Abstract
Introduction: HPV-16 and HPV-18 account for around 70% of cases of invasive cervical cancer worldwide. These are highly pathogenic and high-risk genotypes. The most prevalent sexually transmitted disease is human high-risk papillomavirus (HR-HPV), which can infect anyone who engages in sexual activity at any time in their life. To evaluate the possibility that the virus causes malignant lesions in infected women with high-risk HPV types who have normal and abnormal Pap smears, the current study examined the methylation pattern of CpG islands in the oncogene promoter of high-risk HPV E6.
Methods: Forty-eight Pap smears were collected from women referred to Shahid Sadoughi Hospital in Yazd for this case-control study. The Sinaclon kit was used for DNA extraction. PCR amplification with specific primers allowed the identification of HPV-16 and HPV-18 types. The bisulfite modification method, based on the EpiTect Bisulfite Conversion Kit, was then used to identify methylation in the E6 gene. Version 22 of the SPSS program was used to collect the data, and the corresponding statistical tests were used for analysis.
Results: In this study, methylation at position 506 in the HPV-16 E6 gene was detected in 10.4% (n = 5/48) of cases; however, methylation in the 1985 region for the E6 promoter of HPV-18 was 20.8% (n = 10/48). Comparison of the methylation rate in the HPV-18 nucleotide 1985 in normal and abnormal Pap smear samples showed that the methylation rate in normal and abnormal samples was 55.8% and 44.2%, respectively. There was no significant difference between the frequency of HPV-18 and HPV-16 DNA methylation in all selected CpG nucleotide regions (P ≥ 0.05).
Conclusion: Our results indicate that the methylation change in the E6 gene promoter was not involved in the development of infection with HPV-16 and HPV-18 types in the study population.
Introduction
Worldwide, cervical cancer is the fourth most common cancer affecting women, with developing countries being disproportionately affected1, 2. The world is struggling to implement effective preventive measures such as vaccinations and screenings in underdeveloped countries3, 4. Cervical cancer is usually caused by certain human papillomaviruses (HPV), which are classified as high-risk viruses (hr-HPV)5, 6. These viruses are transmitted through sexual contact and belong to the genus Alphapapillomaviruses. The strongest hr-HPVs are types 16 and 18, which are responsible for around 70% of cases of cervical cancer7, 8, 9, 10. These species remain in the body for long periods of time due to their high persistence rate. The development of HPV-induced cancer is dependent on the ongoing synthesis of the oncoproteins E6 and E711, 12, 13. These proteins cause and maintain uncontrolled cell growth (immortality) in infected cells by interacting with various cellular proteins, including cellular tumor suppressor proteins14. Through its interaction with the early promoter located in the long control region (LCR) of the HPV viral genome, the E2 protein is essential for controlling the production of E6 and E715. The origin of viral DNA replication, early gene promoter sequences (such as those for oncogenes E6 and E7), and regulatory regions that promote viral gene transcription are all located in the LCR region16, 17, 18. One method that could influence the function of the E6 and E7 genes is the methylation of the LCR19, 20.
The process of attaching a methyl group to certain DNA regions — in particular to the cytosine bases within CpG islands — is known as DNA methylation21, 22. By altering the chromatin structure, this methylation — which is often mediated by enzymes called DNA methyltransferases (DNMTs) — can lead to gene silencing. Methylation of the viral LCR is one possible method of silencing certain viral genes associated with hr-HPV infections and cervical cancer. However, if the methylation pattern affects regulatory regions, it can also lead to increased expression of other viral genes23, 24, 25. This complicated interplay shows how DNA methylation influences the severity of the disease.
Hr-HPV infection is a significant risk factor for cervical cancer, but it is not sufficient for the disease to develop solely due to its presence. Precancerous lesions and cervical cancer have DNA methylation patterns that indicate involvement in the disease process26, 27. Increased expression and activity of DNMT1, an enzyme important for DNA methylation, may be related to the methylation of the E6 oncogene promoter in hr-HPV strains. This process may lead to the degradation of the important tumor suppressor protein p5328. An effective method for identifying or predicting the risk of cervical cancer at an early stage of diagnosis is to examine DNA methylation patterns, particularly in the E6 promoter region. In this study, the frequency of E6 gene promoter methylation was compared in HPV-16 and HPV-18 infections. The propensity of these common HPV strains to form malignant tumors should be assessed by analyzing their methylation patterns. Accurate prognosis, efficient disease surveillance, and the development of treatment plans for women with abnormal Pap smears could benefit from this knowledge. In the present study, the methylation pattern of CpG islands in the hr-HPV E6 oncogene promoter was compared to evaluate the potential of the virus for malignant lesions in women infected with high-risk HPV types who have normal and abnormal Pap smears.
Methods
Sampling
In this cross-sectional study, a total of 48 Pap smears were collected over a period of one year (2019) from women referred to the Obstetrics and Gynecology Clinic of Shahid Sadoughi Hospital in Yazd, Iran. In all cases, written informed consent was obtained, and the research protocol was approved by the local ethics committee. Based on the Pap smear results, samples were categorized into two groups: normal (n = 24/48; control) and abnormal (n = 24/48; case). All samples were stored at -20 ºC until further examination. Inclusion criteria included women with normal and abnormal Pap smears who were infected with high-risk HPV (Hr-HPV) types, such as HPV-16 and HPV-18, and were between 15 and 45 years of age. Exclusion criteria included pregnancy, a history of hysterectomy or abnormal cervical epithelial lesions, infection with low-risk HPV types (Lr-HPV), and age not between 15 and 45 years.
DNA Extraction and Hr-HPV Detection
Template DNA extraction from the paraffin-embedded blocks was performed as follows: Heated xylene (1 ml; 60˚C) was added to the samples, followed by incubation at 58˚C for 20 minutes and centrifugation at 15,000 rpm for 10 minutes. Ethanol (99%) was added to all samples and incubated at 60°C for 20 minutes. Subsequently, 300 μl of digestion buffer was added and incubated overnight at 58°C. After centrifugation, the supernatant was transferred to another sterile tube. To obtain a pure template, the extraction procedure was supplemented by a phenol/chloroform assay. The concentration and quality of the template DNA were determined using a NanoDrop 1000 spectrophotometer and gel electrophoresis, respectively. The presence of the Hr-HPV genome (HPV-16 and -18) was determined using MolecuTech REBA HPV-ID.
Methylation Pattern of the E6 Gene by Bisulfite DNA Modification
To investigate the methylation pattern of the E6 gene in the Hr-HPV samples, a bisulfite modification technique using the EpiTect Bisulfite Conversion Kit was performed. In brief, sodium bisulfite was prepared according to the kit protocol, and the sample DNA was treated. In the bisulfite DNA modification method, a pair of forward primers with CpG (F2/F3) and without CpG positions (F1) are used (Table 1). For all forward primers, a reverse primer (R) is also used. Each pair of forward and reverse primers was used separately in one tube. The presence of one of the CpG loci results in a negative PCR for that primer pair. Primer F1 generates the desired band in all cases with R. However, the forward primers (F2, F3) are formed without generating the desired band, indicating that the CpG site is present at that sequence point.
The DNA treated with sodium bisulfite was subjected to a PCR reaction. The primer sequences are listed in Table 2. PCR was performed to amplify E6 methylation in HPV-16 and HPV-18 using the following program: Denaturation at 95°C for 8 minutes, 36 cycles of denaturation at 95°C for 35 seconds, annealing at 56°C for 30 seconds, extension at 72°C for 30 seconds, and final extension at 72°C for 10 minutes. The amplified products were visualized by electrophoresis in 1.5% agarose gels stained with Gel Red™.
Statistical Data Analysis
In this study, participant data was collected confidentially using questionnaires and incorporating the results of the experiments. All patient data were then entered into SPSS software for statistical analysis. A Student's t-test was performed to compare between groups. A p-value of less than 0.05 was considered statistically significant, indicating a statistically relevant difference between the groups compared.
| Primer type | Sequence of primers (5'-3') |
| HPV16- F1 | ATGGGAATTTATATGTTGTATGTGAT |
| HPV16- F2 | ATGGGAATCTATATGTTGTATGTGAT |
| HPV16- F3 | ATGGGAATTTATATGCTGTATGTGAT |
| HPV16- R | CTCCTCCTCTGAGCTATCATTTAATTGCTC |
| HPV18_F1 | ATGATTAAGTTGTGTATATATAGTTTATGT |
| HPV18_F2 | ATGACTAAGTTGTGTATATATAGTTTATGT |
| HPV18_F3 | ATGATTAAGTTGTGTATATACAGTTTATGT |
| HPV18_R | TTCAAATACCTCTATAAATTCCAATACTG |
| E6 Proto-oncogene | Sequence of primers | Product size (bp) |
| HPV-16F | 5'-ATGGGAATTTATATGTTGTATGTGAT-3' | 379 |
| HPV-16R | 5'-CTCCTCCTCTAAACTATCATTTAATTACTC-3' | |
| HPV-18F | 5'-ATGATTAAGTTGTGTATATATAGTTTATGT-3' | 309 |
| HPV-18R | 5'-CTCCTCCTCTAAACTATCATTTAATTACTC-3' |
Results
In this study, the nucleotide pattern at positions 494 and 506 for HPV-16 and 1985 and 2003 for HPV-18 was examined. The mean age of the patients examined was 34.5 ± 7.23 years. The incidence of HPV-16 and HPV-18 was 64.6% and 35.4%, respectively. The incidence of HPV-16 was higher than that of HPV-18 in both groups (cases/controls).
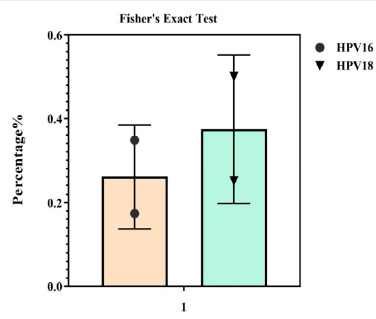
Twenty-four HPV-16 infected samples with abnormal Pap smears were analyzed for CpG methylation patterns in the E6 gene promoter. Of all subjects in this study, 5 cases (10.4%) were found with methylation at position 506 in the HPV-16 E6 gene. Of the 24 samples in each group, only 4 samples in the abnormal Pap smear group and 1 sample in the normal Pap smear group had methylation in the 506 region of the E6 gene promoter of the HPV-16 genotype. It is noteworthy that none of the samples showed methylation at nucleotide site 494 of the promoter of the E6 gene of this genotype. The frequency of HPV-16 DNA methylation at all analyzed CpG sites ranged from 0% to 4.1% in the different subgroups and was not statistically significant in any of them (P = 0.348). The results of DNA methylation by the modified bisulfite method show that the 92, 104, and 106 methylation sites have no methylation after bisulfite treatment, and the cytosine is converted to uracil, with thymine finally being found in the PCR product. Comparison of the methylation rate of 506 HPV-16 nucleotides in normal and abnormal Pap smear samples showed that the methylation rate was 53.5% in normal Pap smear samples and 43.5% in abnormal Pap smear samples (Figure 1, Figure 2 ).
The HPV-18 methylation pattern was analyzed at the CpG site 1985 of the selected E6 gene promoter using 24 pure DNA samples from HPV-18 infected cervical samples with abnormal Pap smears. In 24 samples, non-methylated cytosines at the 1981-2010 nucleotide position were analyzed using methyl primers similar to those used for HPV-16. In total, 10.4% of the samples showed methylation at this position. In each group, only 5 samples in the group of abnormal Pap smears showed methylation in the 1985 region of the E6 promoter of genotype 18 (20.8%). None of the samples showed methylation in the nucleotide locus of the 2003 E6 promoter of this genotype. According to the results, no methylated cytosine was detected in other selected DNA fragments isolated from cervical samples with HPV-18 infection. According to the statistical analysis, there was no significant difference (P ≥ 0.05) in the frequency of HPV-18 DNA methylation at all selected 1985 CpG nucleotide sites in HPV-18 DNA samples isolated from specimens with different degrees of cervical pathology. The results of the methylation modification study showed that no methylation occurred at methylation sites 152 and 159 after bisulfite treatment, and the cytosine was converted to uracil, eventually being incorporated into the thymine PCR product. Comparison of the methylation rate of 1985 HPV-18 nucleotides in normal and abnormal Pap smear samples showed that the methylation rate was 55.8% in normal tissue and 44.2% in abnormal tissue (Figure 3, Figure 4, Figure 5).
In the normal and abnormal Pap smears, methylation at the position of the 506 nucleotide in the CpG region was 54.8% and 45.2% for HPV-16, respectively, but 58.8% and 41.2% for HPV-18 in 1985.
Discussion
An estimated 71% of cases of uterine cancer are associated with HPV-16 and HPV-18 infections29, 30. Different ethnic groups have varying rates of HPV prevalence and types; infections with HPV-52 and HPV-58 are more common in Asia than on other continents31, 32. Numerous studies establish a link between host genes and hyperimmunity to human papillomavirus and uterine cancer. However, the relationship between the severity of cervical lesions and the methylation rate of different HPV strains is controversial. Research has investigated the relationship between the methylation status of the HPV genome, early and late genes, and disease severity, with some results suggesting a negative association and others suggesting a positive association.
One of the key players in gene silencing is increased methylation in CpG islands. Several genes are impacted by CIHM (CpG island hypermethylation) in a large number of tumors. Examining E6 gene promoter methylation in patients with HPV-16 and HPV-18 Pap smears was the goal of this investigation. Despite the contentious discoveries on the pattern of HPV DNA methylation in cervical disease, the various results are nearly equivalent. The enhancer region's nucleotide 7862 (CpG) has a very low rate of DNA methylation in the majority of earlier studies. These results indicate that all HPV DNA samples that lacked methylation at nucleotide position 7862 were examined in this investigation. There is overlap between the CpG location (7862 nucleotides) and the E2 crossing. The transcription of the oncogenes E6 and E7 has been demonstrated to be repressed by E2 gene products. Consequently, loss of E2 suppression and stable E6/E7 expression can result from methylation of the E2 cross13, 16. Among the several investigations identifying the two proteins E6 and E7 as candidates, it is important to highlight the study by Yan et al., which demonstrated that cellular immune responses to peptides E7 and E6 were strongly related to illness progression. High diversity of cytosine methylation at selected CpG sites and between various amplicons was not found when DNA methylation abundance in specific CpG nucleotides was examined. Cytology is still utilized for the early detection of cervical cancer despite its very low sensitivity. Both hypomethylation and hypermethylation are examples of incompatible DNA methylation in cervical lesions. In high-grade intrauterine neoplasia and cervical carcinoma, only three promoter regions are hypomethylated, while most genes are hypermethylated. The most significant risk factor for cervical cancer and its antecedents is hrHPV infection. Earlier research demonstrated that the HPV-16 E6 protein binds to p53, potentially releasing more Sp1, activating more DNMTs, and altering DNA methylation. Identifying disease-specific gene methylation is a new approach to cancer screening, enhancing early cancer diagnosis as the best diagnostic. Previous research has shown that methylation of certain genes, such as PAX1, SOX1, ZNF582, and POU4F3, in uterine cancer is therapeutically valuable for identifying INCIN3 cancer samples. In the present study, due to the frequency of methylation in patients with HPV-16 and HPV-18 infection, methylation changes in the E6 gene promoter did not play an important role in the development of infection with high-risk HPV-16 and HPV-18 virus types in the study population. No significant association was found between GG genotype and E6 gene polymorphism (P ≥ 0.05), although further studies are needed to confirm this.
Conclusions
In the current study, the methylation pattern in the E6 gene promoter was determined using tissue samples from the cervix of 48 women who were referred to the clinic at Shahid Sadoughi Hospital in Yazd and had abnormal or normal Pap smears, in addition to the usual high-risk HPV types. The results show that there is a poor correlation coefficient for the methylation of nearby CpG sites in HPV-16 and HPV-18 for both the 1985 and the 506 sites. This finding could indicate the existence of methylation boundaries. After considering repeated testing, there was no discernible correlation between the methylation of individual CpG sites and viral load in the diagnostic CIN-II or CIN-III samples or the control group.
Abbreviations
CIN-II: Cervical Intraepithelial Neoplasia II, CIN-III: Cervical Intraepithelial Neoplasia III, CIHM: CpG Island Hypermethylation, CpG: Cytosine-phosphate-Guanine, DNMT: DNA Methyltransferase, DNMTs: DNA Methyltransferases, HPV: Human Papillomavirus, HR-HPV: Human High-risk Papillomavirus, hr-HPV: High-risk Human Papillomavirus, LCR: Long Control Region, Lr-HPV: Low-risk Human Papillomavirus, PCR: Polymerase Chain Reaction, SPSS: Statistical Package for the Social Sciences
Acknowledgments
None.
Author’s contributions
All authors equally contributed to this work, read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
In all cases, written informed consent was obtained, and the research protocol was approved by the local ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Perkins
R.B.,
Wentzensen
N.,
Guido
R.S.,
Schiffman
M.,
Cervical cancer screening: a review. Journal of the American Medical Association.
2023;
330
(6)
:
547-58
.
View Article PubMed Google Scholar -
Viveros-Carreño
D.,
Fernandes
A.,
Pareja
R.,
Updates on cervical cancer prevention. International Journal of Gynecological Cancer.
2023;
33
(3)
:
394-402
.
View Article PubMed Google Scholar -
Illah
O.,
Olaitan
A.,
Updates on HPV Vaccination. Diagnostics (Basel).
2023;
13
(2)
:
243
.
View Article PubMed Google Scholar -
Khodadadian
A.,
Varghaiyan
Y.,
Babakhanzadeh
E.,
Alipourfard
I.,
Haghi-Daredeh
S.,
Ghobadi
A.,
Fertility preservation in women with ovarian cancer: Finding new pathways: A case-control study. International Journal of Reproductive Biomedicine.
2021;
19
(2)
:
157-66
.
View Article PubMed Google Scholar -
Choi
S.,
Ismail
A.,
Pappas-Gogos
G.,
Boussios
S.,
HPV and cervical cancer: A review of epidemiology and screening uptake in the UK. Pathogens (Basel, Switzerland).
2023;
12
(2)
:
298
.
View Article PubMed Google Scholar -
Bowden
S.J.,
Doulgeraki
T.,
Bouras
E.,
Markozannes
G.,
Athanasiou
A.,
Grout-Smith
H.,
Risk factors for human papillomavirus infection, cervical intraepithelial neoplasia and cervical cancer: an umbrella review and follow-up Mendelian randomisation studies. BMC Medicine.
2023;
21
(1)
:
274
.
View Article PubMed Google Scholar -
Cho
E.H.,
Park
M.S.,
Woo
H.Y.,
Park
H.,
Kwon
M.J.,
Evaluation of clinical usefulness of HPV-16 and HPV-18 genotyping for cervical cancer screening. Journal of Gynecologic Oncology.
2024;
35
(6)
:
35
.
View Article PubMed Google Scholar -
Aimagambetova
G.,
Babi
A.,
Issanov
A.,
Akhanova
S.,
Udalova
N.,
Koktova
S.,
The distribution and prevalence of high-risk HPV genotypes other than HPV-16 and HPV-18 among women attending gynecologists' offices in Kazakhstan. Biology (Basel).
2021;
10
(8)
:
794
.
View Article PubMed Google Scholar -
Jahangir
M.,
Nazari
M.,
Babakhanzadeh
E.,
Manshadi
S.D.,
Where do obesity and male infertility collide?. BMC Medical Genomics.
2024;
17
(1)
:
128
.
View Article PubMed Google Scholar -
Nazari
M.,
Babakhanzadeh
E.,
Mohsen Aghaei Zarch
S.,
Talebi
M.,
Narimani
N.,
Dargahi
M.,
Upregulation of the RNF8 gene can predict the presence of sperm in azoospermic individuals. Clinical and Experimental Reproductive Medicine.
2020;
47
(1)
:
61-7
.
View Article PubMed Google Scholar -
Basukala
O.,
Banks
L.,
The not-so-good, the bad and the ugly: HPV E5, E6 and E7 oncoproteins in the orchestration of carcinogenesis. Viruses.
2021;
13
(10)
:
1892
.
View Article PubMed Google Scholar -
Ahmed
S.,
Vajeeha
A.,
Idrees
M.,
Hussain
A.,
Munir
R.,
Zaidi
G.,
Genotypic distribution of human papillomavirus and phylogenetic analysis of E6 and E7 gene of HR-HPV variants isolated from Pakistani population. Medicine.
2023;
102
(2)
:
e32651
.
View Article PubMed Google Scholar -
Skelin
J.,
Sabol
I.,
Tomaić
V.,
Do or die: HPV E5, E6 and E7 in cell death evasion. Pathogens (Basel, Switzerland).
2022;
11
(9)
:
1027
.
View Article PubMed Google Scholar -
Pal
A.,
Kundu
R.,
Human papillomavirus E6 and E7: the cervical cancer hallmarks and targets for therapy. Frontiers in Microbiology.
2020;
10
:
3116
.
View Article PubMed Google Scholar -
Wang
J.,
Guo
Y.,
Wang
H.,
Li
Y.,
Zhang
L.,
Wang
Z.,
Genetic diversity of E6, E7 and the long control region in human papillomavirus type 16 variants in Beijing, China. Biochemistry and Biophysics Reports.
2022;
31
:
101286
.
View Article PubMed Google Scholar -
Santa
S.,
Brown
C.A.,
Akakpo
P.K.,
Edusei
L.,
Quaye
O.,
Tagoe
E.A.,
HPV18 L1 and long control region sequences variation and E6/E7 differential expression in nasopharyngeal and cervical cancers: a comparative study. Infectious Agents and Cancer.
2023;
18
(1)
:
78
.
View Article PubMed Google Scholar -
Song
Z.,
Cui
Y.,
Li
Q.,
Deng
J.,
Ding
X.,
He
J.,
The genetic variability, phylogeny and functional significance of E6, E7 and LCR in human papillomavirus type 52 isolates in Sichuan, China. Virology Journal.
2021;
18
(1)
:
94
.
View Article PubMed Google Scholar -
Hoseini
S.H.,
Enayati
P.,
Nazari
M.,
Babakhanzadeh
E.,
Rastgoo
M.,
Sohrabi
N.B.,
Biomarker Profile of Colorectal Cancer: Current Findings and Future Perspective. Journal of Gastrointestinal Cancer.
2024;
55
(2)
:
497-510
.
View Article PubMed Google Scholar -
Yanatatsaneejit
P.,
Chalertpet
K.,
Sukbhattee
J.,
Nuchcharoen
I.,
Phumcharoen
P.,
Mutirangura
A.,
Promoter methylation of tumor suppressor genes induced by human papillomavirus in cervical cancer. Oncology Letters.
2020;
20
(1)
:
955-61
.
View Article PubMed Google Scholar -
Ekanayake Weeramange
C.,
Tang
K.D.,
Vasani
S.,
Langton-Lockton
J.,
Kenny
L.,
Punyadeera
C.,
DNA methylation changes in human papillomavirus-driven head and neck cancers. Cells.
2020;
9
(6)
:
1359
.
View Article PubMed Google Scholar -
Mattei
A.L.,
Bailly
N.,
Meissner
A.,
DNA methylation: a historical perspective. Trends in Genetics.
2022;
38
(7)
:
676-707
.
View Article PubMed Google Scholar -
Villicaña
S.,
Bell
J.T.,
Genetic impacts on DNA methylation: research findings and future perspectives. Genome Biology.
2021;
22
(1)
:
127
.
View Article PubMed Google Scholar -
Zhang
L.,
Tan
W.,
Yang
H.,
Zhang
S.,
Dai
Y.,
Detection of host cell gene/HPV DNA methylation markers: a promising triage approach for cervical cancer. Frontiers in Oncology.
2022;
12
:
831949
.
View Article PubMed Google Scholar -
Zygouras
I.,
Leventakou
D.,
Pouliakis
A.,
Panagiotou
S.,
Tsakogiannis
D.,
Konstantopoulos
G.,
Human Papillomavirus 16 DNA Methylation Patterns and Investigation of Integration Status in Head and Neck Cancer Cases. International Journal of Molecular Sciences.
2023;
24
(19)
:
14593
.
View Article PubMed Google Scholar -
Huang
C.,
Esfani Sarafraz
P.,
Enayati
P.,
Mortazavi Mamaghani
E.,
Babakhanzadeh
E.,
Nazari
M.,
Circular RNAs in renal cell carcinoma: from mechanistic to clinical perspective. Cancer Cell International.
2023;
23
(1)
:
288
.
View Article PubMed Google Scholar -
Chakraborty
S.,
Ramasubbu
K.,
Banerjee
M.,
Balaji
M.P.,
Vinayagam
Y.,
v
D.R.,
A systematic review on the molecular and clinical association between Human Papillomavirus and Human Immunodeficiency Virus co-infection in Head, Neck and Oral squamous cell carcinoma. Reviews in Medical Virology.
2023;
33
(5)
:
e2462
.
View Article PubMed Google Scholar -
Babakhanzadeh
E.,
Danaei
H.,
Abedinzadeh
M.,
Ashrafzadeh
H.R.,
Ghasemi
N.,
Association of miR-146a and miR196a2 genotype with susceptibility to idiopathic recurrent pregnancy loss in Iranian women: A case-control study. International Journal of Reproductive Biomedicine.
2021;
19
(8)
:
725-32
.
View Article PubMed Google Scholar -
Nicolò
S.,
Antonelli
A.,
Tanturli
M.,
Baccani
I.,
Bonaiuto
C.,
Castronovo
G.,
Bacterial species from vaginal microbiota differently affect the production of the E6 and E7 Oncoproteins and of p53 and p-Rb Oncosuppressors in HPV16-infected cells. International Journal of Molecular Sciences.
2023;
24
(8)
:
7173
.
View Article PubMed Google Scholar -
John
J.H.,
Halder
A.,
Purwar
S.,
Pushpalatha
K.,
Gupta
P.,
Dubey
P.,
Study to determine efficacy of urinary HPV 16 & HPV 18 detection in predicting premalignant and malignant lesions of uterine cervix. International Journal of Gynaecology and Obstetrics: the Official Organ of the International Federation of Gynaecology and Obstetrics.
2023;
161
(1)
:
79-85
.
View Article PubMed Google Scholar -
Alipourfard
I.,
Khorshidian
A.,
Babakhanzadeh
E.,
Nazari
M.,
Susceptibility to azoospermia by haplotype analysis of protamine 1 and protamine 2 variants. Human Gene.
2023;
37
:
201200
.
View Article Google Scholar -
Yan
X.,
Shen
L.,
Xiao
Y.,
Wang
Q.,
Li
F.,
Qian
Y.,
Prevalence, characteristics, and distribution of HPV genotypes in women from Zhejiang Province, 2016-2020. Virology Journal.
2021;
18
(1)
:
208
.
View Article PubMed Google Scholar -
Nazari
M.,
Khorshidian
A.,
Alizadeh
S.,
Falahati
A.M.,
Haghparast
A.,
Ghasemifar
S.,
Association between peroxisome proliferator activated receptor gamma coactivator 1 gene with overweight and obesity risk: case-control study and meta-analysis. Human Gene.
2022;
34
:
201123
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 10 (2024)
Page No.: 6838-6844
Published on: 2024-10-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 1412 times
- PDF downloaded - 583 times
- XML downloaded - 91 times
 Biomedpress
Biomedpress






