Abstract
Introduction: COVID-19 is a highly contagious and deadly disease. However, there is no accurate diagnostic test to predict its severity. The aim of this study was to determine the relationship between the lymphocyte counts and CRP levels and the severity of pulmonary involvement shown in the CT scan of the patients with pneumonia caused by COVID-19.
Methods: In this cross-sectional study, demographic information and laboratory findings of the patients with COVID-19 were collected. Then, each patient's pulmonary involvement was scored based on the CT scan results. The data were analyzed using SPSS 16 software as well as ANOVA, chi-square test, Pearson correlation coefficient, and ROC curve.
Results: Data of 125 COVID-19 patients with the mean age of 59.37 ± 1.5 showed that the prevalence of lymphocytes < 1100 × 10⁹/L and CRP > 100 mg/L was higher in the patients with severe pulmonary involvement than in those with mild involvement (p < 0.001). Furthermore, an increase in pulmonary involvement severity observed in HRCT led to decreased absolute blood lymphocyte count and increased CRP levels (p < 0.001). The CRP test with an area under the ROC curve of 0.76 could be an acceptable test for predicting the severity of pulmonary involvement in patients with pneumonia caused by COVID-19.
Conclusion: It was found out in this study that there was a significant positive correlation between CRP levels and the severity of COVID-19 pneumonia. The CRP test could also be an acceptable test for predicting the severity of pulmonary involvement in COVID-19.
Introduction
The outbreak of COVID-19 caused by the SARS-CoV-2 virus began in December 2019 in Wuhan, China, and on March 11, 2020, the World Health Organization declared it a pandemic and an emergency threatening public health1, 2. The most common clinical symptoms of the disease are fever and cough, along with other non-specific symptoms including dyspnea, headache, muscle aches, and fatigue3. The severity of the disease ranges from an asymptomatic condition to acute respiratory syndrome, organ failure, and even death in critical cases1, 2, 3, 4. Laboratory findings are also variable in this disease. Normal or low white blood cell counts, lymphopenia or thrombocytopenia, prolonged thromboplastin, and elevated C-reactive protein levels have been reported in COVID-19 patients5, 6.
Early detection of severe cases of COVID-19 is very important for all health care systems to predict the need for ICU beds and ventilators and to improve patient prognosis in the pandemic situation where medical resources face shortages4. Hence, researchers have always been seeking solutions for the early detection of severe cases. To this end, patients' clinical symptoms, laboratory findings, and imaging can be helpful. Various studies have used lymphocyte counts and CRP levels, as well as the severity of pulmonary involvement in CT scans, to diagnose and determine the severity of the disease3, 4, 5.
In some countries, such as China and Italy, the use of respiratory symptoms and fever along with lung CT scans, leukopenia or lymphopenia, and high CRP has been recommended for patient screening2. On the other hand, lung CT scans are used to diagnose and determine the severity of the disease in many international protocols3, 4, 5. Given that polymerase chain reaction testing (RT-PCR) may not be available in emergencies, and as lung CT scans are very expensive and can impose radiation and future complications for patients, the severity of pulmonary involvement due to COVID-19 might be determined through simpler and more accessible tests. Accordingly, the present study aimed to determine the relationship between lymphocyte counts and CRP levels and the severity of pulmonary involvement in CT scans of patients with pneumonia caused by COVID-19 in Imam Reza Hospital of Mashhad University Of Medical Sciences in 2020.
Methods
During the COVID-19 Epidemic in Iran, with randomized sampling, 165 COVID-19 patients entered the study based on clinical symptoms and positive PCR or CT scan findings at Imam Reza Hospital in Mashhad from March to May 2020, and a cross-sectional study was designed (This study was approved by the Ethics Committee of Mashhad University of Medical Sciences, with the code of ethics IR.MUMS.REC.1399.093). The inclusion criterion was a definitive diagnosis of COVID-19 based on clinical findings, plus PCR results, or positive lung CT scan findings on the first day of hospitalization. On the other hand, the exclusion criteria were defective information in patients' records, taking glucocorticoids or immunosuppressive drugs, undergoing chemotherapy, having an autoimmune disease, blood malignancies, osteomyelitis, and chronic infections. Figure 1).
Statistical analysis
The variables were described using central tendency (percentage and frequency, mean and standard deviation), and the data were analyzed (after assessing the normality condition for quantitative variables) through ANOVA and chi-square statistical tests. The Pearson correlation coefficient and the ROC curve were also used to determine the relationship between the two quantitative variables. The significance level in this study was considered less than 0.05.
| Group (The severity of lung involvement is based on HRCT) | Mild (%) N | Moderate (%) N | Sever (%) N | Very Sever (%) N | Total (%) N | P-value |
| Sex | ||||||
| Male (%) N | (51/7) 15 | (65/7) 23 | (68/8) 22 | (65/5) 19 | (63/2) 79 | 0.526* |
| Female (%) N | (48/3) 14 | (34/3) 12 | (31/2) 10 | (34/5) 10 | (36/8) 46 | |
| Lymphopenia ( × 10⁹/L) | ||||||
| > 1100 (%) N | (31) 9 | (51/4) 18 | (78/1) 25 | (69) 20 | (57/6) 72 | 0.001 |
| < 1100 (%) N | (69) 20 | (48/6) 17 | (21/9) 7 | (31) 9 | (42/4) 53 | |
| CRP (mg/L) | ||||||
| > 100 (%) N | (20/7) 6 | (22/9) 8 | 953/1) 17 | 975/9) 22 | (42/4) 53 | 0.001 |
| < 100(%) N | (79/3) 23 | (77/10) 27 | (46/9) 15 | (24/1) 7 | (57/6) 72 | |
| AGE (year) (mean ± SD) | 58/37 ± 18/28 | 55/76 ± 16/47 | 60/25 ± 15/64 | 63 ± 12/08 | 59/22 ± 15/84 | 0/325** |
| Lymphocyte count (× 10⁹/L) (mean ± SD) | 1468/9 ± 617/55 | 1083/9 ± 423/79 | 912/8 ± 445/7 | 921/1 ± 456/4 | 1091/7 ± 529/5 | 0/001 |
| CRP level (mg/L) (mean ± SD) | 41/96 ± 53/34 | 74/79 ± 76/56 | 128/11 ± 89/96 | 155/87 ± 86/79 | 99/63 ± 88/74 | 0/001 |
| Total | (23/2) 29 | (28) 35 | (25/6) 32 | (23/2) 29 | (100) 125 |
| Variable | B | SD | *β | Statistics of t | P-value |
| Lymphocyte count (× 10⁹/L) | - 0/004 | 0/001 | - 0/345 | - 4/078 | 0/001 |
| CRP level (mg/L) | 0/038 | 0/006 | 0/505 | 6/486 | 0/001 |
| Lymphopenia** < 1100 (× 10⁹/L) | 4/737 | 1/163 | 0/351 | 4/075 | 0/001 |
| CRP ** >100 (mg/L) | 6/689 | 1/074 | 0/497 | 6/226 | 0/001 |
Results
In this study, after excluding 40 patients due to the lack of qualification or having defects in their medical records, the data were collected from 125 patients with Covid-19, of whom 79 (63.2%) were male and 46 (36.8%) were female. The mean age of the participants was 59.37 ± 1.5 years (with a minimum age of 26 and a maximum of 93 years). Besides, the mean lymphocyte count in the total number of patients was 1091/17 ± 52 cells/µl (with a minimum of 154 cells/µl and a maximum of 2600 cells/µl) and the mean CRP level of the patients was 99.36 ± 8.8 mg/l (at least 0.2 mg/l and at most 423 mg/l) (Figure 2). Patients were divided into 4 groups based on the lung involvement in CT scan. 29 patients (23.2%) were in the mild involvement group, 35 ones (20%) in the moderate involvement group, 32 patients (25%) in the severe involvement group, and 29 ones (23.2%) in the very severe involvement group. Another classification was also performed and the patients were divided into two groups of lymphocyte count < 1100×10⁹/L and lymphocyte count > 1100×10⁹/L, and CRP <100 mg/L and CRP >100 mg/L. Table 1 shows demographic information of the patients in each group.
The results of the present study showed that the prevalence of lymphocytes < 1100×10⁹/L and CRP >100 mg/L was higher in patients with severe and very severe lung involvement than in those with mild and moderate involvement, which is statistically significant (p < 0.001). The comparison of the groups in terms of the difference between the mean absolute number of lymphocytes and the CRP levels using the ANOVA and post hoc showed that the patients with severe and very severe lung involvement had fewer lymphocytes (p < 0.001) and higher CRP levels (p < 0.001) than other groups (Figure 3, Figure 4).
The results of the Pearson correlation analysis (after normality assessment) used to examine the relationship between absolute lymphocyte count and CRP levels and the patients' scores of lung involvement in HRCT indicated a significant negative correlation between the absolute number of lymphocytes and the CT scan findings so that increased lung involvement in HRCT was associated with decreased absolute lymphocyte count (p < 0.001). On the contrary, there was a positive correlation between the severity of pulmonary involvement and the CRP levels. In other words, an increased percentage of pulmonary involvement in HRCT led to an elevated CRP level (p < 0.001). The results of linear regression analysis (after making the assumptions) also showed that absolute lymphocyte count and CRP levels were the factors used to predict the severity of lung involvement based on HRCT in patients with pneumonia caused by Covid-19 (Table 2).
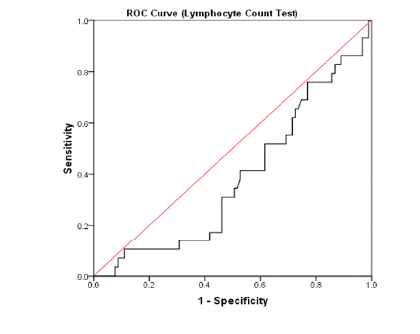
In this study, the ROC curve (Receiver Operating Characteristic) was used to determine the diagnostic value of laboratory tests such as blood lymphocyte count and CRP in predicting the severity of pulmonary involvement in patients with pneumonia caused by COVID-19. Considering HRCT as a standardized test in blood lymphocyte counting, we found the area under the ROC curve to be 0.38 with a confidence interval of 0.27 - 0.49, showing the lack of accuracy and strength of this test to diagnose the severity of lung involvement in COVID-19 patients with pneumonia.
The results of the diagnostic value analysis for the CRP test showed that the area under the ROC curve was 0.76 with a confidence interval of 0.67 - 0.85. Thus, for the CRP = 98 mg/L, the highest sensitivity and the highest specificity were 72% and 71%, respectively, suggesting that the CRP measurement test could be an acceptable test for predicting the severity of lung involvement in patients with COVID-19 pneumonia (Figure 5, Figure 6).
Discussion
Based on the results of this study, which aimed to determine the relationship between lymphocyte counts and CRP levels and the lung CT scan findings of 125 patients with pneumonia caused by COVID-19, the patients with severe pulmonary involvement due to COVID-19 pneumonia had a higher prevalence of lymphopenia and a positive CRP than those with mild and moderate lung involvement. Guan et al. examined the data from 1,099 cases of COVID-19 in China and found that 83.2% of patients had lymphocytopenia and 60.7% of the patients had increased CRP7, 8, 9. Similarly, Gemin Zhang stated in his study that lymphocyte percentage < 10%, and CRP > 150 mg/L were associated with severe coronavirus pneumonia in 20197.
Lymphopenia has been reported in various viral diseases, such as SARS, where the main mechanism of lymphopenia might be an increase in vascular permeability, but its mechanism is unknown in patients with COVID-1910, 11, 12. However, the results obtained in the present study could be justified based on four possible pathophysiological mechanisms that researchers have considered for the lack of lymphocytes in these patients.
In this study, a significant negative correlation was found between the absolute number of lymphocytes and the intensity of lung CT scan findings in patients with COVID-19 pneumonia. Contrary to this finding, the correlation between CRP and COVID-19 pneumonia severity was positive. Analysis of clinical data from 115 patients with pneumonia caused by COVID-19 yielded similar results in studies by Jiheng Liu and Liu Y in China. They found that lymphopenia was associated with pneumonia severity and CRP levels10, 11, 12, 13. In line with this finding, the results of a meta-analysis by Qianwen Zhao on the latest COVID-19 studies from December 2019 to March 22, 2020, in English and Chinese, showed that patients with severe pulmonary involvement due to COVID-19 pneumonia had higher lymphopenia and were at a threefold increased risk of intensive COVID-19 pneumonia compared to the non-severe group14.
The results of a retrospective study by Wu et al., who analyzed the possible risk factors for ARDS and death among 201 patients with COVID-19 pneumonia in Wuhan, China, showed a significant relationship between lymphopenia and increased CRP and acute respiratory distress syndrome15. In their studies, Evangels Terpos and L. Wang also reported that pulmonary nodule diameter in HRCT and the incidence of severe COVID-19 infection had a positive correlation with CRP levels16, 17.
The CRP level is associated with the level of inflammation and indicates the activation of the immune system, penetration of lymphocytes, use of immune molecules, and prevalence of inflammation, and is clinically considered one of the early signs of nosocomial infections in COVID-19 patients4, 18, 19. In the early stages of the disease, CRP can reflect the severity of the disease and be used as an important indicator for early diagnosis and control of COVID-1920, 21, 22.
In this research, the results of examining the diagnostic value of laboratory tests to measure the number of blood lymphocytes and CRP levels for determining the severity of pulmonary involvement in patients with pneumonia caused by COVID-19 (standard HRCT test) using the ROC curve (Receiver Operating Characteristic) showed that the CRP measurement test, unlike the blood lymphocyte count test, could be acceptable for diagnosing the severity of lung involvement in COVID-19 pneumonia patients.
A similar result was found by Wei Chen et al., showing that the correlation between plasma CRP levels and COVID-19 pneumonia severity in HRCT (independent of age and lymphocyte count) was positive. They also analyzed the CRP diagnostic function using the ROC curve and compared it with HRCT intensity in COVID-19 pneumonia. The area under the curve was 0.898, and the P value was < 0.001. In another study by Chaochao Tan to analyze the CRP diagnostic function in predicting severe COVID-19 pneumonia, the area under the ROC curve was found to be 0.87, and the P value was < 0.001. The results of both studies show the good accuracy and strength of CRP in predicting the severity of COVID-19 pneumonia in the early stages of the disease, which is consistent with the results of the present study23, 24.
Although previous studies introduced CT scans as a quick and accurate way of screening for lung infections with unique diagnostic benefits, due to the limitations of performing CT scans under pandemic conditions including high cost, requirement for specialist staff, and risks associated with patient transportation and examination, the CRP assay test can be an acceptable test to diagnose the severity of lung involvement in patients with pneumonia17. There are limitations to undergoing CT scans in COVID-19 pandemic conditions, including high cost, the need for specialized staff, and the risks associated with transporting and examining patients. Alternatively, the CRP measurement test can be an acceptable test to diagnose the severity of lung involvement in patients with COVID-19 pneumonia14, 15, 17.
One of the weaknesses of the present research is the small sample size and the collection of data from only one hospital, which can lead to bias in the results. In the end, more extensive studies on other laboratory indices and inflammatory factors are recommended to predict the severity of CT scan involvement in patients with COVID-19. Optimal resource utilization is also recommended.
Conclusions
In this study, a significant positive correlation was found between CRP levels and the severity of pneumonia caused by COVID-19. The CRP measurement test can also be an acceptable test for predicting the severity of lung involvement in COVID-19 pneumonia patients.
Abbreviations
ANOVA: Analysis of Variance, ARDS: Acute Respiratory Distress Syndrome, CBC: Complete Blood Count, COVID-19: Coronavirus Disease 2019, CRP: C-reactive protein, CT scan: Computed Tomography scan, HRCT: High-Resolution Computed Tomography, ICU: Intensive Care Unit, PCR: Polymerase Chain Reaction, ROC curve: Receiver Operating Characteristic curve, SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2, SPSS: Statistical Package for the Social Sciences
Acknowledgments
None.
Author’s contributions
MP, EB, HF designed and implemented the study, performed analysis, interpreted the data, and contributed to drafting the manuscript. PL and MF interpreted the data, drafted and revised the manuscript. SSH and MP extracted the data and contributed to the data management. All authors read and approved the submitted manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences, with the code of ethics IR.MUMS.REC.1399.093.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Huang
C.,
Wang
Y.,
Li
X.,
Ren
L.,
Zhao
J.,
Hu
Y.,
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet.
2020;
395
(10223)
:
497-506
.
View Article PubMed Google Scholar -
Zhu
N.,
Zhang
D.,
Wang
W.,
Li
X.,
Yang
B.,
Song
J.,
China Novel Coronavirus Investigating
Research Team
A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine.
2020;
382
(8)
:
727-33
.
View Article PubMed Google Scholar -
World Health Organization. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. 2020. at https://www.who.int/dg/speeches/detail/who- director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Published February 11, 2020..
.
-
Guan
W.J.,
Ni
Z.Y.,
Hu
Y.,
Liang
W.H.,
Ou
C.Q.,
He
J.X.,
Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv.
2020
.
View Article Google Scholar -
World Health Organization. Coronavirus Disease 2019(COVID-19): situation report—30. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200219- sitrep-30-covid-19.pdf?sfvrsn=6e50645_2. Published February 20, 2020. Accessed February 20, 2020. 2020
.
-
Wang
D.,
Hu
B.,
Hu
C.,
Zhu
F.,
Liu
X.,
Zhang
J.,
Wang
B.,
Xiang
H.,
Cheng
Z.,
Xiong
Y.,
Zhao
Y.,
Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA.
2020;
323
(11)
:
1061-9
.
View Article Google Scholar -
Zhang
G.,
Zhang
J.,
Wang
B.,
Zhu
X.,
Wang
Q.,
Qiu
S.,
Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respiratory Research.
2020;
21
(1)
:
1-0
.
View Article PubMed Google Scholar -
Tan
L.,
Wang
Q.,
Zhang
D.,
Ding
J.,
Huang
Q.,
Tang
Y.Q.,
Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduction and Targeted Therapy.
2020;
5
(1)
:
1-3
.
PubMed Google Scholar -
Guan
W.J.,
Ni
Z.Y.,
Hu
Y.,
Liang
W.H.,
Ou
C.Q.,
He
J.X.,
China Medical Treatment Expert Group for Covid-19
Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine.
2020;
382
(18)
:
1708-20
.
View Article PubMed Google Scholar -
Liu
J.,
Li
H.,
Luo
M.,
Liu
J.,
Wu
L.,
Lin
X.,
Li
R.,
Wang
Z.,
Zhong
H.,
Zheng
W.,
Zhou
Y.,
Lymphopenia acted as an adverse factor for severity in patients with COVID-19: a single-centered, retrospective study. Research Square.
2020;
2020
.
View Article Google Scholar -
Chen
R.F.,
Chang
J.C.,
Yeh
W.T.,
Lee
C.H.,
Liu
J.W.,
Eng
H.L.,
Role of vascular cell adhesion molecules and leukocyte apoptosis in the lymphopenia and thrombocytopenia of patients with severe acute respiratory syndrome (SARS). Microbes and Infection.
2006;
8
(1)
:
122-7
.
View Article PubMed Google Scholar -
Liu
C.Y.,
Huang
L.J.,
Lai
C.H.,
Chen
H.P.,
Chen
T.L.,
Fung
C.P.,
Clinical characteristics, management and prognostic factors in patients with probable severe acute respiratory syndrome (SARS) in a SARS center in Taiwan. Journal of the Chinese Medical Association.
2005;
68
(3)
:
110-7
.
View Article PubMed Google Scholar -
Liu
Y.,
Yang
Y.,
Zhang
C.,
Huang
F.,
Wang
F.,
Yuan
J.,
Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Science China. Life Sciences.
2020;
63
(3)
:
364-74
.
View Article PubMed Google Scholar -
Zhao
Q.,
Meng
M.,
Kumar
R.,
Wu
Y.,
Huang
J.,
Deng
Y.,
Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. International Journal of Infectious Diseases.
2020;
96
:
131-5
.
View Article PubMed Google Scholar -
Wu
C.,
Chen
X.,
Cai
Y.,
Xia
J.,
Zhou
X.,
Xu
S.,
Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine.
2020;
180
(7)
:
934-43
.
View Article PubMed Google Scholar -
Terpos
E.,
Ntanasis-Stathopoulos
I.,
Elalamy
I.,
Kastritis
E.,
Sergentanis
T.N.,
Politou
M.,
Hematological findings and complications of COVID-19. American Journal of Hematology.
2020;
95
(7)
:
834-47
.
View Article PubMed Google Scholar -
Wang
L.,
C-reactive protein levels in the early stage of COVID-19. Medecine et Maladies Infectieuses.
2020;
50
(4)
:
332-4
.
View Article PubMed Google Scholar -
Feng
G.,
Zheng
K.I.,
Yan
Q.Q.,
Rios
R.S.,
Targher
G.,
Byrne
C.D.,
COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. Journal of Clinical and Translational Hepatology.
2020;
8
(1)
:
18-24
.
View Article PubMed Google Scholar -
Bilgir
O.,
Bilgir
F.,
Calan
M.,
Calan
O.G.,
Yuksel
A.,
Comparison of pre- and post-levothyroxine high-sensitivity c-reactive protein and fetuin-a levels in subclinical hypothyroidism. Clinics.
2015;
70
(2)
:
97-101
.
View Article PubMed Google Scholar -
Warusevitane
A.,
Karunatilake
D.,
Sim
J.,
Smith
C.,
Roffe
C.,
Early diagnosis of pneumonia in severe stroke: clinical features and the diagnostic role of C-reactive protein. PLoS One.
2016;
11
(3)
:
e0150269
.
View Article PubMed Google Scholar -
Chalmers
S.,
Khawaja
A.,
Wieruszewski
P.M.,
Gajic
O.,
Odeyemi
Y.,
Diagnosis and treatment of acute pulmonary inflammation in critically ill patients: the role of inflammatory biomarkers. World Journal of Critical Care Medicine.
2019;
8
(5)
:
59-71
.
View Article PubMed Google Scholar -
Matsumoto
H.,
Kasai
T.,
Sato
A.,
Ishiwata
S.,
Yatsu
S.,
Shitara
J.,
Association between C-reactive protein levels at hospital admission and long-term mortality in patients with acute decompensated heart failure. Heart and Vessels.
2019;
34
(12)
:
1961-8
.
View Article PubMed Google Scholar -
Chen
W.,
Zheng
K.I.,
Liu
S.,
Yan
Z.,
Xu
C.,
Qiao
Z.,
Plasma CRP level is positively associated with the severity of COVID-19. Annals of Clinical Microbiology and Antimicrobials.
2020;
19
(1)
:
18
.
View Article PubMed Google Scholar -
Tan
C.,
Huang
Y.,
Shi
F.,
Tan
K.,
Ma
Q.,
Chen
Y.,
C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. Journal of Medical Virology.
2020;
92
(7)
:
856-62
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 10 (2024)
Page No.: 6831-6837
Published on: 2024-10-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 1446 times
- PDF downloaded - 506 times
- XML downloaded - 59 times
 Biomedpress
Biomedpress







