Abstract
Introduction: Mesenchymal stem cells (MSCs) are increasingly used for the treatment of various diseases. However, the treatment cost remains relatively high. One reason for this is the complexity and expense involved in the transportation and use of MSCs. Currently, most of the transport of stem cells from the production site to the hospital is carried out under cold conditions with CO2 ice or liquid nitrogen. This transportation is difficult and costly, especially from one country to another. At the site of use, the use of products is also complicated, requiring cells to be properly thawed and sometimes washed to remove cold storage medium before use. Therefore, this study aimed to investigate the transport and storage medium of MSCs at room and cool temperature (2-8 oC).
Methods: MSCs from adipose tissue (ADSCs) and from the human umbilical cord (UCMSCs) were sufficiently proliferated to serve the research under appropriate conditions. The cells were suspended in an injectable, and chemically defined storage medium (CellTravel) at a density of 5.106 cells/mL. Cells were stored at room temperature (RT) and cool temperature 2-8 oC. The cell viability and viable cell counts were monitored after 12, 24, 36, and 48 h of storage. After 48 h, the cells were cultured to assess cell proliferation in vitro. After 72 h of culture, the cells were re-assessed for cell markers using flow cytometry.
Results: The results showed that in CellTravel, cells maintained a high survival rate regardless of preservation at RT or 2-8 oC up to 48 h. After 48 h, the cells maintained a high viability when cultured in vitro. The cells exhibited the particular phenotype of MSCs, similar to that before preservation.
Conclusion: The results of this study indicate that cells can be transported at RT or 2-8 oC for up to 48 h in CellTravel medium with a survival rate of > 80%. This suggests that cells can be transported from one place to another and from one country to another at RT or 2-8 oC, and the cells at the destination can be used directly for treatment.
Introduction
Mesenchymal stem cells (MSCs) are the most common type of stem cell in the body. They are found in the majority of the tissues. In recent years, MSCs have been obtained from bone marrow, umbilical cord blood, umbilical cord, adipose tissue, and dental pulp1, 2, 3, 4, 5, 6, 7. Although MSCs exist in various tissues, they maintain the universal characteristics of MSCs. Therefore, in 2006, Dominici et al. proposed minimum standards for identifying MSCs8; and this standard has been widely used to this day.
The mechanisms of action of MSCs in disease treatment are becoming clearer9, 10, 11, 12. After more than 20 years of research, scientists have summarized the main mechanisms of MSCs in disease treatment, including (1) differentiation into functional cells, (2) immunomodulation, (3) anti-inflammatory effects, (4) stimulation of angiogenesis, (5) stimulation of endogenous stem cells, (6) inhibition of apoptosis in damaged cells, and (7) antimicrobial properties. These mechanisms are due to the self-renewal and differentiation properties of MSCs and their ability to secrete factors (including secretomes and exosomes) that act on the target cells13, 14, 15. Although there are many such mechanisms, MSCs are widely accepted for disease treatment owing to their immunomodulatory properties. Therefore, they have been licensed as treatment drugs for graft-versus-host disease (GVHD)16, 17, 18. In reality, MSCs are used in the treatment of many different diseases through various mechanisms such as chronic obstructive pulmonary disease19, 20, 21, 22, diabetes23, 24, 25, diabetic ulcers26, 27, 28, 29, diabetic kidney disease30…
Despite their great therapeutic potential, the use of MSCs has not been widespread because of the relatively high cost of treatment and complex process of implementation. MSCs produced in the laboratory are transported to hospitals for treatment. The process of transporting cells to hospitals far from the production site or from one country to another usually requires freezing the cells and transporting them under deep-cold conditions. This transportation process not only affects the survival of MSCs if the temperature rises, but also significantly increases the cost of the treatment process. Additionally, for freezing shipping, MSCs need to be stored in a cryopreservation medium with cryoprotectants such as DMSO, so the cells should be washed before use in humans.
Therefore, with the aim of simplifying cell transportation while ensuring high cell survival, reducing transportation costs, and reducing the costs of cell processing before use, this study aimed to evaluate the effectiveness of transporting and storing MSCs in injectable, defined medium — CellTravel over a short period (24h and 48h). The research results aim to provide a recommendation for transporting cells in a short time without necessarily having to freeze or transport frozen cells.
Methods
Proliferative Culture of Mesenchymal Stem Cells
This study was conducted on two types of MSCs: human umbilical cord MSCs (UCMSC) and human adipose tissue-derived MSCs (ADSC). Cells were obtained from the Cell Bank of the Stem Cell Institute (University of Natural Sciences, VNU-HCMC, Vietnam). Cells were thawed using ThawBest solution (Regenmedlab, HCMC, VN). UCMSCs were cultured in MSCCult I medium (Regenmedlab, HCMC, VN) and ADSCs were cultured in ADSCCult I medium (Regenmedlab, HCMC, VN). Cells were subcultured upon reaching 80% confluence using Deattachment solution (Regenmedlab, HCMC, VN). Cells were cultured in a CO2 incubator at 37 °C, 5% CO2, and absolute humidity.
Cell Preservation with CellTravel
CellTravel, an injectable and defined solution made of phosphate saline buffer supplemented with amino acids and human serum albumin (injectable grade), was used for storing UCMSCs and ADSCs. UCMSCs and ADSCs were suspended in CellTravel at a density of 5 × 106 cells/mL. Cells were placed in 5 mL vials and tightly capped. The vials were stored under ambient condition and at 2-8 oC. At 12, 24, 36, and 48h, three vials of cells were used to assess the survival rate (cell viability) and the actual number of living cells in the vial. After 48h, the cells were collected for culture and determination of cell marker levels post-culture. The experiment was repeated independently 3 times.
Survival Rate Assessment
The survival rates of UCMSC and ADSC were determined using two independent methods. Method 1: Flow cytometry technique with 7-AAD staining. Briefly, cells were stained with 7-ADD dye, and the survival rate was analyzed after 5 min of staining on a FACSmelody system (BD Bioscience). Method 2: Using a hemocytometer with trypan blue dye.
Determining Population Doubling Time (PDT)
The population doubling time (PDT) was determined for the cells after various storage conditions and durations. After storage, cells were cultured at an appropriate culture condition at a density of 5000 cells/cm2; after 72 h, cells were harvested to determine cell count. PDT value was determined based on the formula available here: https://www.omnicalculator.com/biology/cell-doubling-time
Cell Marker Evaluation by Flow Cytometry
The expression of MSC markers in both UCMSCs and ADSCs after 72h of culture was assessed using flow cytometry. The cells were stained with antibodies against anti-CD14, CD34, CD45, HLA-DR, CD44, CD73, CD90, and CD105 (BD Bioscience). The cells were analyzed using a FACSMelody flow cytometer (BD Bioscience). An isotype control sample was used for all the tests.
Statistical Analysis
All experiments were independently repeated three times. The results were calculated as the mean ± SD. Statistical significance was set at p < 0.05. All data were processed and plotted using the Prism software.
Results
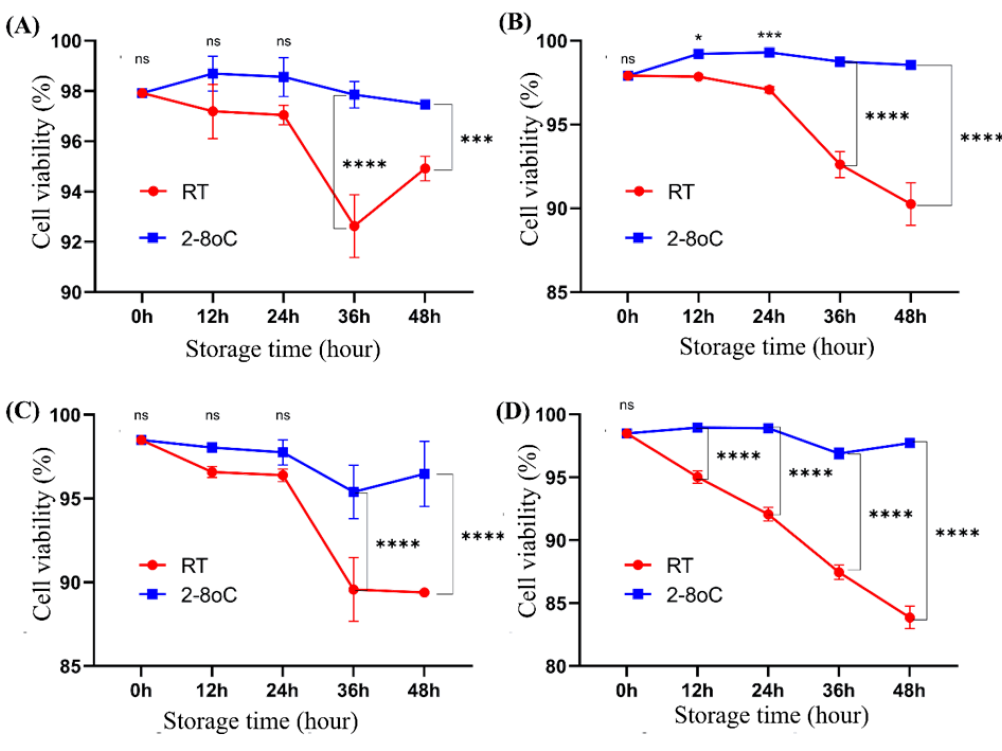
The cell survival rate at 0, 12, 24, 36, and 48 h when stored at room temperature and at 2-8 oC
The survival rates of ADSCs and UCMSCs at the time points of 0, 12, 24, 36, and 48 h stored at RT and 2-8 oC are presented in Figure 1. Compared to transportation conditions at RT, transporting at 2-8 oC helps maintain a high and relatively stable cell survival rate throughout 48 h. Determining the cell survival rate using the trypan blue counting method with a hemocytometer often yields higher survival rates than cell counting using flow cytometry techniques with 7-AAD. After 36 h of storage onwards, for both cell types, cells stored at 2-8 oC show a significantly higher survival rate than those stored at RT (p < 0.05). These results also indicate that ADSCs have a higher survival ability in CellTravel at RT than UCMSCs. The lowest survival rate of ADSCs was 92.63 ± 1.25% at 36 h when stored at RT and counted using trypan blue.
Absolute viable cell count at 0, 12, 24, 36, and 48 h when stored at RT and 2-8 oC
To determine the absolute count of viable cells over the storage period, cells at each time point and under the two storage conditions were counted using a hemocytometer with trypan blue. The results shown in Figure 2 indicate that the absolute number of viable cells stored at RT did not change significantly over storage time. The number of UCMSCs showed a significant increase at the 12h mark when stored at 2-8 oC (7.92 x 106), but at subsequent time points, the number remained relatively stable. However, unlike UCMSCs, ADSCs were capable of proliferating when stored at 2-8 oC. Indeed, the number of ADSCs increased from 5 x 106 at 0 h to 6.41 ± 0.47 x 106 at 12 h, 5.56 ± 0.28 x 106 at 24 h, 6.3 ± 0.57 x 106 at 36 h, and reached 8.10 ± 0.33 x 106 at 48 h. At the time points 12h, 24h, 36h, and 48h, the number of ADSCs stored at 2-8 oC was significantly greater than the number of ADSCs stored at RT (p < 0.01).
Cell proliferation rate after storage
ADSCs and UCMSCs, after storage at RT and 2-8 oC for various time points, maintained their shapes after culturing (Figure 3). The cell proliferation rate was evaluated using the population doubling time (PDT) parameter described in Figure 4. The PDT value did not significantly change when cells were stored in CellTravel at 2-8 oC throughout the 48 h of storage; however, the PDT value statistically increased when stored at RT after 48 h for both ADSCs and UCMSCs. Indeed, data shows that for ADSCs, the PDT value increased from 17.15 ± 0.56 h before storage to 18.88 ± 0.54 h (p < 0.05) in cells after 48 hr storage at RT, while this value was 17.80 ± 0.52 h when stored at 2-8 oC after 48 h (p > 0.05). For UCMSCs, the PDT value increased from 15.32 ± 0.44 h before storage to 16.58 ± 0.54 h after 48 h storage at RT (p < 0.05), while this value was 15.98 ± 0.52 h after 48hr storage at 2-8 oC (p > 0.05).
Cell marker expression after culturing
The marker profile of MSCs showed stable gene expression. This study evaluated MSC cells (ADSCs and UCMSCs) after storage under various conditions and durations in CellTravel and were recultured after 72 h to obtain sufficient cells for marker assessment tests. The results presented in Figure 5 show that although marker expression did change, these changes were not statistically significant. Under all test conditions for both storage durations, ADSCs and UCMSCs after 72 h of culturing maintained the characteristic phenotype of MSCs, where the ratio of cells expressing markers CD14, CD34, CD45, and HLA-DR was below 2%, and markers CD44, CD73, CD90, and CD105 were expressed above 95%.
Discussion
Recently, MSCs have been increasingly utilized owing to their advantages in treating diseases. Notably, MSCs have low antigenicity and immunogenicity, making their allogeneic use more feasible. This has turned MSCs into a cell type that can be produced on a large scale and used for treatment. Consequently, MSCs have been licensed for use as therapeutic agents in several countries. This highlights the importance of cell transportation as a critical issue in the use of MSCs in treatment. This study aimed to evaluate a procedure for storing and transporting MSCs at room and cool temperatures (2 – 8 °C). The results showed that MSCs could be stored in the CellTravel solution with a high survival rate after 48 h at room temperature and cool temperatures (2 – 8 °C). These findings open new avenues for the storage and transportation of MSCs over a short period of time.
In this study, we evaluated two different MSC lines, adipose tissue (ADSCs) and umbilical cord tissue (UCMSCs), which represent different types of MSCs. The cell survival rate was assessed using two different methods: trypan blue staining and 7-AAD staining, with the aim of increasing the accuracy of the results. In general, cell counting using trypan blue staining showed a higher live cell ratio than counting using the flow cytometry technique with 7-AAD staining. However, for both techniques, the storage of MSCs at room temperature (RT) and 2 – 8 °C for ADSCs and UCMSCs resulted in a survival rate of more than 80% after 48 h of transportation. With this high survival rate, the goal of transporting MSCs using CellTravel was fully met for the direct use of MSCs in treatment. Indeed, according to the global standards for cell survival in treatment, cells must achieve a survival rate of >70% to >80%. Combined with the fact that CellTravel is a transfusion-standard medium with defined components, it facilitated the transportation of MSCs usable in treatment after 48 h of transportation at room temperature.
During 48 h of storage at room temperature and 2 – 8 °C, there was a slight fluctuation in cell numbers. This is because some cells continued their cell cycle, which paused upon harvesting. As cells remained active at these storage temperatures, albeit less intensely than at 37 °C, the overall cell number slightly increased after 48 h at 2 – 8 °C, whereas a minor reduction was observed at room temperature. The results also showed that the survival of ADSCs was higher than that of UCMSCs at both RT and 2 – 8 oC after 48h. Despite survival according to the membrane damage assessment methods, we still question whether these cells might enter apoptosis or senescence. Therefore, the next tests we conducted involved culturing the stored cells under regular culturing conditions and analyzing the changes in the PDT values. Surprisingly, at all points examined, the post-storage cells showed similar PDT values (p > 0.05). Although there was a tendency for the PDT value to increase slightly at the 48-hour mark compared to the 24- and 36-hour marks, storage at RT tended to increase PDT values compared to storage at 2 – 8 °C. This experiment showed that storing cells in CellTravel allows MSCs to maintain a high survival rate and retain their proliferation capability after storage, similar to before storage. However, the following question arises: do cells change their gene expression after storage? Do they lose their MSC phenotypes? This question was answered in the final experiment to examine MSC marker expression after various storage times and proliferation cultures. The cells, after being stored at different time points and at different temperatures, continued to be cultured for 72 h and showed that the cells maintained the MSC cell phenotype similar to that before storage at different temperatures throughout 48 h. Summarizing the results, we see that MSCs from adipose tissue and umbilical cord can be stored for transportation for 48 h at room temperature or 2 – 8 °C, and cell transportation at 2 – 8 °C provides better results than at RT.
Along with the idea of transporting cells at room temperature, Buick et al. (2024) conducted a similar study on the CellShip medium using immortal cell lines (HEK293, CHO, HepG2, K562, and Jurkat). The findings from this study showed that immortal cells can be transported under RT conditions for up to 96 h31. The concept of a cool temperature transport medium was introduced by Buick et al. in 201932. Although CellShip is designed as an animal-free medium, it is not recommended whether it meets transfusion standards. Most reports on CellShip transport efficiency are based on immortal or cancer cell lines.
For transporting stem cells at room temperature or cool conditions, Ye et al. (2020) reported that mouse embryonic stem cells (ESCs) could maintain high vitality for 3-5 days at cool temperatures or RT when transported in 100% FBS solution33. Ye et al. (2020) compared the transportation efficiency of ESCs under dry ice (CO2 ice) conditions. The results showed that under room temperature or cool transport conditions, ESCs do not significantly change their characteristics compared with transport in dry ice conditions33. For MSCs, transport under ambient temperature conditions resulted in high survival rates after three days using HemSol34. HemSol is a gelatin-based gel. Therefore, during transport, the cells are encapsulated and fixed within the gel layer. Upon heating the gel to 40 °C, the gel layer melted and released cells. Transporting HemSol resulted in a very high survival rate (up to > 95% after 3 days). However, this rate was only determined using a cell-counting chamber with a red blood cell chamber and trypan blue. Despite its high survival rate, the use of HemSol has several limitations compared to CellTravel. Indeed, HemSol exists as a gel at room temperature; therefore, liquification is a necessary step before use. However, HemSol cannot be a transfusion-standard solution. Cells transported in HemSol need to be centrifuged to remove the HemSol before use. The idea of HemSol represents a significant step forward compared to the use of semi-solid gels from Matrigel35 or agarose and DMEM mixtures36.
This study, despite its results on two types of MSCs, ADSCs and UCMSCs, suggests that transportation efficacy may differ significantly among different types of MSCs from various tissues and among different cell types. However, a major limitation of this study is that we only investigated two MSC lines, and the results may not fully reflect the efficiency of different MSC lines. In addition, this study did not compare the conditions of transportation at room temperature and 2 – 8 °C with those of typical transportation in dry ice. Additionally, the cell storage time in this study was relatively short (2 days); therefore, expanding the investigation period would be necessary to align with the reality of international cell transportation.
Conclusion
This study evaluated the effectiveness of storing MSCs, represented by ADSCs and UCMSCs, in CellTravel solution at room and cool temperatures (2 – 8 °C). The results showed that the cell survival rates remained high after 48 h at both room temperature and 2 – 8 °C. The cells continued to proliferate and expressed normal markers before storage. These results have significant potential for using CellTravel in cell transportation at room temperature and for directly using cells in clinical applications. Using this method, the cost of cell transportation is reduced, making cell transportation simpler. Alongside prior studies, this study once again supports the method of transporting cells at room temperature for short-term cell transportation. However, to facilitate the use of CellTravel, exploring its cell survival capability for longer periods, such as 3 and 5 days, is also necessary.
Abbreviations
7-AAD - 7-Aminoactinomycin D, ADSC - Adipose-Derived Stem Cell, ADSCCult I - (Name of a culture medium for ADSCs, manufactured by Regenmedlab), CD - Cluster of Differentiation (e.g., CD14, CD34, CD45, HLA-DR, CD44, CD73, CD90, CD105), CHO - Chinese Hamster Ovary cells, CO2 - Carbon Dioxide, DMSO - Dimethyl Sulfoxide, ESC - Embryonic Stem Cell, FBS - Fetal Bovine Serum, FCM - Flow Cytometry, GVHD - Graft-versus-Host Disease, HEK293 - Human Embryonic Kidney 293 cells, HepG2 - Hepatocellular carcinoma Cell Line G2, HLA-DR - Human Leukocyte Antigen - DR isotype, Jurkat - A human T lymphocyte cell line, K562 - A human chronic myelogenous leukemia cell line, MSC - Mesenchymal Stem Cell, MSCCult I - (Name of a culture medium for ADSCs, manufactured by Regenmedlab), oC - Degrees Celsius, PDT - Population Doubling Time, RT - Room Temperature, UCMSC - Human Umbilical Cord Mesenchymal Stem Cells, VN - Vietnam, VNU-HCMC - Vietnam National University Ho Chi Minh City.
Acknowledgments
Thank you to the Cell Bank of the Stem Cell Institute at the University of Natural Sciences, VNU-HCMC, Viet Nam, for providing the ADSCs and UCMSCs for this study.
Author’s contributions
Pham Van Phuc (PVP): Conceived and designed the experiments; Wrote the paper. Phan Tuan Kiet (PTK): Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data. Vu Bich Ngoc (VBN): Assisted with the design of the study and provided critical feedback on the manuscript. Nguyen Thi Hien Trang (NTHT): Performed the experiments; Participated in the research design and helped to draft the manuscript. Nguyen Thuan Phat (NTP): Contributed to sample preparation and data collection; Performed the experiments. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Yang
Y.,
Zhang
C.,
Sheng
X.,
Isolation and Culture of Three Kinds of Umbilical Cord Mesenchymal Stem Cells. Journal of Visualized Experiments.
2022;
186
:
e64065
.
View Article Google Scholar -
Gupta
S. Dutta,
Sen
A.,
Priyadarshi
P.,
Ta
M.,
Enzyme-free isolation of mesenchymal stem cells from decidua basalis of the human placenta. STAR Protocols.
2023;
4
(3)
:
102498
.
View Article PubMed Google Scholar -
Naeem
A.,
Gupta
N.,
Naeem
U.,
Khan
M.J.,
Elrayess
M.A.,
Cui
W.,
A comparison of isolation and culture protocols for human amniotic mesenchymal stem cells. Cell Cycle (Georgetown, Tex.).
2022;
21
(15)
:
1543-56
.
View Article PubMed Google Scholar -
Doornaert
M.,
Maere
E. De,
Colle
J.,
Declercq
H.,
Taminau
J.,
Lemeire
K.,
Xenogen-free isolation and culture of human adipose mesenchymal stem cells. Stem Cell Research.
2019;
40
:
101532
.
View Article PubMed Google Scholar -
Saito
T.,
Sato
T.,
Suzuki
K.,
Isolation and culture of human adipose-derived mesenchymal stromal/stem cells harvested from postmortem adipose tissues. Journal of Forensic and Legal Medicine.
2020;
69
:
101875
.
View Article PubMed Google Scholar -
Zheng
S.,
Gao
Y.,
Chen
K.,
Liu
Y.,
Xia
N.,
Fang
F.,
A Robust and Highly Efficient Approach for Isolation of Mesenchymal Stem Cells From Wharton's Jelly for Tissue Repair. Cell Transplantation.
2022;
31
:
9636897221084354
.
View Article PubMed Google Scholar -
Xu
M.,
Xu
J.,
Cheng
D.,
Chen
X.,
Lin
S.,
Si
X.,
Isolation of Umbilical Cord-Derived Mesenchymal Stem Cells with High Yields and Low Damage. Journal of visualized experiments.
2024;
209
.
View Article PubMed Google Scholar -
Dominici
M.,
Le Blanc
K.,
Mueller
I.,
Slaper-Cortenbach
I.,
Marini
F.,
Krause
D.,
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy.
2006;
8
(4)
:
315-7
.
View Article PubMed Google Scholar -
Naji
A.,
Eitoku
M.,
Favier
B.,
Deschaseaux
F.,
Rouas-Freiss
N.,
Suganuma
N.,
Biological functions of mesenchymal stem cells and clinical implications. Cellular and Molecular Life Sciences.
2019;
76
(17)
:
3323-48
.
View Article PubMed Google Scholar -
Yu
X.,
Liu
P.,
Li
Z.,
Zhang
Z.,
Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Frontiers in Endocrinology (Lausanne).
2023;
14
.
View Article PubMed Google Scholar -
Jiang
W.,
Xu
J.,
Immune modulation by mesenchymal stem cells. Cell Proliferation.
2020;
53
(1)
:
e12712
.
View Article PubMed Google Scholar -
Al-Ghadban
S.,
Bunnell
B.A.,
Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology (Bethesda, MD).
2020;
35
(2)
:
125-33
.
View Article PubMed Google Scholar -
M\Huzes
G.,
Sipos
F.,
Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells.
2022;
11
(15)
:
2300
.
View Article PubMed Google Scholar -
Giovannelli
L.,
Bari
E.,
Jommi
C.,
Tartara
F.,
Armocida
D.,
Garbossa
D.,
Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: risk-benefit profile and next steps for the market access. Bioactive Materials.
2023;
29
:
16-35
.
View Article PubMed Google Scholar -
Fan
X.L.,
Zhang
Y.,
Li
X.,
Fu
Q.L.,
Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cellular and Molecular Life Sciences.
2020;
77
(14)
:
2771-94
.
View Article PubMed Google Scholar -
Kebriaei
P.,
Hayes
J.,
Daly
A.,
Uberti
J.,
Marks
D.I.,
Soiffer
R.,
A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation.
2020;
26
(5)
:
835-44
.
View Article PubMed Google Scholar -
Okada
K.,
Miyata
T.,
Sawa
Y.,
Insurance systems and reimbursement concerning research and development of regenerative medicine in Japan. Regenerative Medicine.
2017;
12
(2)
:
179-86
.
View Article PubMed Google Scholar -
Matsushita
S.,
Tachibana
K.,
Kusakabe
T.,
Hirayama
R.,
Tsutsumi
Y.,
Kondoh
M.,
The Roadmap to Approval under Japan's Two-Track Regulatory System: Comparing Six Regenerative Medical Products. Cell Stem Cell.
2020;
27
(4)
:
515-8
.
View Article PubMed Google Scholar -
Bich
P. Le Thi,
Thi
H. Nguyen,
Chau
H. Dang Ngo,
Van
T. Phan,
Do
Q.,
Khac
H. Dong,
Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Research & Therapy.
2020;
11
(1)
:
60
.
View Article PubMed Google Scholar -
Weiss
D.J.,
Casaburi
R.,
Flannery
R.,
LeRoux-Williams
M.,
Tashkin
D.P.,
A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest.
2013;
143
(6)
:
1590-8
.
View Article PubMed Google Scholar -
Weiss
D.J.,
Segal
K.,
Casaburi
R.,
Hayes
J.,
Tashkin
D.,
Effect of mesenchymal stromal cell infusions on lung function in COPD patients with high CRP levels. Respiratory Research.
2021;
22
(1)
:
142
.
View Article PubMed Google Scholar -
de Oliveira
H.G.,
Cruz
F.F.,
Antunes
M.A.,
de Macedo Neto
A.V.,
Oliveira
G.A.,
Svartman
F.M.,
Combined Bone Marrow-Derived Mesenchymal Stromal Cell Therapy and One-Way Endobronchial Valve Placement in Patients with Pulmonary Emphysema: A Phase I Clinical Trial. Stem Cells Translational Medicine.
2017;
6
(3)
:
962-9
.
View Article PubMed Google Scholar -
Izadi
M.,
Nejad
A. Sadr Hashemi,
Moazenchi
M.,
Masoumi
S.,
Rabbani
A.,
Kompani
F.,
Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Research & Therapy.
2022;
13
(1)
:
264
.
View Article PubMed Google Scholar -
Zang
L.,
Li
Y.,
Hao
H.,
Liu
J.,
Cheng
Y.,
Li
B.,
Efficacy and safety of umbilical cord-derived mesenchymal stem cells in Chinese adults with type 2 diabetes: a single-center, double-blinded, randomized, placebo-controlled phase II trial. Stem Cell Research & Therapy.
2022;
13
(1)
:
180
.
View Article PubMed Google Scholar -
Lian
X.F.,
Lu
D.H.,
Liu
H.L.,
Liu
Y.J.,
Yang
Y.,
Lin
Y.,
Safety evaluation of human umbilical cord-mesenchymal stem cells in type 2 diabetes mellitus treatment: A phase 2 clinical trial. World Journal of Clinical Cases.
2023;
11
(21)
:
5083-96
.
View Article PubMed Google Scholar -
Zhang
C.,
Huang
L.,
Wang
X.,
Zhou
X.,
Zhang
X.,
Li
L.,
Topical and intravenous administration of human umbilical cord mesenchymal stem cells in patients with diabetic foot ulcer and peripheral arterial disease: a phase I pilot study with a 3-year follow-up. Stem Cell Research & Therapy.
2022;
13
(1)
:
451
.
View Article PubMed Google Scholar -
Zhang
J.,
Zhao
B.,
Wei
W.,
Wang
D.,
Wang
H.,
Zhang
A.,
Prospective, Randomized, and Controlled Study of a Human Umbilical Cord Mesenchymal Stem Cell Injection for Treating Diabetic Foot Ulcers. Journal of visualized experiments.
2023;
193
.
View Article PubMed Google Scholar -
Arango-Rodríguez
M.L.,
Solarte-David
V.A.,
Becerra-Bayona
S.M.,
Callegari
E.,
Paez
M.D.,
Sossa
C.L.,
Role of mesenchymal stromal cells derivatives in diabetic foot ulcers: a controlled randomized phase 1/2 clinical trial. Cytotherapy.
2022;
24
(10)
:
1035-48
.
View Article PubMed Google Scholar -
Andersen
J. Ask∅,
Rasmussen
A.,
Frimodt-M∅ller
M.,
Engberg
S.,
Steeneveld
E.,
Kirketerp-M∅ller
K.,
Novel topical allogeneic bone-marrow-derived mesenchymal stem cell treatment of hard-to-heal diabetic foot ulcers: a proof of concept study. Stem Cell Research & Therapy.
2022;
13
(1)
:
280
.
View Article PubMed Google Scholar -
Perico
N.,
Remuzzi
G.,
Griffin
M.D.,
Cockwell
P.,
Maxwell
A.P.,
Casiraghi
F.,
Trial Consortium
NEPHSTROM,
Safety and Preliminary Efficacy of Mesenchymal Stromal Cell (ORBCEL-M) Therapy in Diabetic Kidney Disease: A Randomized Clinical Trial (NEPHSTROM). Journal of the American Society of Nephrology.
2023;
34
(10)
:
1733-51
.
View Article PubMed Google Scholar -
Buick
E.,
Mead
A.,
Alhubaysh
A.,
Bou Assi
P.,
Das
P.,
Dayus
J.,
CellShip: An Ambient Temperature Transport and Short-Term Storage Medium for Mammalian Cell Cultures. Biopreservation and Biobanking.
2024;
22
(3)
:
275-85
.
View Article PubMed Google Scholar -
Buick
J.M. Emma,
Sebastien Farnaud, Derek Renshaw. Cell shipment without a cold chain. SLTB 2019 Meeting And Joint Workshop Organised By The Stem Cells User GroupAndalusian Initiative For Advanced Therapies And The Society For Low Temperature Biology: Spain; 2019.
Google Scholar -
Ye
Y.,
Wang
X.,
Ma
C.,
Chen
X.,
Liang
H.,
Zhang
W.,
Transporting ESCs in FBS at ambient temperature. Stem Cell Research.
2020;
49
:
102009
.
View Article PubMed Google Scholar -
Stefansson
S.,
Han
S.,
Jeon
Y.I.,
Chung
D.S.,
Hwang
P.,
Le
H.,
Transporting mammalian cells at ambient temperature: A viable alternative to dry ice. Advances in Bioscience and Biotechnology.
2017;
8
(04)
:
127-33
.
View Article Google Scholar -
Wang
J.,
Chen
P.,
Xu
J.,
Zou
J.X.,
Wang
H.,
Chen
H.W.,
Transporting Cells in Semi-Solid Gel Condition and at Ambient Temperature. PLoS One.
2015;
10
(6)
:
e0128229
.
View Article PubMed Google Scholar -
Wheatley
S.P.,
Wheatley
D.N.,
Transporting cells over several days without dry-ice. Journal of Cell Science.
2019;
132
(21)
:
jcs238139
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 7 (2024)
Page No.: 6633-6641
Published on: 2024-07-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2999 times
- PDF downloaded - 893 times
- XML downloaded - 106 times
 Biomedpress
Biomedpress







