulating therapies such as therapeutic plasma exchange (TPE) and intravenous immunoglobulin (IVIG) are unavailable or not administered in a timely manner. This case report emphasizes the potential benefits of early TPE and IVIG administration.
Case Presentation: A 28-year-old Rhesus D (RhD) negative woman (G3P2) experienced recurrent fetal loss due to severe HDFN and had a history of RhD alloimmunization and fetal loss in her second pregnancy. In her third pregnancy, high anti-D titers (1:2,024) were identified at weeks 12 and 16. Despite the need, TPE and IVIG were not administered properly due to the patient missing follow-ups and the absence of IUT facilities, culminating in macerated intrauterine death (IUD) at 27 weeks.
Discussion: The failure to implement early TPE and IVIG interventions, alongside systemic treatment inadequacies, contributed to the adverse pregnancy outcome. This case accentuates the necessity of accessible and timely intervention in pregnancies complicated by high anti-D titers.
Conclusion: For RhD alloimmunized pregnancies with high anti-D titers, early administration of TPE and IVIG before 20 weeks of gestation is crucial in reducing fetal morbidity and mortality. This case underscores the importance of early intervention and continuous monitoring in managing sensitized pregnancies.
Introduction
Maternal red blood cell (RBC) alloimmunization may lead to the production of harmful antibodies that cause hemolytic disease of the fetus and newborn (HDFN), resulting in fetal morbidity and mortality. Severe HDFN can lead to hydrops fetalis and significant jaundice, potentially resulting in kernicterus, permanent brain damage, or infant death. In contrast, mild HDFN typically causes only mild neonatal jaundice, which is often effectively treated with phototherapy alone1. Before the introduction of anti-D immunoglobulin (RhIG) in 1968, it was estimated that HDFN affected approximately 1% of all pregnancies worldwide, with anti-D being a major contributor to fetal morbidity and mortality2. Approximately 0.1 to 0.4% of pregnant women remain sensitized despite the implementation of RhIG, likely due to antigens other than RhD3.
The related morbidity and mortality of HDFN due to anti-D have significantly reduced after routine antenatal and postnatal RhIG prophylaxis. However, Rhesus D (RhD) alloimmunization still occurs due to failure in identifying the mother’s Rhesus status or detecting fetal-maternal hemorrhage (FMH), and non-compliance with RhIG prophylaxis guidelines. These were evidenced in our published data on RBC alloimmunization and HDFN in pregnant Malay women, besides reported cases of severe anti-D HDFN in primigravida women4, 5.
The risk and management of HDFN have improved with advanced diagnostic methods and treatment modalities. Nowadays, the fetal RhD genotype can be detected with a high-sensitivity method using polymerase chain reaction (PCR) techniques on free fetal DNA (ffDNA) extracted from maternal plasma, eliminating the need for paternal testing6. Detailed ultrasonography scanning (USS) and Doppler assessment of fetal middle cerebral artery-peak systolic velocity (MCA-PSV) have significantly increased the success of accurately diagnosing HDFN without placing the fetus at risk7. Meanwhile, therapeutic plasma exchange (TPE), intravenous immunoglobulin (IVIG) injections, and intrauterine transfusion (IUT) have provided excellent outcomes in treating severe HDFN7, 8. Here we report the case of a woman with recurrent fetal loss due to high anti-D titers and highlight the benefit of administering TPE and IVIG as early as 20 weeks of gestation to avoid sudden intrauterine death (IUD). However, the patient missed the opportunity to undergo those treatments and our institution also lacked the experience to perform an IUT.
CASE PRESENTATION
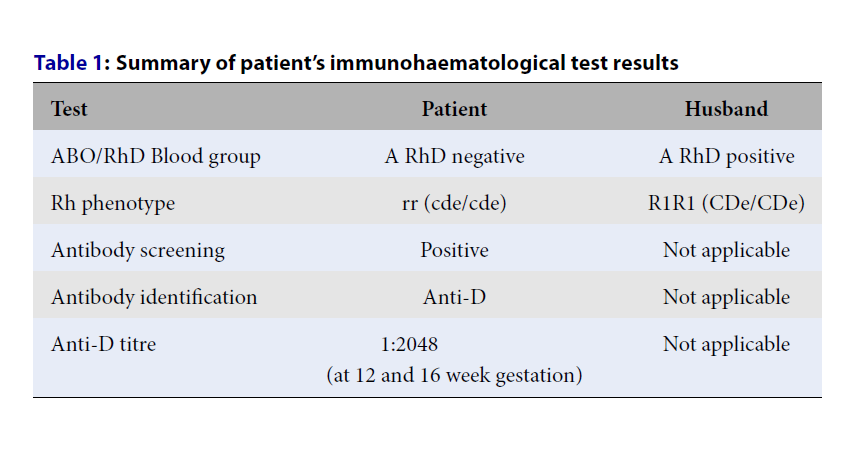
A 28-year-old gravida 3 para 2 (G3P2) patient with RhD negative status was referred to our healthcare institution for fetal monitoring and pregnancy management. She had one surviving child from her first pregnancy in 2015, when she was 22 years old. RhD alloimmunization was detected in her second pregnancy in 2017, at the age of 24, when she delivered a macerated fetus at 38 weeks with a high anti-D titer of 1:>4,096. Her RhD negative status was identified as a rr (cde/cde) phenotype during her first pregnancy. Her husband’s blood type was O RhD positive with a R1R1 (CDe/CDe) phenotype. She claimed that she was given only one dose of antenatal anti-D prophylaxis during her first and second pregnancy at 28 weeks gestation, and no postnatal prophylaxis was given. She also said she did not receive any blood transfusion, suffered miscarriage, or experienced FMH events during her first pregnancy. With regard to her RhD alloimmunization, she and her husband were provided counseling on future pregnancy plans and the possible complications that entailed. Thus, she chose to remain in voluntary subfertility for four years by taking intramuscular Depo Provera. However, after three years, in October 2021, she was referred to our institution at 16 weeks of gestation when complications arose in her latest pregnancy.
The patient had early antenatal booking at six weeks gestation (July 2021) and uneventful follow-ups at a district hospital. However, a positive indirect antiglobulin test at the 12th week of gestation detected a high anti-D titer of 1:2048 (September 2021). She was referred to our center on the 16th week (October 2021), where another test also yielded the same result (Table 1). Initially, she was scheduled for bi-weekly check-ups and adhered to the schedule very well. Detailed ultrasound scanning was performed at 21 weeks gestation, which showed good fetal growth, but there was a presence of minimal pericardial effusion with no cardiomegaly or pleural effusion. Despite having a high anti-D titer, fetal MCA-PSV Doppler assessment remained normal throughout the follow-up. After the obstetrician and transfusion team discussion, it was decided to proceed with therapeutic plasma exchange (TPE) to reduce her anti-D titer during her next follow-up appointment (in early December 2021). In the meantime, referral to a maternal-fetal specialist for intrauterine transfusion (IUT) was planned in case her MCA-PSV Doppler assessments showed abnormal results.
However, TPE could not be carried out as the patient missed her follow-up appointment for unknown reasons after 23 weeks gestation (December 2023). Later, the clinicians were informed of the termination of her pregnancy at 27 weeks (January 2022) after she delivered a macerated stillborn by induction at a district hospital near her home. Her postnatal recovery went well physically and mentally because she was already aware of the poor outcome. On further discussion, she and her husband were still keen to attend appointments at our institution for future pregnancies. Details of the patient’s pregnancies are summarised in Table 2.
| Test | Patient | Husband |
| ABO/RhD Blood group | A RhD negative | A RhD positive |
| Rh phenotype | rr (cde/cde) | R1R1 (CDe/CDe) |
| Antibody screening | Positive | Not applicable |
| Antibody identification | Anti-D | Not applicable |
| Anti-D titre | 1:2048 (at 12 and 16 week gestation) | Not applicable |
| Pregnancy | Year | Gestation (weeks) | Anti-D status | Anti-D prophylaxis | Foetal monitoring | Management / pregnancy outcome |
| 1 | 2015 | 37 | Not detected | Given at 28 weeks Not given postnatal | IUGR and oligohydramnios of unknown cause | Boy, 1.4 kg via LSCS at 37 weeks due to acute foetal distress, the only surviving child. |
| 2 | 2017 | 28 | Unknown | Given at 28 weeks | Unknown | Girl, 2.8 kg, vaginal delivery. MSB at 38 th week of gestation. |
| 37-38 | Detected, Anti-D titre of 1: > 4,096 | Not given postnatal | IUD after 37 weeks | |||
| 3 (current) | 2021 July-September | 6-15 | Detected, anti-D titre of 1:2,048 at 12 th week | Not given, not indicated | Booking and follow-up at district hospital. Foetal growth corresponding to age. | Referred to our centre at 16 weeks gestation. |
| Oktober-December | 16-23 | Anti-D titre of 1:2,048 at 16 th week | Foetal growth corresponding to age, normal MCA Doppler. | Opted for TPE. IUT is planned if abnormal MCA Doppler results. | ||
| Late December | After 23 | Missed follow-up at our centre | unknown | |||
| 2022 January | 27 | Delivered at district hospital after being diagnosed with an IUD | MSB | |||
Discussion
The risk of an RhD negative woman becoming allosensitized could be reduced from 16% to less than 0.2% through the administration of antenatal and postnatal RhIG prophylaxis9. RhD alloimmunization in this patient clearly occurred due to non-compliance with guidelines on RhIG prophylaxis, since the patient was given a single dose of RhIG only during her first and second pregnancies without postnatal prophylaxis. There was also no indication that she was treated in her latest pregnancy. The guidelines suggest that allosensitized pregnant women should receive either two doses of 500 IU RhIG at 28 and 34 weeks gestation, or a single dose of 1,500 IU RhIG between 28 and 30 weeks gestation, as well as within 72 hours post-delivery10, 11.
In addition to RhIG compliance, immunogenicity of RBC antigens, FMH and related events, and the recipient’s inflammation status, several studies have explored the influence of genetic polymorphisms on RBC alloimmunization among responders, particularly in transfused patients. However, there is a notable lack of research on the impact of genetic polymorphisms on RBC alloimmunization in pregnant women. Among transfused patients, variants of human leukocyte antigen (HLA) class II, especially HLA-DRB1, have been linked to RBC alloimmunization. For instance, HLA-DRB1*04 and HLA-DRB1*15 have been associated with anti-Fya, HLA-DRB1*10, HLA-DRB1*11, and HLA-DRB1*13 with anti-K, and HLA-DRB1*01 with anti-Jka. In the case of Rh and RhD alloimmunization, only limited studies have shown an association with HLA-DRB1*01, HLA-DRB1*15, and HLA-DQB1*06 with anti-D and HLA-DRB1*09 with anti-E12, 13.
The diagnosis and management of pregnancy in a highly sensitized RhD negative condition require close cooperation from the patient, her partner, and healthcare providers. As the condition might be genetically influenced, poor outcomes could recur in future pregnancies. Our center does not have its own guidelines; instead, we follow the Royal College of Obstetricians and Gynaecologists guidelines for managing pregnant women with RBC alloantibodies, including those who are RhD negative (Figure 1)6. There were different critical titers of anti-D antibodies associated with significant fetal risk. Most used a titer of more than 16 as an indication for close monitoring, and a repeated titer of ≥32 as an indication for MCA-PSV Doppler assessment14, 15. The higher anti-D titers in RhD negative pregnant women predicted more severe HDFN and increased need for IUT16. A titer higher than 1,000 was a clear indication that severe HDFN was inevitable if preventive measures were not provided properly as reported in this case15. Besides the anti-D titer, the IgG subclass might also determine the severity of HDFN, in which the maternal anti-D antibodies were usually associated with IgG1 and/or IgG3 subclasses. Maternal serum containing both IgG1 and IgG3 was reportedly associated with more severe HDFN than the presence of a single subclass15. Unfortunately, despite a very high titer (more than 1,000), we did not determine the IgG subclass in our patient to observe this association due to a lack of resources.
There were many reported cases of RhD alloimmunization with very high anti-D titer in pregnancies (>1,000). Recently, they were effectively treated with a multidisciplinary approach with gynecologist, neonatal, immune-hematological and apheresis specialists which included intensive TPE, followed by high dose IVIG (400 mg/kg per day for five days) and with or without IUT7, 8, 17. Previous studies reported that early application of TPE and IVIG treatment in severe RhD incompatible pregnancy could potentially avoid the need for IUT16, 17, 18. The previous report utilized a Plasma Flow OP-05W and a Cascade Flow EC-20W for TPE. Each procedure processed 9,000 ml of whole blood at a flow rate of 60 mL/min, resulting in a total plasma volume of 2,600 ml19. The anti-D titer should be measured both before and after the TPE. This non-invasive approach effectively reduced the anti-D titers significantly and subsequently reduces the placental transfer of maternal anti-D16, 19. Thus, TPE and IVIG administration should be considered early in pregnancy from the 7th to 20th week and continued until IUT could safely be administered if indicated. If the current pregnancy is managed with intensive TPE followed by IVIG in the earlier weeks, it is expected to have a different outcome in terms of fetal survival, as demonstrated in previous case reports7, 8, 17, 19.
There have also been reported cases where the use of TPE and/or immunoadsorption in combination with IVIG proved to be an effective and safe treatment strategy for extremely high anti-D alloimmunization in pregnant women20. Recently, there are potential and awaiting more formal evaluation of emerging strategies using B cell depletion or plasma cell targeting approaches to prevent alloantibody formation following RBC transfusion in at-risk patients21. These treatment modalities could be beneficial in preventing and managing RBC alloimmunization in pregnant women in the future.
In our patient, the fetus was at high risk of hydrops fetalis based on previous obstetric history and very high anti-D titer. She should have received counseling and been managed with a combination of antibody treatment modalities (TPE and IVIG) as early as possible, despite her normal serial MCA-PSV Doppler findings, as the delay increased the risk of IUD. In the absence of fetal anemia and without the observation of hydrops in a detailed USS, it was possible to modulate the maternal immune response using TPE and IVIG as they could delay the onset of fetal anemia and even avoid the need for IUT. Because of the lack of experience and expertise in dealing with alloimmunized pregnant women with high anti-D titer, the intervention and management plans for the patient were delayed until a late stage of gestation, and this was compounded when she missed her appointment for treatment at our center. Therefore, it was no surprise that her latest pregnancy also ended in fetal loss.
Since the patient and her husband planned to have another baby, our institution learned from the shortcomings and presented the couple with a proper management plan. The management of pregnant women with RhD alloimmunization and high anti-D titers should include early booking, close fetal monitoring using MCA-PSV Doppler assessment, and anti-D titer measurements. Intensive TPE should be carried out, followed by IVIG (<20 weeks gestation) and IUT if there was evidence of fetal anemia. While TPE and/or IVIG with IUT offers a promising fetal outcome in managing pregnant women with high-titer anti-D, it is essential to counsel patients on the risks and benefits of the opted procedures, as well as the potential consequences of refusal or delayed procedures. Antibody titers might rebound following TPE, but this effect is variably suppressed if TPE is repeated or followed by IVIG16. IUT may still be necessary if MCA-PSV Doppler findings indicate fetal anemia, even if the patient has already undergone intensive TPE and/or IVIG in early pregnancy. Although IUT is considered a relatively safe treatment, adverse events persist such as preterm labor, infection, and fetal death, especially if performed before 20 weeks of gestation22. The importance of patient compliance in attending follow-ups must also be emphasized. The patient’s full compliance with treatment would have effectively reduced the anti-D antibody titer to minimal levels so that fetal anemia could be prevented, and an early delivery should be carried out whenever possible.
Conclusions
Early identification of RhD negative pregnant women, coupled with timely detection of anti-D alloimmunization, regular monitoring of anti-D titres and fetal anaemia, and a multidisciplinary approach to managing HDFN due to anti-D alloimmunization, enhances the likelihood of a positive pregnancy outcome and fetal survival. Intensive TPE with high-dose IVIG should be started as early as possible in highly sensitized RhD negative pregnant women. TPE and IVIG could delay IUT or prevent severe HDFN with a good pregnancy outcome. Future research should explore the role of genetic polymorphisms that contribute to elevated anti-D titres, aiming to enhance precision medicine in preventing and managing anti-D alloimmunization with high anti-D titres in pregnant women and its consequences.
Abbreviations
ffDNA: free foetal DNA, FMH: Foetal-maternal haemorrhage, HDFN: Haemolytic disease of foetus and new born, IUD: Intrauterine death, IUT: Intrauterine transfusion, IVIG: Intravenous immunoglobulin, MCA-PSV: Foetal middle cerebral artery-peak systolic velocity, RBC: Red blood cell, RhD: Rhesus D, RhIG: anti-D immunoglobulin, TPE: Therapeutic plasma exchange, USS: Ultrasound sonography
Acknowledgments
We thank to Ministry of Higher Education Malaysia for Fundamental Research Grant Scheme (FRGS) with Project code: FRGS/1/2020/SKK06/USM/02/3 for funding support of this case report.
Author’s contributions
Conceptualization: Mohd Nazri Hassan; Collection and processing of material: Mohd Nazri Hassan, Marini Ramli, Marne Abdullah; Text writing: Mohd Nazri Hassan, Noor Haslina Mohd Noor; Editing: Salfarina Iberahim, Zefarina Zulkafli, Wan Suriana Wan Ab Rahman, Final approval of the version to be submitted: Zefarina Zulkafli, Rosnah Bahar, Shafini Mohamed Yusoff. All authors read and approved the final manuscript.
Funding
Fundamental Research Grant Scheme (FRGS) with Project code: FRGS/1/2020/SKK06/USM/02/3.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying image. A copy of the written consent is available for review by Editor-in-chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Eder
A.F.,
Update on HDFN: new information on long-standing controversies. Immunohematology.
2006;
22
(4)
:
188-95
.
View Article Google Scholar -
Bowman
J.,
Thirty-five years of Rh prophylaxis. Transfusion.
2003;
43
(12)
:
1661-6
.
View Article Google Scholar -
Yu
D.,
Ling
L.E.,
Krumme
A.A.,
Tjoa
M.L.,
Moise
K.J.,
Live birth prevalence of hemolytic disease of the fetus and newborn in the United States from 1996 to 2010. AJOG Global Reports.
2023;
3
(2)
:
100203
.
View Article Google Scholar -
Hassan
M.N.,
Mohd Noor
N.H.,
Johan Noor
S.R.,
Sukri
S.A.,
Mustafa
R.,
Luc Aster
H.V.,
Hemolytic disease of fetus and newborn due to maternal red blood cell alloantibodies in the Malay population. Asian Journal of Transfusion Science.
2014;
8
(2)
:
113-7
.
View Article Google Scholar -
Hassan
M.Z.,
Iberahim
S.,
Abdul Rahman
W.S.,
Zulkafli
Z.,
Bahar
R.,
Ramli
M.,
Severe anti-D haemolytic disease of fetal and newborn in rhesus D negative primigravida. The Malaysian Journal of Pathology.
2019;
41
(1)
:
55-8
.
-
Gynaecologists
undefined Royal College of Obstetrician &,
Green-top guideline No 65: the management of women with red cell antibodies dueing pregnancy. Obstetrician & Gynaecologist.
2014;
16
(3)
:
224
.
View Article Google Scholar -
Novak
D.J.,
Tyler
L.N.,
Reddy
R.L.,
Barsoom
M.J.,
Plasmapheresis and intravenous immune globulin for the treatment of D alloimmunization in pregnancy. Journal of Clinical Apheresis.
2008;
23
(6)
:
183-5
.
View Article Google Scholar -
Tara
F.,
Maleki
A.,
Taheri
N.,
Moein Darbari
S.,
A case of D alloimmunization in pregnancy: successfully treated solely with therapeutic plasma exchange (TPE). Journal of Blood Medicine.
2019;
10
:
251-3
.
View Article Google Scholar -
MacKenzie
I.Z.,
Bowell
P.,
Gregory
H.,
Pratt
G.,
Guest
C.,
Entwistle
C.C.,
Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. British Journal of Obstetrics and Gynaecology.
1999;
106
(5)
:
492-7
.
View Article Google Scholar -
Karim
F. Abdul,
Abdullah
J.,
Ahmad
N.H.,
Handbook on clinical use of bloodNational Blood Centre: Kuala Lumpur; 2020.
Google Scholar -
Qureshi
H.,
Massey
E.,
Kirwan
D.,
Davies
T.,
Robson
S.,
White
J.,
British Society for Haematology
BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfusion Medicine (Oxford, England).
2014;
24
(1)
:
8-20
.
View Article Google Scholar -
Gerritsma
J.J.,
Oomen
I.,
Meinderts
S.,
van der Schoot
C.E.,
Biemond
B.J.,
van der Bom
J.G.,
consortium
SCORE,
Back to base pairs: what is the genetic risk for red bloodcell alloimmunization?. Blood Reviews.
2021;
48
.
View Article Google Scholar -
Hendrickson
J.E.,
Jeanne
E.,
Eisenbarth, Stephanie C, Tormey, Christopher A. Red blood cll alloimmunization: new findings at the bench and new recommendations for the bedside. Current Opinion in Hematology.
2016;
23
(6)
:
543-9
.
View Article Google Scholar -
Bettelheim
D.,
Panzer
S.,
Reesink
H.W.,
Csapo
B.,
Pessoa
C.,
Guerra
F.,
Monitoring and treatment of anti-D in pregnancy. Vox Sanguinis.
2010;
99
(2)
:
177-92
.
View Article Google Scholar -
Velkova
E.,
Correlation between the amount of anti-D antibodies and IgG subclasses with severity of haemolytic disease of foetus and newborn. Open Access Macedonian Journal of Medical Sciences.
2015;
3
(2)
:
293-7
.
View Article Google Scholar -
Tang
T.H.,
Guo
C.Y.,
Li
X.Y.,
Hu
Y.X.,
Liu
W.K.,
Yu
M.X.,
Effect of Anti-D titers in RhD-negative pregnant women on fetuses and newborns: A retrospective study. Pediatrics and Neonatology.
2024;
65
(3)
:
288-92
.
View Article Google Scholar -
Kamei
K.,
Yamaguchi
K.,
Sato
M.,
Ogura
M.,
Ito
S.,
Okada
T.,
Successful treatment of severe rhesus D-incompatible pregnancy with repeated double-filtration plasmapheresis. Journal of Clinical Apheresis.
2015;
30
(5)
:
305-7
.
View Article Google Scholar -
Maisonneuve
E.,
Dugas
A.,
Friszer
S.,
Toly-Ndour
C.,
Cariot
L.,
Dhombres
F.,
Effect of intravenous immunoglobulins to postpone the gestational age of first intrauterine transfusion in very severe red blood cell alloimmunization: A case-control study. Journal of Gynecology Obstetrics and Human Reproduction.
2021;
50
(7)
:
102119
.
View Article Google Scholar -
Isojima
S.,
Hisano
M.,
Suzuki
T.,
Sago
H.,
Murashima
A.,
Yamaguchi
K.,
Early plasmapheresis followed by high-dose γ-globulin treatment saved a severely Rho-incompatible pregnancy. Journal of Clinical Apheresis.
2011;
26
(4)
:
216-8
.
View Article Google Scholar -
Colpo
A.,
Tison
T.,
Gervasi
M.T.,
Vio
C.,
Vicarioto
M.,
De Silvestro
G.,
Personalized treatment with immunoadsorption and intravenous immunoglobulin in a case of severe Rh alloimmunization during pregnancy unresponsive to plasma - exchange. Transfusion and Apheresis Science : Official Journal of the World Apheresis Association : Official Journal of the European Society for Haemapheresis.
2017;
56
(3)
:
480-3
.
View Article Google Scholar -
Arthur
C.M.,
Stowell
S.R.,
The development and consequences of red blood cell alloimmunization. Annual Review of Pathology.
2023;
18
(1)
:
537-64
.
View Article Google Scholar -
Zwiers
C.,
Lindenburg
I.T.,
Klumper
F.J.,
de Haas
M.,
Oepkes
D.,
Van Kamp
I.L.,
Complications of intrauterine intravascular blood transfusion: lessons learned after 1678 procedures. Ultrasound in Obstetrics {&}amp; Gynecology.
2017;
50
(2)
:
180-6
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 10 (2024)
Page No.: 6845-6851
Published on: 2024-10-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 1663 times
- PDF downloaded - 572 times
- XML downloaded - 75 times
 Biomedpress
Biomedpress



