The ornithine decarboxylase, NO-synthase activities and phospho-c-Jun content under experimental gastric mucosa malignancy
Abstract
Ornithine decarboxylase is the first and key regulatory enzyme in synthesis of polyamines, which are essential for cell proliferation and differentiation, so its aberrant regulation is reported to play a role in neoplastic transformation and tumours growth. That’s why, there were analysed some major links of metabolic pathways that are closely related to tumorigenesis: ornithine decarboxylase, and the NADPH-dependent enzyme nitric oxide synthase, the nuclear phosphoprotein c-Jun, that could play an important role in the development of gastric cancer malignancy.The gastric carcinogenesis was initiated in rats by 10-week replacement of drinking water by 0.01% N-methyl-N'-nitro-N-nitrosoguanidine solution, at the same time they were redefined on the diet containing 5% NaCl. After this period expiry the animals were fed with standard diet till the end of the 24th week. The gastric mucosa cells were extracted at the end of the 4th, 6th, 8th, 10th, 12th, 18th and 24th week and underwent biochemical examinations. It was established the elevated phospho-c-Jun content, ornithine decarboxylase and inducible nitric oxide synthase activities from 6th to 24th week of gastric cancer development compared to the control references. The increasing of ornithine decarboxylase activity could probably be caused by the growth of phospho-c-Jun, it is also belonging to an ornithine decarboxylase transactivation effects. Thus, it was shown that the increase of ornithine decarboxylase and inducible nitric oxide synthase activities, phospho-c-Jun and nitrite-ions accumulation in gastric mucosa epithelial cells were associated with the gastric malignant progression. The complex relationships between the examined enzymes and transcription activator that pointed to an aggravation of pathological disturbances due to reciprocal action between ornithine decarboxylase and c-Jun and nitric oxide synthase participation.
Introduction
Normal structure and functioning of gastric mucosa are determined by regulated division rate of proliferating cells in neck regions of glands. Increasing evidence has indicated that cellular organic cationspolyamines are necessary for normal epithelial proliferation Liu, 2004Timmons, 2013. Polyamine level is regulated by the enzyme ornithine decarboxylase (ODC,EC 4.1.1.17) Timmons, 2013. ODC is a key enzyme of polyamine synthesis and catalyzes the decarboxylation of ornithine to putrescine. It is one of the enzymes necessary for progression into S phase Bello-Fernandez et al.,1993, but it aberrant regulation plays an important role in the wide variety of neoplastic transformations Pegg, 2006.
It is clear that polyamines affect the expression of growth-related genes and these effects depend on a cell type. Polyamines are absolutely required for c-myc and c-junmRNA synthesis and their depletion significantly decreases the transcription rates of these genes in IEC-6 cells Liu, 2004Timmons, 2013. Also, polyamine deficiency results in a significant decrease in c-fos, c-myc and c-junеxpression in іntestinal crypt cells Wang et al., 1993.
The proto-oncogenec-junencodes for a resident nuclear protein c-Junthat is being the target of serine protein kinase cascade has also been implicated in the control of cell cycle by cyclin D1 promoter activation during M-G1 transition. Phosphorylation of c-Jun by c-Jun N-terminal kinase (JNK) is the most important activity regulation that affects on DNA-binding, stability, аbility to interact with other proteins, and transactivation potential.c-Jun is expressed at the low level in different cells, but growth factors, oncogenes, cytokines, environmental stress, bacterial and viral infections upregulate expression of c-Jun Eriksson , 2005. It is believed that c-Jun is a critical promoter of proliferation and deregulated expression and activation of its oncogene are frequently observed in wide variety of cancers Taira et al., 2012. There is report that endogenous nitric oxide (NO) can suppress JNK through a thiol-redox mechanism and modulate c-Jun phosphorylation rate. Redox regulation of JNK by NO may be important for revealing the mechanism by which inducible nitric oxide synthase (iNOS) induction and NO mediate intracellular signalling leading to various cellular functions Park et al.,2000.
Besides that polyamines stimulate DNA synthesis and increase growth-related genes transcription, there is data that increasing of nitric oxide synthase (NOS, EC1.14.13.39) activity and addition exogenous NO levels have been recognized to result in inhibition of cell proliferation. It was shown that NO inhibits tumour cell proliferation by inhibiting ODC by Snitrosylation. The cytostatic effects of NO were reversed by the exogenous polyamines adding but not by ornithine, that points on ODC inhibition by NO Bauer et al., 2001.
So one of the major links of metabolic pathway, that are closely related to tumorigenesis, is the NADPHdependent enzyme nitric oxide synthase catalyzing the production of nitric oxide fromL-arginine. Nitric oxide has conflicting actions on different tumours. The role of NO as an importantcellular signallingmolecule in tumour cell apoptosis and survival depends on its concentration, cell type, duration of exposure and other factors Ma et al., 2007.
The present study was focused on the hypotheses that changes of ODC and NO-synthase activity, NO and phospho-c-Jun contents might play role in the mechanisms of chemical-induced gastric mucosa malignancy, thus, the aim of the study was to determine the activities of ODC, NOS, NO and phospho-c-Jun contents in gastric mucosa cells during the gastric cancer development in rats. The study demonstrated the increase of ODC and iNOS activities and phospho-c- Jun and nitrite-ions accumulation in gastric mucosa epithelial cells that were associated with gastric malignant progression.
Materials and methods
Experimental design
Investigations were carried out on white male rats (n=112) with initial weight of 100±20 g.
Gastric carcinogenesis was initiated by 10-week replacement of drinking water by 0.01% solution of carcinogen N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) while the rats were fed with fodder containing 5% Sodium Chloride (NaCl). Then the animals were fed standard vivarium diet for the following 14 weeks Kuroiwa et al., 2007Takahashi et al., 1994. Control animal group was fed standard diet during the whole experiment period.Samplings of experimental material were performed at the end of the 4th, 6th, 8th, 10th, 12th, 18th, and 24th week. Pathology development was verified by histological methods. The mucosa of isolated stomachs was turned outward and mucous membrane cells were extracted for analysis.
Cell isolation
Gastric mucosa cells were isolated by enzymatic disaggregation method with pronase usage TAIROV et al., 1983.
ODC activity assay
ODC activity was measured in supernatant obtained by cell homogenate centrifugation at 20 000 g for 15 min at 4ºC. Spectrophotometric assay for ODC was based on solubility in 1-petanol of coloured product of its activity, putrescine, with 2,4,6- trinitrobenzenesulfonic acid Ngo et al., 1987. ODC activity was represented in nM of trinitrophenyl adducts of putrescine per 1 mg of protein per 1 min.
NOS activity assay
Evaluation of NOS activity (Сa2+-dependent and independent) was based on the combination of classic method Salter et al., 1991 with its modification Boyde and Rahmatullah, 1980Chin et al., 1999 that was adapted to the spectrophotometric assay of one of the reaction product L-citrulline in CaCl2 present. The activity was represented in μM of L-citrulline per 1 mg of protein per 1 min. iNOS activity was determined similarly, but there was added 2 μМ ЕDТА instead of CaCl2.
Determination of nitrite-ions content
Nitrite-ions content was detected in deproteinizated homogenate aliquots by Griess reagent Moshage et al., 1995. Absorbance was registered at λ=540 nm and nitrite-ions content was expressed in nM per 1 mg of protein.
Determination of phospho-c-Jun content
Phospho-c-Jun content was determined in gastric mucosa cell lysate prepared with the use of Cell Lysis Buffer (Cell Signaling Technology, USA). Phospho-c- Jun content was measured by Sandwich ELISA method using the assay kit PathScan Phospho-c-Jun (Ser63) (Cell Signaling Technology, USA) and represented in conventional unit of absorbancy (λ=450 nm) on mg of protein.
Protein concentration assay
Protein concentration was measured by Bradford’s method Bradford, 1976.
Statistical analysis
Experimental data were processed by common methods of variance analysis with 7 repeats. Reliability of discrepancies between two samplings was determined using Student’s criterion. The results are presented in the values of the arithmetic mean and mean square error, M±s Brandt, 1975.
Ethics statement
Investigations were carried out in compliance with the main standards of keeping and working with laboratory animals and the rules of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes as well as with the ethic norms specified in Ukrainian legislation.
Results and discussion
The assay of the activity of polyamine metabolism enzyme as biological marker of malignant growth is useful in view of polyamine rate affecting ion channels, cell-cell interactions, cytoskeleton, signalling via phosphorylation and other mechanisms. Increased polyamine levels and ODC activity are the first event detectable during cell transformation provides reliable marker in estimating the cancer progression Ngo et al., 1987Pegg, 1988Pegg, 2006.
There was the task to measure the ODC activity in gastric mucosa cells during the 10 weeks of MNNG administration with the following-up 14-week carcinogen- free period. It was established in average 2.3- fold increase in ODC activity in all stages of gastric cancer malignancystudy compared to the control ( Figure 1 ).
It should be notedthat the termination of the gastric carcinogen exposure on 10 weeks of experiment was not accompanied by marked changes in activity of investigated enzyme. So the MNNG despite its carcinogenic effect has no direct influence on ODC activation, like it was established by O’Brien (1967) for the active carcinogenic component of croton oil 12-O-tetracanoyl- phorbol-13-acetate.
Perhaps the one of the principle factors of the revealed increase in ODC activity during the experiment is the stage of carcinogenesis under MNNG, thus initial period characterised ODC activation in gastric cells but the last timepoint when adenocarcinomas were registered and MNNG treatment was ceased, there wasn’t ODC activity normalization. An increased ODC activity has also been reported to occur in many cells exposed to chemical carcinogens or tumour promoters, including MNNG Okuzumi et al., 1991. The induction of ODC activity plays an importantrole in initiation and promotion steps of the model and prevention of this rise by using specific inhibitors such as DFMO reduces tumour formation Shantz and Pegg, 1994.
Initially, a correlation between ODC activity and tumour- promoting ability was shown in mouse skin carcinogenesis O’Brien, 1976. In the rat colon and in surgically resected human colon bearing colon cancer ODC was elevated not only in colon cancer but also in adenoma Rozhin et al., 1984. Concerning ODC activity in human stomach, there was concluded that gastric cancer tissue had significantly elevated ODC levels over those of mucosa and among mucosa of the stomach, that of the pyloric glands had higher ODC activity than that of the fundic glands Okuzumi et al., 1991. It is considered that ODC plays an important role in gastric carcinogenesis, but the mechanism of ODC activity elevation and its influence on human gastric cancer development is not well understood.
It is known that comprehensive alterations in enzyme activities occur very rapidly and are the result of changes in quantity of enzyme protein. ODC is transcriptionally regulated and many factors increase its mRNA synthesis. The promoter region of odcgene includes multiple sequences susceptible to hormones, growth factors and tumour promoters, including сАМР response element, AP-1, AP-2 sites, GC-rich Sp1 binding sites andТАТА box Pegg, 2006.
So it has been supposed a participation of protooncogene c-jun in ODC transactivation. c-Jun is a major component of AP-1 transcription complex and it dominants in many cells despite there are other variants of AP-1 complexes. It is believed, that c-Jun is a critical promoter of cellular proliferation and deregulated expression and activation of its oncogene are observed in wide variety of cancers including gastric malignancy Eriksson , 2005.
There was no any change of the content of activated phosphorylated form of c-Jun at the beginning (4-th week) stage of gastric malignancy, but it was established the relationbetween rising phospho-c-Jun content and ODC activity from the 6th to 24thweek of gastric cancer development ( Figure 2 ): when the gastric mucosa cells were characterized by increased phospho-c- Jun content at average 3.8-fold and the increased ODC activity at average 2.3-fold over the control ( Figure 1 ).
Concerning probable mechanism of ODC-induced cell transformation, constitutive activation of JNK and constitutively increased phosphorylation rate of c-Jun on Ser 63 and Ser 73 was detected. It was established that cell transformation induced by ODC and c-Ha-ras oncogene is accompanied by constitutively increased phosphorylation of c-JunKielosto, 2004.
The 4th week of MNNG- stimulated gastrocarcinogenesis was characterised by ODC activity growth with the reference value of phospho-c-Jun in gastric mucosa cells. The established increase of ODC activity is accorded to the data about malignant transformations including those were initiated by chemical agents.
There is no doubt that cellular polyamines produced by ODC activity are required for proto-oncogene transcription. Polyamines affect the expression of growthrelated genes and these effects depend on a cell type. It has been reported that polyamines stimulatec-myc and c-jun transcription in COLO 320 human colon carcinoma cells. Also,polyamines are essential for c-myc and c-jun mRNA synthesis in intestinal crypt cells IEC-6 cells and their depletion significantly decreases the transcription of these genes but doesn’t effect on post-transcription Liu, 2004Timmons, 2013.
Thus, it is assumed the importance of increasing the activity of ODC in the first place, which triggers the synthesis of mRNA of such oncogenes as c-Jun and consequent it translation, that we apparently observed at the 4th week, but post-translational modification leading to activated a form of c-Jun probably occurred lately.
The growth of the phosphorylated form of c-Jun content from the 6th to 24th weeks of gastric cancer development ( Figure 2 ) was probably caused by its high expression, amplification of c-Jun N-terminal kinasemediated phosphorylation or inactivation of phoshpatases.In contrast to the established increase of the ОDC activity level, the quantity of the phosphorylated form of c-Jun changed at the time of carcinogen treatment cessation (10 week): it decreased but remained high comparing with the control. It is believed that c-Jun has an ability to positively regulate cell proliferation positively regulate cell proliferation by the way of repression of tumour supprеssor gene expression and functіon, and іnduction of cyclin D1 transcription Eriksson , 2005.
These results confirm other findings of immunohistochemical analysis that revealed activation of JNK in human gastric cancer tissue and are consistent with those of others who demonstrated that mice lacking JNK1, a major JNK isozyme, demonstrated a noticeable decrease in gastric cancer induced by N-methyl- N-nitrosourea Shibata et al., 2008.
Although under physiological proliferating stimuli such as hormones, growth factors, the activity of ODC in normally rested cells rises briefly and dramatically for passage from G1 into S phase Timmons, 2013, werevealed that the prolonged increase of this enzyme activity might be an exhibition of long-term carcinogen influence and following-up pathological proliferation. These data corresponded to those obtained from in vivoexperiment where constitutive expression of ODC was observed under the influence of tumor growth promotors after administration of carcinogen initiation dose.
So, unlike normal physiological stimuli, carcinogens, tumor growth promotors, as bile acids, free radicals and some oncogens cause in cells dramatic constitutiverising of ODC activity which in these conditions functions as oncogen. The gene coding for ODC may act as an oncogene since expression of ODC at very high level from appropriate plasmid vectors leads to a transformed phenotype Shantz and Pegg, 1994.
In addition, the ornithine decarboxylase activity is modulated by S-nitrosylation under nitric oxide synthase activation Bauer et al., 2001. So we also studied the major link of metabolic pathway that is closely related to tumorigenesis: the NADPH-dependent enzyme NOS.
A semiessential amino acid, L-arginine, is assumed to be a major precursor of ornithine in mammalian cells through the arginase to yield ornithine and polyamines. But also L-arginine is metabolized by the oxidative desaminase pathway to form NO by the action of nitric oxide synthase. Three isoforms of NOS have been identified: two constitutive, i.e., endothelial NOS and neuronal NOS, and one inducible NOS (iNOS). NO as an intracellular messenger participates in various metabolic processes like immunologic function and proliferation Ma et al., 2007.
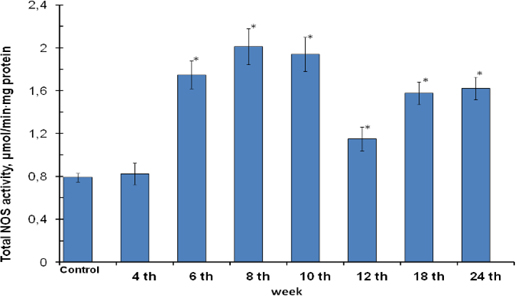
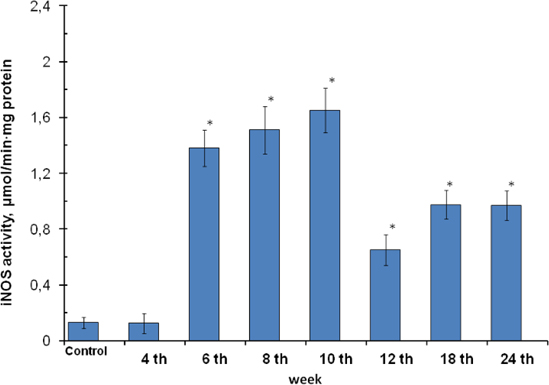
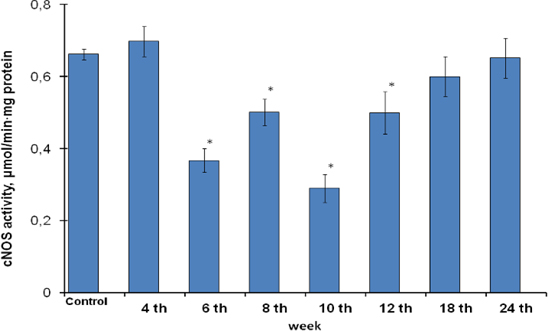
The submitted studies showed the increased total (constitutive and inducible) NOS activity at 2.1-fold over the control in gastric mucosa cells from the 6th to 24th weeks of gastric cancer development ( Figure 3 ). It was generally determined by rising of inducible isoforms which activity increased at 9.2-fold over the control ( Figure 4 ) but didn’t by constitutive isoforms whose activities had fallen or were at the control reference ( Figure 5 )), and accompanied by the rising of nitrite- ions content ( Figure 6 ).
The results obtained by Ma et al., 2007 from human colon biopsy specimens shown the expression of endothelial NOS in the normal and paratumour mucosa; however, it was not expressed in the tumour cells. The pattern of expression of neuronal NOS was similar to that of endothelial NOS. The tumour cells showed moderate or intense staining for iNOS, whereas the glandular cells in normal and paratumour mucosa did not express iNOS Ma et al., 2007, that didn’t contradict with our results. It has been postulated the elevated nitric oxide synthase activity and its expression in gastric cancer cells Kielosto, 2004. The role of nitric oxide in tumour cell apoptosis and survival is cell type and duration exposure dependent. High NO concentration induces cytostasis and cytotoxicity in tumour cells both in vitro and in vivo, however, low NO concentration have a pro-tumour effect in the carcinogenesis and angiogenesis of solid tumours Ma et al., 2007.
The conducted by us histological investigations pointed to the gastric cancer progression and hence the inefficiency of NO to stop the malignancy. The inflammation signs, vessels extension and plethora, the epithelial cell desquamation, and the increased mucus production at the end of the 6th week of experimental gastric carcinogenesis were shown Tymoshenko et al., 2012. After the influence of MNNG during the 8 weeks, gastric mucosa demonstrated atrophic changes. The metaplastic modifications, sites with hyperplasia and atypia, were observed after 10 weeks of MNNG treatment. Adenomas and one adenocarcinoma were detected at the end of the 12th week of the experiment. Such neoplasms were visualized in stomach pyloric regions in 70% rats at the end of the 18th week of experimental gastric carcinogenesis.
According to the literature data MNNG causes alkylation of DNA and nitroamidination of proteins in mucosa epithelial cells, leading to mutations. MNNG methylates the bases of nucleic acids, yielding 7- methylguanine and 3-methyladenine. The guanidino- 14C-labeled carbon of MNNG is efficiently incorporated into proteins due to the conversion of a lysine residue of protein to a nitrohomoarginine residue by transfer of the nitroamidino group from MNNG (Sugimura et al., 1970). In addition, MNNG interacts with membrane lipids and induces free radical production. Also free radical formation is generated by exposure of MNNG in acidic conditions such as that predominant in stomach Gunassekaran et al., 2010.
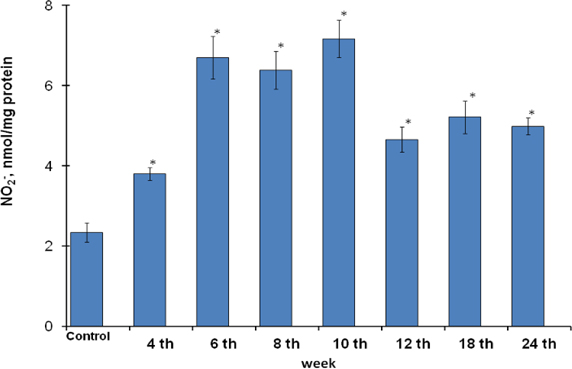
Although the gastric mucosa cells were characterized by control reference of NOS activity at the end of the 4th week of MNNG and NaCl consumption ( Figure 3 ) and significant histological changes at the same time point weren’t observed, but accumulation of the nitrite- ions in gastric mucosa epithelial cells at this time point were observed ( Figure 6 ).
The increased level of NO2- content was noticed during the MNNG consumption from the 6th to 10th weeks compared to the periods when the rats were fed with standard diet till the end of the 24th week. The6th, 8th and 10th weeks were characterized bynitrite-ions content growthin gastric mucosa cells at 2.9, 2.7 and 3.1 times respectively. There was increasing of NO2- at 2.0, 2.2 and 2.1 times at the end of the 12th, 18th and 24thweeks of gastric cancer development.
Also, it was noticeable the highest rate of nitrite-ions at the end of the 4th week. It is known that MNNG transforms into the active form N-methyl-N´- nitroguanidine in gastric acidic conditions and is accompanied by realising of nitrous acid Sugimura and Fujimura, 1967 that might determine the highest nitrite- ions content in times of MNNG consumption. After the end of the 10th week this exogenous infusion of nitrous acid discontinued and was reflected in the decreasing of NO2- content compared to the previous weeks. It should be noted if the rise of NO2- at the 4th week might be determined only by MNNGinfluence, the established changes of NO2- content from the 6th to 24th weeks were caused by the increase of the inducible NOS activity ( Figure 3 ).
In addition, clinical and preclinical studies have shown that eflornithine (DMFO), an ODC inhibitor, has significant chemopreventive and cytostatic effects against colon, skin, cervical and pancreatic cancers and should be evaluated in combination with other drugs in anticipation of future clinical trials Bailey et al., 2010Laukaitis and Gerner, 2011Mohammed et al., 2014Vlastos et al., 2005. Also the antioxidant supplementation was associated with the decrease of mucosal ODC in patients with premalignant changes in gastric mucosa Marotta et al., 2004.
Conclusion
The increase of ODC and iNOS activities, phospho-c- Jun content, and accumulation of the nitrite-ions in gastric mucosa epithelial cells from the 6th to 24th week were observed and shown the complex ambiguous relationships between ODC and c-Jun during the process of gastric cancer development, that pointed to an aggravation of pathological disturbances due to reciprocal action between ODC and c-Jun.
Abbreviations
AP-1: Activator protein-1
cNOS: Constitutive nitric oxide synthase
iNOS: Inducible nitric oxide synthase
JNK: c-Jun N-terminal kinase
MAP: kinase Mitogenactivated protein kinase
MNNG: N-Methyl-N'-nitro- N-nitrosoguanidine
NO: Nitric oxide
NOS: nitric oxide synthase
ODC: Ornithine decarboxylase
References
-
H.H.
Bailey,
K.
Kim,
A.K.
Verma,
K.
Sielaff,
P.O.
Larson,
S.
Snow,
T.
Lenaghan,
J.L.
Viner,
J.
Douglas,
N.E.
Dreckschmidt.
A Randomized, Double-Blind, Placebo-Controlled Phase 3 Skin Cancer Prevention Study of -Difluoromethylornithine in Subjects with Previous History of Skin Cancer. Cancer Prevention Research.
2010;
3
:
35-47
.
-
P.M.
Bauer,
G.M.
Buga,
J.M.
Fukuto,
A.E.
Pegg,
L.J.
Ignarro.
Nitric Oxide Inhibits Ornithine Decarboxylase viaS-Nitrosylation of Cysteine 360 in the Active Site of the Enzyme. Journal of Biological Chemistry.
2001;
276
:
34458-34464
.
-
C.
Bello-Fernandez,
G.
Packham,
J.L.
Cleveland.
The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proceedings of the National Academy of Sciences.
1993;
90
:
7804-7808
.
-
T.R.C.
Boyde,
M.
Rahmatullah.
Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Analytical Biochemistry.
1980;
107
:
424-431
.
-
M.M.
Bradford.
A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical.
1976;
Biochemistry72
:
248-254
.
-
Z.
Brandt.
Statistical Methods for Analysis of Observations [Russian translation]. Mir, Moscow.
1975;
87
.
-
S.Y.
Chin,
K.N.
Pandey,
S.-J.
Shi,
H.
Kobori,
C.
Moreno,
L.G.
Navar.
Increased activity and expression of Ca2+-dependent NOS in renal cortex of ANG II-infused hypertensive rats. American Journal of Physiology-Renal Physiology.
1999;
277
:
F797-F804
.
-
M.
Eriksson.
АР-1 transcription factor in cell differentiation and survival. HBGS, Helsinki.
2005
.
-
G.R.
Gunassekaran,
R.
Gayathri,
D.K.D.
Priya,
S.
Muruga,
D.
Sakithsekaran.
Protective role of gossypol against N-methyl-Nnitro- N-nitrosoguanidine (MNNG) induced gastric carcinogenesis in experimental rats. International Journal of Medicine and Medical Sciences.
2010;
2
:
121-127
.
-
M.
Kielosto.
Reversible Regulation of the Transformed Phenotype of Ornithine Decarboxylase- and Ras-Overexpressing Cells by Dominant- Negative Mutants of c-Jun. Cancer Research.
2004;
64
:
3772-3779
.
-
Y.
Kuroiwa,
Y.
Ishii,
T.
Umemura,
K.
Kanki,
K.
Mitsumori,
A.
Nishikawa,
H.
Nakazawa,
M.
Hirose.
Combined treatment with green tea catechins and sodium nitrite selectively promotes rat forestomach carcinogenesis after initiation with N-methyl-N’- nitro-Nnitrosoguanidine. Cancer Science.
2007;
98
:
949-957
.
-
C.M.
Laukaitis,
E.W.
Gerner.
DFMO: Targeted risk reduction therapy for colorectal neoplasia. Best Practice & Research Clinical Gastroenterology.
2011;
25
:
495-506
.
-
L.
Liu.
Polyamine-modulated expression of c-Myc plays a critical role in stimulation of normal intestinal epithelial cell proliferation. AJP: Cell Physiology.
2004
.
-
Q.
Ma,
Y.
Wang,
X.
Gao,
Z.
Ma,
Z.
Song.
L-Arginine Reduces Cell Proliferation and Ornithine Decarboxylase Activity in Patients with Colorectal Adenoma and Adenocarcinoma. Clinical Cancer Research.
2007;
13
:
7407-7412
.
-
F.
Marotta,
R.
Barreto,
H.
Tajiri,
J.
Bertuccelli,
P.
Safran,
C.
Yoshida,
E.
Fesce.
The Aging/Precancerous Gastric Mucosa: A Pilot Nutraceutical Trial. Annals of the New York Academy of Sciences.
2004;
1019
:
195-199
.
-
A.
Mohammed,
N.B.
Janakiram,
V.
Madka,
R.L.
Ritchie,
M.
Brewer,
L.
Biddick,
J.M.R.
Patlolla,
M.
Sadeghi,
S.
Lightfoot,
V.E.
Steele.
Eflornithine (DFMO) Prevents Progression of Pancreatic Cancer by Modulating Ornithine Decarboxylase Signaling. Cancer Prevention Research.
2014;
7
:
1198-1209
.
-
H.
Moshage,
B.
Kok,
J.R.
Huizenga,
P.
Jansen.
Nitrite and nitrate determinations in plasma: a critical evaluation. Clinical Chemistry.
1995;
41
:
892-896
.
-
T.T.
Ngo,
K.L.
Brillhart,
R.H.
Davis,
R.C.
Wong,
J.H.
Bovaird,
J.J.
Digangi,
J.L.
Ristow,
J.L.
Marsh,
A.P.H.
Phan,
H.M.
Lenhoff.
Spectrophotometric assay for ornithine decarboxylase. Analytical Biochemistry.
1987;
160
:
290-293
.
-
T.
O’Brien.
The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Research.
1976;
36
:
2644-2653
.
-
J.
Okuzumi,
T.
Yamane,
Y.
Kitao,
K.
Tokiwa,
T.
Yamaguchi,
Y.
Fujita,
H.
Nishino,
A.
Iwashima,
T.
Takahashi.
Increased mucosal ornithine decarboxylase activity in human gastric cancer. Cancer research.
1991;
51
:
1448-1451
.
-
H.S.
Park,
S.H.
Huh,
M.S.
Kim,
S.H.
Lee,
E.J.
Choi.
Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proceedings of the National Academy of Sciences.
2000;
97
:
14382-14387
.
-
A.E.
Pegg.
Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy. Cancer research.
1988;
48
:
759-774
.
-
A.E.
Pegg.
Regulation of Ornithine Decarboxylase. Journal of Biological Chemistry.
2006;
281
:
14529-14532
.
-
J.
Rozhin,
P.S.
Wilson,
A.W.
Bull,
N.D.
Nigro.
Ornithine decarboxylase activity in the rat and human colon. Cancer research.
1984;
44
:
3226-3230
.
-
M.
Salter,
R.G.
Knowles,
S.
Moncada.
Widespread tissue distribution, species distribution and changes in activity of Ca 2+ - dependent and Ca 2+ -independent nitric oxide synthases. FEBS Letters.
1991;
291
:
145-149
.
-
L.M.
Shantz,
A.E.
Pegg.
Overproduction of ornithine decarboxylase caused by relief of translational repression is associated with neoplastic transformation. Cancer Research.
1994;
54
:
2313-2316
.
-
W.
Shibata,
S.
Maeda,
Y.
Hikiba,
A.
Yanai,
K.
Sakamoto,
H.
Nakagawa,
K.
Ogura,
M.
Karin,
M.
Omata.
c-Jun NH2-Terminal Kinase 1 Is a Critical Regulator for the Development of Gastric Cancer in Mice. Cancer Research.
2008;
68
:
5031-5039
.
-
T.
Sugimura,
S.
Fujimura.
Tumour Production in Glandular Stomach of Rat by N-Methyl-N-Nitro-N-Nitrosoguanidine. Nature.
1967;
216
:
943-944
.
-
N.
Taira,
R.
Mimoto,
M.
Kurata,
T.
Yamaguchi,
M.
Kitagawa,
Y.
Miki,
K.
Yoshida.
DYRK2 priming phosphorylation of c-Jun and c- Myc modulates cell cycle progression in human cancer cells. Journal of Clinical Investigation.
2012;
122
:
859-872
.
-
M.
Tairov,
R.
Bersimbaev,
S.
Argutinskaya,
R.
And Salganik.
Cellular Location Of Adenylate Cyclases Stimulated By Histamine And Prostaglandin-E2 In Rat Gastric-Mucosa And Their Role In Regulation Of Gastric-Secretion. Biochemistry-Moscow.
1983;
48
:
888-894
.
-
M.
Takahashi,
A.
Nishikawa,
F.
Furukawa,
T.
Enami,
T.
Hasegawa,
Y.
Hayashi.
Dose-dependent promoting effects of sodium chloride (NaCI) on rat glandular stomach carcinogenesis initiated with Nmethyl- N’-nitro-N-nitrosoguanidine. Carcinogenesis.
1994;
15
:
1429-1432
.
-
J.
Timmons.
Polyamines and Gut Mucosal Homeostasis. Journal of Gastrointestinal & Digestive System.
2013;
3
.
-
M.O.
Tymoshenko,
O.O.
Kravchenko,
L.M.
Gaida,
O.V.
Lynchak,
N.I.
Ruzhytska,
L.I.
Ostapchenko.
Глутатіонтрансферазнаактивністьівміствідновленогоглутатіону вцитозоліклітинслизовоïоболонкишлункащурівпіслявпливукан церогену N-метил-N’-нітро-N-нітрозогуанідину. Biopolym Cell.
2012;
28
:
374-380
.
-
A.-T.
Vlastos,
L.A.
West,
E.N.
Atkinson,
I.
Boiko,
A.
Malpica,
W.K.
Hong,
M.
Follen.
Results of a phase II double-blinded randomized clinical trial of difluoromethylornithine for cervical intraepithelial neoplasia grades 2 to 3. Clinical cancer research.
2005;
11
:
390-396
.
-
J.-Y.
Wang,
S.
McCormack,
M.
Viar,
H.
Wang,
C.-Y.
Tzen,
R.
Scott,
L.
Johnson.
Decreased expression of protooncogenes c-fos, cmyc, and c-jun following polyamine depletion in IEC-6 cells. American Journal of Physiology-Gastrointestinal and Liver Physiology.
1993;
265
:
G331-G338
.
Comments
Downloads
Article Details
Volume & Issue : Vol 3 No 04 (2016)
Page No.: 596-604
Published on: 2016-04-15
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4527 times
- Download PDF downloaded - 1738 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress