Abstract
Introduction: Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation (MCLMR) is an autosomal dominant rare syndrome. The characteristic features include microcephaly, eye disorders, and mild-to-moderate intellectual disability. Recent evidence has revealed that mutations in the kinesin family member 11 (KIF11) gene are associated with MCLMR.
Methods: Herein, we present the case of a four-year-old Iranian girl with clinical manifestations of MCLMR and a literature review of previous cases with attributes of MCLMR. Considering her developmental delay, microcephaly, small face, low-set ears, hearing loss, visual defects, and seizures, MCLMR was suspected, and whole-exome sequencing (WES) was performed. The candidate variant was screened in the proband, her parents, and other family members using Sanger sequencing. The variant's pathogenicity and the effect of amino acid substitution on protein stability and 3D structure were evaluated by in silico analysis and different bioinformatics prediction tools.
Results: The WES analysis identified a de novo novel heterozygous missense mutation in the KIF11 gene [c.629T>C; p.(Val210Ala)], which co-segregated with the phenotype and was consistent with autosomal dominant inheritance. According to in silico protein analysis results, the mutation occurred in the kinesin motor domain, specifically in the BimC/Eg5 spindle pole proteins, that participate in spindle assembly and chromosome segregation during cell division. It had a negative effect on the protein structure and led to a loss of protein function.
Conclusion: The pathogenic mutation in the KIF12 gene could reduce protein activity and lead to the pleiotropic phenotypes of MCLMR syndrome in our patient. To our knowledge, this variant has not been reported previously in the literature, and this report is the first genetic study of an Iranian patient suffering from MCLMR with the novel KIF11 variant. Also, our findings broaden both the understanding of the clinical phenotype and the allelic repertoire of KIF11. Comparing the patient's features with those of other patients reported previously provides future viewpoints for clinical study in this area.
Introduction
Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation (MCLMR; MIM# 152950) is clinically characterized by a broad spectrum of symptoms, including central nervous system involvement, which manifests as mild to severe microcephaly and congenital optical anomalies, especially chorioretinopathy, lower-limb lymphedema, and several characteristic facial features1. Moreover, some patients develop mild to moderate mental retardation2. The prevalence of this rare neonatal disorder is estimated at fewer than 1 per 1,000,000 live births. Furthermore, it is inherited in an autosomal dominant pattern with variable expressions and reduced penetrance3. A mutation in the kinesin family member 11 (KIF11) gene has been recently described as the primary cause of the disease4.
The KIF11 gene (MIM# 148760), also known as EG5; HKSP; KNSL1; MCLMR; TRIP5, is located on 10q23.33. This gene spans 62,266 bases of the genomic sequence, encompassing 22 coding exons and encodes a homotetrameric kinesin motor complex with 1056 residues. The members of this protein family are involved in various microtubule activities, mostly during cell mitosis, which encompasses centrosome separation, chromosome positioning, and the establishment of a bipolar spindle at the time of cell mitosis. Furthermore, in non-mitotic cells, this protein is required for the transportation of secretory proteins from the Golgi apparatus to the cell membrane5, 6.
Next-generation sequencing (NGS) technologies are widely used in various fields of pediatric disorders to better understand cases with unknown origins, identify unexpected clinical manifestations, and recognize complications that may occur in the clinical course of the disease. These technologies have significant advantages in term of predicting disease and symptoms. Also, the association of alterations with genes already known to be pathogenic, the extension of the clinical manifestations of known diseases, the determination of prognosis, and the identification of new genes whose mutations cause disease are among the uses of this technique7.
In the present study, we reported a novel heterozyeous missense mutation in an Iranian family with MCLMR. It is identified by the whole-exome sequencing (WES) approach and segregated with direct sequencing of the coding region of the KIF11 gene. Also, we updated the literature by comparing our patient's features with those of the other patients reported previously and provided future viewpoints for clinical study in this area. Our findings broaden both the understanding of the clinical phenotype and the allelic repertoire of KIF11.
Methods
Case Presentation
A four-year-old girl was referred to our genetic department (Meybod Genetics Research Center, Welfare Organization of Yazd, Iran) due to severe developmental delay. The parents were both healthy and in a non-consanguineous marriage. Familial analysis revealed no similar phenotype in their pedigree.
Exome Sequencing
Genomic DNA was extracted from peripheral blood cells of the proband and normal individuals, including both parents and grandparents. Molecular genetic analysis of the KIF11 gene was conducted in our patient using NGS for the 22 coding exons and all exon-intron boundaries. WES was performed by the Illumina platform using the HiSeq 2000 sequencer and Agilent SureSelectXT2 V7 library preparation kit with a 150X depth. Exome raw data was mapped to GRCh38 using BWA, followed by variant calling and genotyping using GATK Unified Genotyper. Variants (SNVs and INDELs) were processed through our in-house developed pipeline to be classified based on the latest ACMG criteria for the interpretation of short nucleotide variants. The criteria PS2, PM2, and PP1 were applied based on case-level data, proving that the mutation is a de novo mutation, the rarity of the mutation in the general population genome database, and co-segregation with the disease in family members, respectively. Under the ACMG rules for classifying sequence variants, a pathogenic variant was classified by applying one strong pathogenic criterion, one supporting criterion, and one moderate pathogenic criterion. Then, non-benign variants were curated to find those of clinical significance. We further filtered variants by searching online databases related to human genetic variations [gnomAD (https://gnomad.broadinstitute.org/), 1000 Genome Project (http://www.internationalgenome.org), dbSNP137 (https://www.ncbi.nlm.nih.gov/snp/), ExAC (http://exac.broadinstitute.org/)], and only considered variants with a frequency of less than 0.01. Finally, the variant of interest was evaluated via Sanger sequencing in the proband sample.
Segregation Analysis
To confirm the association of the identified variant with this disorder, the healthy parents and grandparents were examined by Sanger sequencing. Primers F: 5'-TGTGAGGCTTTGAGAAGTCAGA-3' and R: 5'-AGAAAATGGGGCTAGGGAAG-3' were designed by Primer3.0 (http://bioinfo.ut.ee/primer3-0.4.0/). Subsequently, the polymerase chain reaction (PCR) was carried out under standard conditions, and products were sequenced by a 3500xL Dx Genetic Analyzer (Thermo Fisher Scientific).
In Silico Analysis of Mutant Protein
Several bioinformatics analysis tools were used to predict the pathogenicity and functional effects of the candidate variant, as well as the protein structure. Possible putative conserved domains of the query protein were searched at http://blast.ncbi.nlm.nih.gov/Blast.cgi against the non-redundant protein database. To find the Crystal Structure, RCSB Protein Data Bank (PDB) (http://www.rcsb.org/pdb/home/home.do) was used. The PDB code 6TA3 represented the Human kinesin-5 motor domain in the GSK-1 state bound to microtubules. The Kinesin-like protein KIF11 structure served as an input file in the ConSurf Server for identification of functional regions at https://consurf.tau.ac.il/ to determine conserved functional and structural amino acids. Moreover, InterProSurf (http://curie.utmb.edu/pattest9.html), which predicts functional sites on protein surfaces using patch analysis, was employed.8. HOPE (https://www3.cmbi.umcn.nl/hope/) is a web service that analyzes the structural effects of a point mutation in a protein sequence by combining information obtained from a series of web services and databases9.
| Types of variants | No. of SNVs |
|---|---|
| Total number of variants | 118,824 |
| Variants remaining on exonic, exonic-splicing and splicing region | 24530 |
| SNP variant remaining after filtering for synonymous | 12548 |
| Variants after filtering for 1000 genoms (MAF ≤ 0.01) | 2268 |
| Variants after filtering for Exome Variant Server and gnomAD (MAF ≤ 0.01) | 1322 |
| Number of rare and novel homozygous variant | 172 |
| Number of novel heterozygous variant | 322 |
Results
Clinical Presentation
The proband was born underweight through vaginal delivery at 33 weeks gestation in the first pregnancy. Her birth weight, length, and head circumference were measured at 1.250 kg, 43 cm, and 26 cm, respectively.
After birth, she had a low head circumference and was slightly underweight but did not have any other problems. Up to 16 months, she experienced delays in sitting and walking, and she started walking at the age of 16 months. At the age of 3 years, she was admitted to the paediatrician for poor hearing and eyesight. The results of the audiological evaluation, using OAE (Otoacoustic Emission), ABR (Auditory Brainstem Response), and ASSR (Auditory Steady-State Response), showed that she was sedated with chloral hydrate. Moreover, the audiological evaluation revealed that TEOAE responses were abnormal in both ears. Additionally, ABR wave V in the right and left ears was traced down to 70 Db and 90 Db NHL, respectively, and ASSR threshold measurement indicated moderate to severe hearing loss in both ears. Besides, MRI evaluation showed that the cerebral hemispheres were smaller than normal. Likewise, thick irregular cortical gyri in both parietal and occipital regions were observed. Furthermore, eye examination revealed that the right and left eye prescriptions were +8 and +9, respectively. For poor eyesight, the doctor prescribed appropriate eyeglasses, and for managing the poor hearing, cochlear implantation was performed. Also, no chromosomal aberrations were detected in the cytogenetic analysis (46,XX).
Based on the mentioned signs, such as developmental delay, microcephaly, small face, low-set ears, hearing loss, visual defects, and seizures, some inconclusive clinical diagnoses were given (Figure 1).
Identification of a Novel Missense KIF11 Variant
WES detected 118,824 variants, of which 24,530 variants were located on the exonic or exonic-splicing region. Variant filtering was conducted based on ACMG guidelines, leaving 1,322 variants for further analysis. The details of the filtering strategy are summarized in Table 1. Given the autosomal dominant inheritance, a likely pathogenic missense mutation (c.629T>C) in the KIF11 gene was discovered at amino acid position 210 (p.Val210Ala). The variant was not documented in population variant databases, including ClinVar, 1000 Genomes (October 2015), dbSNP, Exome Aggregation Consortium (ExAC), Cambridge, and IRANOME.
The results of Sanger sequencing validated that this variant is well segregated with the disease, as the variant was not found in other healthy family members, including parents and grandparents (Figure 2).
In Silico Protein Analysis
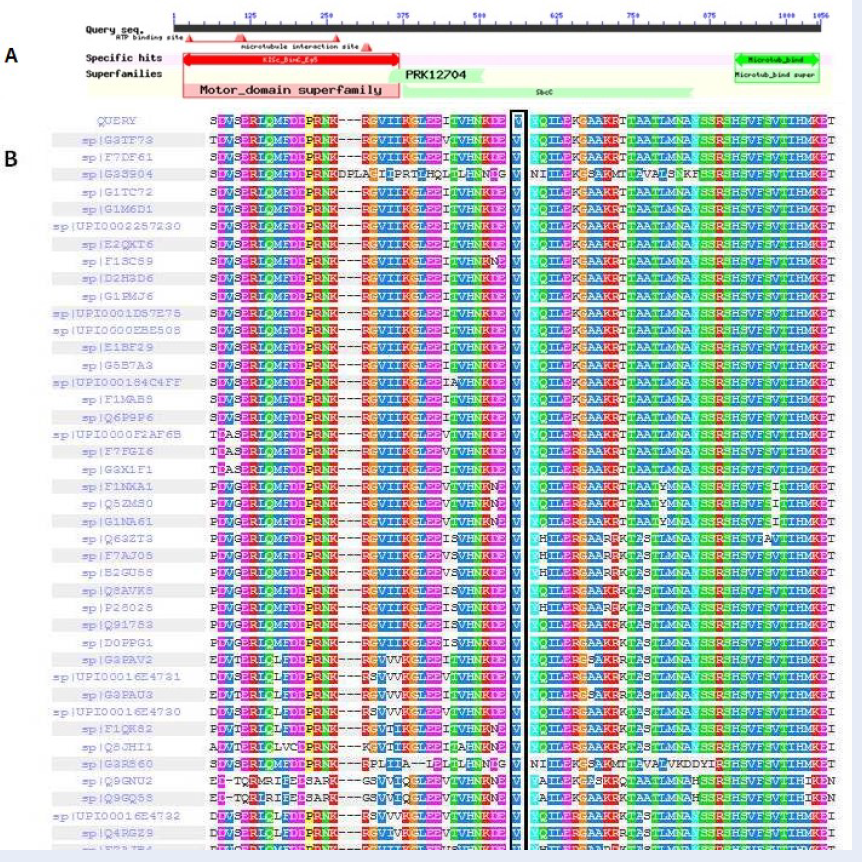
According to BLAST, residues of 16-368 belong to the Kinesin motor domain, BimC/Eg5 spindle pole proteins (accession: cd01364), which participate in spindle assembly and chromosome segregation during cell division (Figure 3A).The 6TA3 Protein Data Bank (PDB) record was detected for the human kinesin-5 motor domain in the GSK-1 state bound to microtubules. The microtubules and all non-protein atoms were removed to attain the kinesin-like protein KIF11 structure using MOE software. Figure 4A shows this protein structure before and after monomerization.
ConSurf and Interprosurf results determine functional sites on the protein structure surfaces. These results show that residues 12, 16, 169, 171, 172, 175, 176, 179, 183, 203, 204, 206, 207, 210, 211, 212, 214, 220, 221, 223, 224, 225, 227, 228, 231, 302, 303, 304, and 327 are the most functional sites in the protein structure (Figure 4B). Using SIFT, the V210A amino acid substitution was predicted as a “tolerated substitution”. However, consistent with PolyPhen-2, PROVEAN analyzer, and PHD-SNPg results, this mutation was predicted to be probably deleterious and damaging. Furthermore, PolyPhen-2 displays a multiple sequence alignment for 75 amino acid residues surrounding the variant’s position in the query sequence (Figure 3B). Moreover, I-Mutant2.0 predicted a decrease in the stability of the mutation.
According to HOPE results, the mutated residue lies in a domain that is vital for the binding of other molecules and is also in contact with residues in another domain that is crucial for binding. The interplay of these two domains could be disturbed by the mutation, exerting an adverse effect on protein function by disrupting the signal transfer from the binding domain to the activity domain (Figure 5).
In addition, based on data obtained from a known database and the estimation of protein-protein interactions, STRING (https://string-db.org/) demonstrated that this protein has several functional partners, which mostly belong to the TRAFAC class myosin-kinesin ATPase superfamily, and are involved in cell cycle cytokinesis through Rho-mediated signaling, chromosome congression, microtubule-kinetochore conjugation, spindle assembly checkpoint activation, and other key processes in cell division and cell cycle progression.
Discussion
MCLMR, a rare autosomal dominant or sporadic condition, is a congenital malformation characterized by variable expression of microcephaly, eye disorders such as chorioretinopathy, congenital lymphedema, and mild-to-moderate intellectual disability. Additionally, the most commonly reported cause of this disorder is KIF11 mutations.
In this study, we describe an Iranian girl from a non-consanguineous marriage with primary microcephaly and a novel heterozygous missense mutation in KIF11 (p.Val210Ala). KIF11, an evolutionarily conserved microtubule-associated protein, is involved in mitotic spindle dynamics, centrosome separation, and microtubule-based movements of vesicles and other organelles in non-dividing cells10, 11. KIF11 protein knockdown, in any form, results in monopolar spindle formation, defects in centrosome separation, and metaphase arrest12.
To date, 67 cases have been described with KIF11 mutations and MCLMR2, 3, 13, 14, 15, 16, 17, 18, 19, 20. The majority of the currently described cases were missense (8/67), splicing (12/67), nonsense (14/67), frameshift (24/67), and deletion (2/67) (Table 2). It demonstrated that aside from point mutations, KIF11 haploinsufficiency caused by deletion is linked to autosomal dominant microcephaly, chorioretinopathy, and mild intellectual disability. In all of them, the missense mutations affected highly conserved residues, or a prematurely truncated protein was formed because of a frameshift mutation. Also, among different reports, most of the mutations were sporadic (1/67), heterozyous (18/67), and maternal (5/67)2, 13, 14, 15, 16, 17, 18, 19, 20, 21. Moreover, several heterozygous de novo (18/67) mutations in the KIF11 gene in MCLMR patients have been reported, and the variant identified in our study is compatible with their results2, 3, 13, 14, 15, 16, 17, 18, 19, 20, 21.
In line with these studies, we detected a heterozygous mutation in KIF11 causing loss of function by affecting the N-terminal domain. This catalytic (head) domain has ATPase activity (between residue 105 and 112) and belongs to the larger group of P-loop NTPases. Additionally, three internal domains in the protein structure contribute to multimerization into a homo-tetramer with coiled-coil linkage22. For kinesin motor domains to move along the microtubule with ATP hydrolysis, kinesin head groups work in pairs. Also, the neck linker binds to the motor domain, which repositions the other head domain through the coiled-coil domain close to a second tubulin dimer. ATP hydrolysis in the kinesin motor domain triggers a conformational change that pulls the first domain forward23, 24.
Moreover, the mutation found is located in a protein α-helix domain in buried regions that are important for the binding of other domains. The wild-type and mutant amino acids differ in size, with the mutant residue being smaller than the wild-type, and the mutation will cause space in the core of the protein. Therefore, the amino acid alteration in this region interferes with protein binding and domain-domain interactions. As this motor protein is very conserved and vital in many species, any structural changes result in a wide range of disturbances. In addition, the pathogenicity degree demonstrated in different predictive ways indicates the deleteriousness of this mutation, and it is compatible with our patient's clinical signs.
Based on the clinical presentations among MCLMR patients, the most prevalent craniofacial features were a broad nose with a rounded tip (26/67), a long philtrum and prominent chin (25/67), prominent ears (24/67), and up-slanting palpebral fissures (24/67) (Table 3). Our case also had different craniofacial attributes related to MCLMR, including a broad nose with a rounded tip, prominent ears, a small face, low-set ears, and up-slanting palpebral fissures. Likewise, among the patients with KIF11 mutations and MCLMR, eye problems, specifically chorioretinopathy, were more prevalent (56/67). Comparatively, our proband showed poor eyesight. Among different CNS disorders, ventricular stenosis (3/67), a small frontal lobe (7/67), intellectual disability (24/67), learning problems (34/67), and behavioral problems (11/67) were common (Table 3). Additionally, microcephaly was present in all except P59, typically resulting from abnormalities in the regular neurogenesis process, leading to inadequate neuron generation during cortical development25.
Intriguingly, previous studies have shown that several genes such as CENPJ, MCPH1-17, ASPM, CDK5RAP2, STIL, CEP152, and WDR62, which are involved in a recessive pattern of microcephaly, play a role in centrosome formation and spindle development26. These encode centrosomal proteins, essential for various processes like centriole duplication, centrosome maturation, spindle assembly, microtubule dynamics, and regulation of the cell cycle27.
The kinesin motor’s function, in cooperation with microtubules and associated proteins, supports the development of neuronal progenitors, the movement of neurons, and the intracellular transport of neurons and dendrites28.
The results of our study highlighted the important role of molecules involved in the function of mitotic spindles in CNS development by showing the presence of mutations in the KIF11 gene that lead to the dominant form of microcephaly. Moreover, the study on zebrafish revealed that the inhibitor mutation in KIF11 led to a mitotic arrest in radial glia, serving as resident neural stem cells. Thus, the loss of function of KIF11 causes a noticeable reduction in oligodendroglia and neurogenesis29.
Other significant clinical presentations in patients with KIF11 mutations were developmental delay (6/67), hearing impairment (4/67), speech delay (8/67), and cardiac anomaly (3/67) (Table 3). Additionally, in 37 cases, lymphedema was presented. The deleterious effect of the identified missense mutation on the function of KIF11 in this study is compatible with our patient's clinical signs.
Conclusions
In conclusion, the c.629T>C (V210A) mutation is pathogenic and can reduce KIF11 protein activity, which is related to intracellular transportation and cell division. This has led to the autosomal dominant and pleiotropic phenotypes of MCLMR syndrome in our patient. To our knowledge, this variant has not been previously reported in the literature, making this report the first genetic study of an Iranian patient suffering from MCLMR with this novel variant of KIF11. The results might have practical implications for the mutation analysis of the KIF11 gene in patients with CNS developmental problems, with or without chorioretinal dysplasia. Furthermore, our findings broaden both the understanding of the clinical phenotype and the allelic repertoire of KIF11. The comparison of our patient's features with those of other patients previously reported offers new perspectives for future clinical studies in this area. We should also mention that WES is a powerful approach for identifying candidate genes in rare sporadic disorders with de novo mutations.
Abbreviations
ACMG - American College of Medical Genetics and Genomics, dbSNP - Single Nucleotide Polymorphism Database, INDELs - Insertions and Deletions, MRI - Magnetic Resonance Imaging, NGS - Next-Generation Sequencing, PCR - Polymerase Chain Reaction, PDB - Protein Data Bank, SNVs - Single Nucleotide Variants, WES - Whole-Exome Sequencing
Acknowledgments
The authors would like to thank to Dr. N. Namiranian and Diabetes Research Center for their valuable support. We are also grateful to the patient and her family members for their participation.
Author’s contributions
S.A: conception and design and performing the main steps of essay and writing the manuscript. HR.J: data extraction and revising the manuscript critically for intellectual content. F.S: All bioinformatics analysis and interpretation of the data. A.M: Contribute to WES and performing PCR. M.A: Analysis of results and interpretation of the data. M.O.: Head of team and monitoring and fixing technical errors during all steps of the study. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study design was approved by the Institutional Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (IR.SSU.MEDICINE.REC.1400.190) and written informed consent was obtained from each participant before the collection of samples.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Jones
G.E.,
Ostergaard
P.,
Moore
A.T.,
Connell
F.C.,
Williams
D.,
Quarrell
O.,
Microcephaly with or without chorioretinopathy, lymphoedema, or mental retardation (MCLMR): review of phenotype associated with KIF11 mutations. European Journal of Human Genetics.
2014;
22
(7)
:
881-7
.
View Article PubMed Google Scholar -
Malvezzi
J.V.,
H Magalhaes
I.,
S Costa
S.,
Otto
P.A.,
Rosenberg
C.,
Bertola
D.R.,
KIF11 microdeletion is associated with microcephaly, chorioretinopathy and intellectual disability. Human Genome Variation.
2018;
5
(1)
:
18010
.
View Article PubMed Google Scholar -
Balikova
I.,
Robson
A.G.,
Holder
G.E.,
Ostergaard
P.,
Mansour
S.,
Moore
A.T.,
Ocular manifestations of microcephaly with or without chorioretinopathy, lymphedema or intellectual disability (MCLID) syndrome associated with mutations in KIF11. Acta Ophthalmologica.
2016;
94
(1)
:
92-8
.
View Article PubMed Google Scholar -
Li
J.K.,
Fei
P.,
Li
Y.,
Huang
Q.J.,
Zhang
Q.,
Zhang
X.,
Identification of novel KIF11 mutations in patients with familial exudative vitreoretinopathy and a phenotypic analysis. Scientific Reports.
2016;
6
(1)
:
26564
.
View Article PubMed Google Scholar -
Rososinski
A.,
Tran
T.,
Galvin
J.,
Patel
C.,
Fung
A.T.,
New findings from multimodal fundus imaging over 3 years of a patient with microcephaly, chorioretinopathy, and Kif11 mutation. Retinal Cases {&}amp; Brief Reports.
2019;
13
(1)
:
79-83
.
View Article PubMed Google Scholar -
Zalenski
A.A.,
Majumder
S.,
De
K.,
Venere
M.,
An interphase pool of KIF11 localizes at the basal bodies of primary cilia and a reduction in KIF11 expression alters cilia dynamics. Scientific Reports.
2020;
10
(1)
:
13946
.
View Article PubMed Google Scholar -
Zhong
Y.,
Xu
F.,
Wu
J.,
Schubert
J.,
Li
M.M.,
Application of Next Generation Sequencing in Laboratory Medicine. Annals of Laboratory Medicine.
2021;
41
(1)
:
25-43
.
View Article PubMed Google Scholar -
Capriotti
E.,
Fariselli
P.,
Casadio
R.,
I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Research.
2005;
33
(Web Server issue)
:
W306-10
.
View Article PubMed Google Scholar -
Venselaar
H.,
Te Beek
T.A.,
Kuipers
R.K.,
Hekkelman
M.L.,
Vriend
G.,
Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinformatics.
2010;
11
(1)
:
548
.
View Article PubMed Google Scholar -
Rapley
J.,
Nicolàs
M.,
Groen
A.,
Regué
L.,
Bertran
M.T.,
Caelles
C.,
The NIMA-family kinase Nek6 phosphorylates the kinesin Eg5 at a novel site necessary for mitotic spindle formation. Journal of Cell Science.
2008;
121
(Pt 23)
:
3912-21
.
View Article PubMed Google Scholar -
Wakana
Y.,
Villeneuve
J.,
van Galen
J.,
Cruz-Garcia
D.,
Tagaya
M.,
Malhotra
V.,
Kinesin-5/Eg5 is important for transport of CARTS from the trans-Golgi network to the cell surface. The Journal of Cell Biology.
2013;
202
(2)
:
241-50
.
View Article PubMed Google Scholar -
Venuto
S.,
Monteonofrio
L.,
Cozzolino
F.,
Monti
M.,
Appolloni
I.,
Mazza
T.,
TRIM8 interacts with KIF11 and KIFC1 and controls bipolar spindle formation and chromosomal stability. Cancer Letters.
2020;
473
:
98-106
.
View Article PubMed Google Scholar -
Guo
Z.,
Huo
X.,
Wu
D.,
Hao
B.,
Liao
S.,
A Novel Variant of the KIF11 Gene, c.2922G>T, Is Associated with Microcephaly by Affecting RNA Splicing. Developmental Neuroscience.
2022;
44
(2)
:
113-20
.
View Article PubMed Google Scholar -
Hazan
F.,
Ostergaard
P.,
Ozturk
T.,
Kantekin
E.,
Atlihan
F.,
Jeffery
S.,
A novel KIF11 mutation in a Turkish patient with microcephaly, lymphedema, and chorioretinal dysplasia from a consanguineous family. American Journal of Medical Genetics. Part A.
2012;
158A
(7)
:
1686-9
.
View Article PubMed Google Scholar -
Güneş
N.,
Taşdemir
E.,
Jeffery
H.,
Yetik
H.,
Ostergaard
P.,
Tüysüz
B.,
A novel mutation of KIF11 in a Child with 22q11. 2 deletion syndrome associated with MCLMR. Molecular Syndromology.
2019;
9
(5)
:
266-70
.
View Article PubMed Google Scholar -
Mirzaa
G.M.,
Enyedi
L.,
Parsons
G.,
Collins
S.,
Medne
L.,
Adams
C.,
Congenital microcephaly and chorioretinopathy due to de novo heterozygous KIF11 mutations: five novel mutations and review of the literature. American Journal of Medical Genetics. Part A.
2014;
164A
(11)
:
2879-86
.
View Article PubMed Google Scholar -
Riedl
J.,
Voβmerbäumer
U.,
Stoffelns
B.,
Elflein
H.,
Total retinal detachment caused by a KIF11 mutation. European Journal of Ophthalmology.
2017;
27
(5)
:
e147-8
.
View Article PubMed Google Scholar -
Ostergaard
P.,
Simpson
M.A.,
Mendola
A.,
Vasudevan
P.,
Connell
F.C.,
van Impel
A.,
Mutations in KIF11 cause autosomal-dominant microcephaly variably associated with congenital lymphedema and chorioretinopathy. American Journal of Human Genetics.
2012;
90
(2)
:
356-62
.
View Article PubMed Google Scholar -
Mears
K.,
Bakall
B.,
Harney
L.A.,
Penticoff
J.A.,
Stone
E.M.,
Autosomal dominant microcephaly associated with congenital lymphedema and chorioretinopathy due to a novel mutation in KIF11. JAMA Ophthalmology.
2015;
133
(6)
:
720-1
.
View Article PubMed Google Scholar -
Schlögl
E.,
Radeva
M.Y.,
Vielmuth
F.,
Schinner
C.,
Waschke
J.,
Spindler
V.,
Keratin retraction and desmoglein3 internalization independently contribute to autoantibody-induced cell dissociation in Pemphigus vulgaris. Frontiers in Immunology.
2018;
9
:
858
.
View Article PubMed Google Scholar -
Alahmadi
G.,
Alshamrani
A.A.,
Albakri
A.,
Novel variant of KIF11 associated with MCLMR syndrome. Ophthalmic Genetics.
2023;
44
(2)
:
205-7
.
View Article PubMed Google Scholar -
Schlögel
M.J.,
Mendola
A.,
Fastré
E.,
Vasudevan
P.,
Devriendt
K.,
de Ravel
T.J.,
No evidence of locus heterogeneity in familial microcephaly with or without chorioretinopathy, lymphedema, or mental retardation syndrome. Orphanet Journal of Rare Diseases.
2015;
10
(1)
:
52
.
View Article PubMed Google Scholar -
Soppina
P.,
Patel
N.,
Shewale
D.J.,
Rai
A.,
Sivaramakrishnan
S.,
Naik
P.K.,
Kinesin-3 motors are fine-tuned at the molecular level to endow distinct mechanical outputs. BMC Biology.
2022;
20
(1)
:
177
.
View Article PubMed Google Scholar -
Mann
B.J.,
Wadsworth
P.,
Kinesin-5 Regulation and Function in Mitosis. Trends in Cell Biology.
2019;
29
(1)
:
66-79
.
View Article PubMed Google Scholar -
Woods
C.G.,
Human microcephaly. Current Opinion in Neurobiology.
2004;
14
(1)
:
112-7
.
View Article PubMed Google Scholar -
Ostergaard
P.,
Simpson
M.A.,
Mendola
A.,
Vasudevan
P.,
Connell
F.C.,
van Impel
A.,
Mutations in KIF11 cause autosomal-dominant microcephaly variably associated with congenital lymphedema and chorioretinopathy. American Journal of Human Genetics.
2012;
90
(2)
:
356-62
.
View Article PubMed Google Scholar -
Maillard
C.,
Roux
C. J.,
Charbit-Henrion
F.,
Steffann
J.,
Laquerriere
A.,
Quazza
F.,
Buisson
N. B.,
Tubulin mutations in human neurodevelopmental disorders. Seminars in Cell & Developmental Biology.
2023;
137
:
87-95
.
View Article Google Scholar -
Zhou
Y.,
Xu
M.F.,
Chen
J.,
Zhang
J.L.,
Wang
X.Y.,
Huang
M.H.,
Loss-of-function of kinesin-5 KIF11 causes microcephaly, chorioretinopathy, and developmental disorders through chromosome instability and cell cycle arrest. Experimental Cell Research.
2024;
436
(1)
:
113975
.
View Article PubMed Google Scholar -
Johnson
K.,
Moriarty
C.,
Tania
N.,
Ortman
A.,
DiPietrantonio
K.,
Edens
B.,
Kif11 dependent cell cycle progression in radial glial cells is required for proper neurogenesis in the zebrafish neural tube. Developmental Biology.
2014;
387
(1)
:
73-92
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 6 (2024)
Page No.: 6532-6547
Published on: 2024-06-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2804 times
- PDF downloaded - 1009 times
- XML downloaded - 150 times
 Biomedpress
Biomedpress







