Abstract
Eosinophilic gastrointestinal diseases (EGIDs) constitute a spectrum of disorders distinguished by the abnormal infiltration of eosinophils into the walls of the gastrointestinal tract, a phenomenon that frequently results in delayed or overlooked diagnoses due to the nonspecific nature of symptoms and endoscopic findings. This article discusses a noteworthy case characterized by chronic diarrhea and mild abdominal discomfort. The presence of peripheral eosinophilia and the evidence obtained from biopsies raised suspicions, ultimately leading to the diagnosis of EGIDs. Despite a two-month diagnostic delay attributed to limited disease awareness, the patient responded well to medical management. The therapeutic regimen comprised methylprednisolone, ketotifen, and montelukast, complemented by dietary modifications, culminating in the full resolution of symptoms. A notable complication was the onset of severe cellulitis during steroid treatment, necessitating an expedited reduction in the steroid dosage while continuing with other medications and undertaking surgical debridement. This infection was successfully controlled, and the corticosteroid therapy was gradually tapered off and discontinued over an eight-week period. The administration of ketotifen and montelukast was maintained, with no further recurrences reported. This case exemplifies the critical need to consider EGIDs in the differential diagnosis of individuals presenting with gastrointestinal symptoms, particularly when peripheral eosinophilia is evident. Furthermore, it emphasizes the imperative of holistic patient care, inclusive of managing infectious complications that may arise during the course of treatment.
Introduction
Eosinophilic gastrointestinal diseases (EGIDs) encompass a group of rare disorders characterized by the atypical infiltration of eosinophils into any section of the gastrointestinal (GI) tract, excluding known secondary causes1. This classification includes disorders such as eosinophilic esophagitis, eosinophilic gastroenteritis, and eosinophilic colitis2. The clinical presentation of EGIDs is varied and depends on the specific GI tract segment involved. Common symptoms include abdominal pain, diarrhea, nausea, vomiting, and early satiety3. A study conducted in Malaysia reported a prevalence rate of 2.6% for eosinophilic gastroenteritis, highlighting its rarity4. Nonetheless, the prevalence of EGIDs in Vietnam and other Southeast Asian countries remains poorly documented. Diagnosing EGIDs is complex and often delayed due to the general nature of the symptoms associated with these conditions and their infrequent occurrence. Managing EGIDs presents a distinct challenge, especially in developing countries, due to the limited available evidence on these rare conditions. This report details the case of a patient diagnosed with eosinophilic colitis and eosinophilic duodenitis, who presented with chronic diarrhea and abdominal pain. The patient initially responded positively to corticosteroid therapy, followed by sustained remission with the administration of ketotifen and montelukast, demonstrating the potential effectiveness of this treatment regimen for EGIDs. This case contributes valuable insights into novel therapeutic strategies for the management of EGIDs.
Case Reports
A 63-year-old male farmer was admitted to the hospital due to a two-month history of persistent, non-bloody, watery diarrhea, with approximately ten daily bowel movements that were not alleviated by fasting. This significantly interfered with his daily activities. He concurrently experienced mild periumbilical abdominal cramps, which were relieved post-defecation. Despite the pain not disrupting his sleep, he was awakened by nighttime bowel movements. He reported no difficulty swallowing, food impaction, heartburn, chest pain, postprandial fullness, or early satiety. No adaptive behaviors, such as prolonged meals, avoidance of hard food, or concurrent water drinking with meals, were noted. He denied symptoms of fever, nausea, vomiting, night sweats, arthralgia, or weight loss. The patient had no known medical history and was not taking any medications or supplements at the time of admission.
The patient presented in good health with stable vital signs: a temperature of 37°C, blood pressure of 120/70 mm Hg, and a heart rate of 70 beats per minute. His body mass index was 21. Physical examinations of the skin, thyroid, lungs, heart, joints, and abdomen were unremarkable, and no peripheral lymphadenopathy was detected.
Initial blood and fecal tests were performed to investigate the patient’s chronic diarrhea (Table 1). The patient exhibited mild anemia with a hemoglobin level of 126 g/L (reference range, 130 to 170) and a normal mean corpuscular volume. The complete blood count revealed an elevated eosinophil count of 5300 per microliter. The patient had a normal C-reactive protein level and mildly low albumin levels at 31 g/L (reference range, 35 to 55). Thyroid hormone levels, cortisol levels, and liver and renal functions were within normal limits. The patient’s immunoglobulin E concentration was significantly elevated at 10000 IU/mL (reference range, <100). Serological tests for parasitic diseases indicated the presence of antibodies against cysticercosis and Echinococcus spp. and the absence of antibodies against Entamoeba histolytica, Toxocara canis, Strongyloides spp., and Fasciola spp. Thorough stool tests were performed to confidently rule out infections as the cause of chronic diarrhea. Microscopic examination of three consecutive stool specimens revealed the presence of fecal leukocytes. The patient tested negative for Clostridioides difficile toxin. The fecal calprotectin level was significantly elevated at 278 micrograms per gram. Stool culture indicated normal flora.
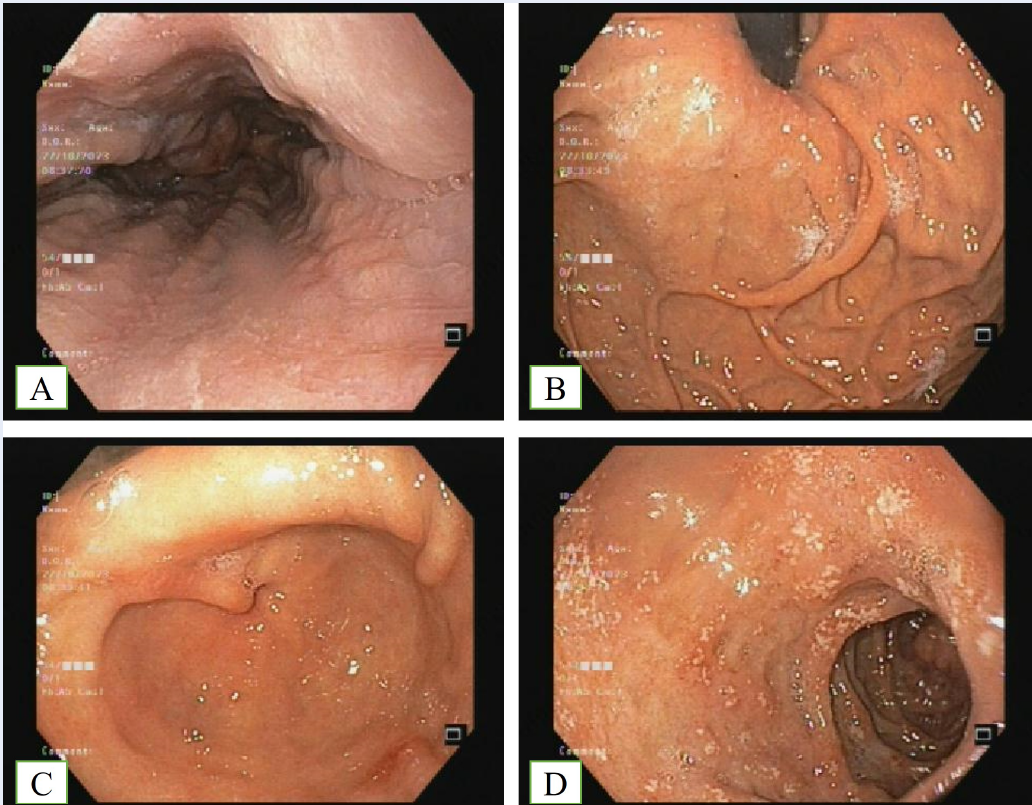
Contrast-enhanced abdominal computed tomography was performed to investigate abdominal pain, revealing circumferential and symmetric colonic wall thickening (Figure 1). Colonoscopy with endoscopic biopsy served as a crucial examination to explore the underlying causes of chronic diarrhea, particularly in elderly patients. Endoscopic findings revealed sporadic superficial ulcerations in rectal, sigmoid, descending, and transverse sections of the colon (as depicted in Figure 2), whereas the mucosa of the ascending colon appeared normal. Biopsy specimens were obtained from sigmoid ulcers and normal ascending colon mucosa. Histology revealed eosinophilic infiltration in the lamina propria, with an estimated 100 eosinophils per high-power field (HPF) (Figure 3). Tuberculosis (TB) polymerase chain reaction (PCR) of the colonic biopsy specimens was negative. Additional blood tests and bone marrow examinations were performed to explore secondary causes of eosinophilia. The patient tested negative for antinuclear antibodies, perinuclear anti-neutrophil cytoplasmic antibodies, and cytoplasmic anti-neutrophil cytoplasmic antibodies. Bone marrow aspiration revealed an elevated eosinophil count (34%) and a normal blast cell count (1%). Fluorescence in situ hybridization of bone marrow cells revealed no rearrangements of PDGFRA, PDGFRB, FGFR1, JAK2, or FLT3. The patient was diagnosed with primary eosinophilic colitis based on histological findings and symptoms in the absence of other specific causes. Tuberculosis was deemed unlikely due to a normal chest radiograph, a negative Quantiferon TB Gold IGRA test, negative tissue TB PCR, and the absence of constitutional symptoms and pathognomonic signs upon histological evaluation.
Eosinophil infiltration within the colon wall suggested a probable diagnosis of eosinophilic gastroenteritis and eosinophilic esophagitis. Consequently, esophagogastroduodenoscopy was conducted. The procedure identified superficial duodenal ulcers and antral erosions, with the esophageal mucosa appearing normal (Figure 4). Concurrently, a negative urease rapid test indicated the absence of Helicobacter pylori infection. The mucosa of the esophagus appeared to be in a normal state. Biopsy specimens were procured from three distinct locations: the distal esophagus, the erosion site of the antrum, and the superficial ulcer of the D1 section of the duodenum. Histological examination revealed eosinophil-predominant inflammation in the esophagus (37 eosinophils per HPF) and duodenum (120 eosinophils per HPF) (Figure 5), with minimal eosinophil presence within the gastric mucosa (approximately 5 per HPF). Initially, due to the presence of serum cysticercosis and Echinococcus spp. antibodies, the patient was administered 400 mg of albendazole bid for 7 days. However, symptoms of diarrhea and abdominal pain persisted. Subsequent histological results from the colon, esophagus, stomach, and duodenum mucosa indicated eosinophil infiltration within the gastrointestinal walls. After ruling out potential secondary causes of eosinophil infiltration, such as inflammatory bowel disease, connective tissue disease, vasculitides, parasitic infection (due to the lack of response to antiparasitic medication), and hypereosinophilic syndrome, a diagnosis of primary eosinophilic colitis and eosinophilic duodenitis was established. Despite the presence of abnormal eosinophil infiltration within the esophageal mucosa, the diagnosis of primary eosinophilic esophagitis could not be conclusively established due to the lack of symptoms typically associated with esophagitis. The patient was administered a systemic corticosteroid regimen, beginning with 32 mg of oral methylprednisolone daily for two weeks, followed by a weekly taper of 4 mg. The patient was administered a daily dose of 40 mg of oral pantoprazole in response to the presence of duodenal ulcers. Prior to the initiation of systemic steroid therapy, the patient tested negative for HBsAg, anti-HBc, and anti-HCV (i.e., markers of hepatitis B and C infections). In an effort to preemptively address concerns regarding potential disease relapse following the cessation or tapering of steroid therapy, a combination of ketotifen (administered twice daily at a dosage of 1 mg) and montelukast (administered once daily at a dosage of 10 mg) was introduced concurrently with the onset of steroid therapy. This therapeutic regimen led to a marked decrease in the frequency of bowel movements, from ten to three per day. By the fifth day of therapy, the patient reported a further reduction to a single daily bowel movement, unaccompanied by abdominal pain. For the best treatment approach, a diet plan was taken into account. A radioallergosorbent test (i.e., a blood test used to check what substances a person is allergic to) was performed on the twelfth day following the initiation of steroid therapy, the results of which are presented in Table 2. Based on the identification of potential food allergens, a dietary elimination protocol was implemented, which included the removal of chocolate, milk, pineapple, shrimp, sardine, barley flour, oat flour, corn, orange, and beer from the patient’s diet. However, the extensive list of dietary restrictions posed challenges to patient adherence to the protocol, leading to occasional lapses in dietary compliance. Notably, rice was identified as a potential allergen, but its central role in the patient’s diet made its elimination impractical. Upon discharge for outpatient monitoring, the patient was advised to adhere to an inactivated vaccination schedule due to the risk of infection associated with corticosteroid therapy. While the patient was on a regimen of 16 mg of methylprednisolone, he sustained a laceration on his right middle finger, which was inflicted by a crab pincer. This injury subsequently progressed to severe cellulitis, necessitating the implementation of an antibiotic treatment protocol and surgical removal of damaged tissue. As a result, the dosage of methylprednisolone was reduced to 8 mg daily for a duration of two weeks, followed by a weekly decrease of 4 mg, while the administration of ketotifen and montelukast was sustained. Over time, the infection was successfully resolved, and complete healing was observed after two months. The patient remained symptom-free from abdominal pain and diarrhea for a period of two months under the steroid regimen. Three months post-steroid cessation, the disease remained in remission under a maintenance regimen of ketotifen (1 mg twice daily) and montelukast (10 mg daily). Figure 6 delineates the patient’s medication dosages and significant occurrences throughout the disease progression.
| Variable | Reference range | Value |
| Blood | ||
| Hemoglobin (g/liter) | 130 – 170 | 126 |
| White-cell count (per µL) | 4000 – 11000 | 12600 |
| Differential count (per µL) | ||
| Neutrophils | 1800 – 8250 | 4200 |
| Lymphocytes | 800 – 4400 | 2300 |
| Monocytes | 160 – 1100 | 800 |
| Eosinophils | 80 – 880 | 5300 |
| Basophils | 0 – 220 | 0 |
| Platelet count (per µL) | 200000 – 400000 | 225000 |
| Glucose (mg/dl) | 70 – 110 | 90 |
| Alanine aminotransferase (U/liter) | 5 – 49 | 8 |
| Aspartate aminotransferase (U/liter) | 9 – 48 | 15 |
| Blood urea nitrogen (mg/dl) | 7 – 20 | 12 |
| Creatinin (mg/dl) | 0.7 – 1.5 | 1.12 |
| Natri (mmol/liter) | 135 – 150 | 136 |
| Kali (mmol/liter) | 3.5 – 5.5 | 3.7 |
| Albumin (g/liter) | 35 – 55 | 31 |
| C-reactive protein (mg/liter) | <6 | 3.2 |
| Free thyroxine (pg/mL) | 8 – 20 | 14.07 |
| Thyroid stimulating hormone (mIU/liter) | 0.4 – 5 | 1.349 |
| Folate (ng/ml) | 5.3 – 14 | 4.9 |
| Vitamin B12 (pg/ml) | 211 – 911 | 406 |
| High-sensitivity troponin I (pg/mL) | <34.2 | <2.5 |
| Anti-nuclear antibodies | Negative | Negative |
| Anti-double stranded DNA (IU/mL) | <25 | 1.12 |
| C3 (mg/dL) | 90 – 180 | 64.4 |
| C4 (mg/dL) | 10 – 40 | 12.1 |
| Cortisol (ng/ml) | 50 – 230 | 88 |
| Anti-neutrophil cytoplasmic antibodies | Negative | Negative |
| Bone marrow | ||
| Blast cells (%) | 1 – 3 | 1 |
| Neutrophil (%) | 10 – 30 | 25 |
| Lymphocytes (%) | 10 – 15 | 11 |
| Eosinophils (%) | 0 – 5 | 34 |
| Stool | ||
| Fecal calprotectin | <50 µg/g | 278 |
| Clostridium difficile PCR | Negative | Negative |
| Culture | Normal flora | |
| Ova and parasites | Negative | Negative |
| Antigen | Specific immunoglobulin E level (kU/liter)* |
| Egg white | 0.22 |
| Egg yolk | 0.14 |
| Codfish | 0.23 |
| Shrimp | 0.39 |
| Tuna | 0.19 |
| Sardine | 0.39 |
| Wheat flour | 0.27 |
| Rye flour | 0.26 |
| Barley flour | 0.53 |
| Oat flour | 1.21 |
| Rice | 0.48 |
| Soybean | 0.20 |
| Corn | 0.56 |
| Gluten | 0.87 |
| Peanut | 0.18 |
| Hazelnut | 0.04 |
| Almond | 0.10 |
| Cow’s milk | 0.28 |
| Chocolate | 1.21 |
| Goat’s milk | 0.48 |
| Tomato | 0.20 |
| Lemon | 0.18 |
| Orange | 0.39 |
| Strawberry | 0.10 |
| Apple | 0.21 |
| Pineapple | 74.06 |
| Cooked pork | 0.08 |
| Beef | 0.12 |
| Chicken | 0.06 |
| Brewer’s yeast | 0.56 |
| Baker’s yeast | 0.24 |
| Latex | 0.11 |
| Dermatophagoides pteronyssinus | 0.12 |
| Dermatophagoides farinae | 0.87 |
| Blomia tropicalis | 0.53 |
| Cat | 0.39 |
| Dog | 0.23 |
| Chicken feathers | 0.26 |
| Penicillium notatum | 0.27 |
| Cladosporium herbarum | 0.17 |
| Aspergillus fumigatus | 0.31 |
| Candida albicans | 0.07 |
| Alternaria alternata | 0.13 |
| Bermuda grass | 0.12 |
| Dandelion | 0.19 |
| Honey bee venom | 1.55 |
| Common wasp venom | 1.21 |
| Fire ant | 0.21 |
| Mosquito | 0.26 |
| Cockroach | 0.03 |
Discussion
EGIDs are uncommon conditions characterized by the unusual infiltration of eosinophils into the GI tract walls, potentially affecting any segment from the esophagus to the colon5. Eosinophils, which are typically absent in the esophageal lamina propria, are found in other segments of the GI tract under normal conditions. Their recruitment to the GI wall is triggered by various inflammatory stimuli, including parasitic infections and allergic diseases. Numerous cytokines facilitate eosinophil proliferation and maturation within the bone marrow, with interleukin-5 being the most specific to the eosinophil lineage and a potential target for therapeutic intervention6. Depending on the extent of eosinophil infiltration in the GI walls, the clinical manifestations of EGIDs can be stratified into three distinct patterns: mucosal, muscular, and serosal7. The mucosal pattern, which is most prevalent, is characterized by symptoms such as abdominal pain, diarrhea, malabsorption, anemia, and weight loss5. Such symptoms are nonspecific, potentially resulting in delayed diagnosis, as evidenced by the patient in this case who experienced two months of chronic diarrhea. A cohort study conducted by Chehade et al. involving 4108 patients revealed an average duration of 3.6 years between symptom onset and diagnosis8. Hence, maintaining a high degree of clinical suspicion is crucial, particularly in patients exhibiting chronic gastrointestinal symptoms9. In our case, the patient exhibited peripheral eosinophilia, a significant clue leading to the diagnosis. However, it is essential to note that the absence of peripheral eosinophilia does not reliably exclude an EGID diagnosis, as approximately 20% of patients do not present with elevated blood eosinophil counts, as demonstrated by Kinoshita et al.10.
Endoscopic findings can range from a normal appearance to nonspecific gastritis or colitis, and from erosions to ulcerations10, 11. Given the patchy nature of eosinophilic infiltration in the gastrointestinal tract, it is necessary to obtain multiple biopsy specimens from both normal and abnormal mucosa12, 13. In our patient, even the normal mucosa of the ascending colon exhibited an abnormal accumulation of eosinophils.
The diagnosis of EGIDs is typically straightforward in patients who present with gastrointestinal symptoms, provided there is evidence of abnormal eosinophil infiltration within the lamina propria of the GI tract wall, and secondary causes have been excluded14, 15. There is no established consensus on the quantification of eosinophils within the gastrointestinal mucosa15. Turner et al. characterized colonic eosinophilia as the presence of more than 50 eosinophils per HPF in the right colon, more than 35 per HPF in the transverse colon, and more than 25 per HPF in the left colon16. Consequently, our patient was diagnosed with eosinophilic colitis, as evidenced by the detection of 100 eosinophils per HPF in the colon mucosa. For reference, the eosinophil thresholds per HPF for the mucosa of the esophagus, stomach, and duodenum are 15, 30, and 52, respectively17. Biopsy specimens from the patient’s esophagus and duodenum satisfied the aforementioned eosinophil thresholds, leading to a concurrent diagnosis of eosinophilic duodenitis. Despite this, a diagnosis of eosinophilic esophagitis was precluded due to the absence of related symptomatic manifestations18. A comprehensive medical history was collected, revealing that the patient did not experience any esophageal symptoms, including dysphagia, heartburn, food impaction, or chest pain. Consequently, the patient was diagnosed with asymptomatic esophageal eosinophilia. Given that approximately 75% of asymptomatic esophageal eosinophilia patients exhibit lamina propria fibrosis and 20% develop eosinophilic esophagitis, it is recommended that these patients undergo clinical, endoscopic, and histological follow-up evaluations19.
A notable limitation of this case report is the absence of small bowel endoscopy with biopsy, which would have facilitated a more comprehensive assessment of the severity of eosinophilic infiltration within the small bowel wall. Nonetheless, findings from contrast-enhanced abdominal computed tomography merely indicated thickening of the colonic wall. Potential etiologies for secondary eosinophilia include hypereosinophilic syndrome, parasitic infections, inflammatory bowel disease, Helicobacter pylori infection, connective tissue disorders, and drug-induced reactions5. Given the distinct treatment strategies for secondary causes, comprehensive investigations to identify these causes are imperative. Our patient underwent an array of laboratory examinations, including blood tests, fecal tests, and bone marrow examinations. These tests did not reveal any secondary causes of eosinophilia, with the exception of positive serology for cysticercosis and Echinococcus spp. Despite this, a regimen of antiparasitic treatment did not ameliorate the patient’s symptoms. Consequently, the final diagnosis was primary eosinophilic duodenitis and eosinophilic colitis.
At present, there are no high-level recommendations for the management of eosinophilic gastroenteritis and eosinophilic colitis. Systemic corticosteroids have been identified as an efficacious initial treatment, particularly for patients exhibiting severe symptoms, as evidenced by a multitude of case reports and studies15. The treatment protocol commences with an equivalent dose of 20–40 mg of prednisone, which is gradually reduced over a period of weeks or months5. Our patient was also administered an initial dosage of 32 mg of methylprednisolone, equating to a 40 mg dosage of prednisone. During corticosteroid treatment, our patient developed severe cellulitis, potentially indicative of systemic side effects associated with steroid use. Furthermore, a study by Pineton de Chambrun G et al. reported a 37% relapse risk following steroid withdrawal, underscoring the necessity for alternative, steroid-sparing therapies in the management of EGIDs20. In the case under consideration, a therapeutic regimen comprising ketotifen and montelukast was initiated concurrently with steroid therapy. Following the discontinuation of steroid treatment, the patient remained in remission for a subsequent period of three months. This was achieved through the administration of a combined regimen of ketotifen (1 mg, administered twice daily) and montelukast (10 mg, administered daily). Ketotifen functions as a mast cell stabilizer, inhibiting the release of toxic mediators from mast cells and attenuating the activation of eosinophils. This is noteworthy, as mast cells and eosinophils exhibit mutual stimulation in the absence of external activators15, 21. In a research study conducted by Melamed et al., six patients diagnosed with eosinophilic gastroenteritis were administered a daily dosage of 2 to 4 mg of ketotifen over a period of 12 months. Notably, all patients exhibited significant clinical improvement. The serum levels of immunoglobulin E decreased substantially during the 4-6 month interval of the treatment regimen. Furthermore, eosinophilic infiltrates were effectively eradicated, underscoring the efficacy of this therapeutic approach22. Montelukast, a leukotriene receptor antagonist, serves to counteract the effects of leukotrienes, which are released by eosinophils and influence vascular permeability and chemotaxis. There are case reports that illustrate the achievement of long-term remission in instances of recurrent or steroid-dependent eosinophilic gastroenteritis through the administration of montelukast at daily dosages ranging from 10 to 40 mg. These findings underscore the potential therapeutic efficacy of montelukast in the management of this condition23, 24. Nonetheless, empirical data derived from those case reports underscored the significance of ketotifen or montelukast in patients experiencing recurring symptoms subsequent to steroid discontinuation, as opposed to the utilization of these two medications in a preemptive strategy, as exemplified in our patient. A study by Hui et al. revealed that an initial treatment regimen of ketotifen and montelukast induced a response in approximately 90% of patients4. This study demonstrated the benefits of combining ketotifen and montelukast in treating eosinophilic gastroenteritis. However, in our case report, this combination was used concurrently with a systemic steroid rather than as the initial treatment. In a previous case report by Nguyen et al., a 59-year-old Vietnamese patient with EGID exhibited an initial partial response to systemic corticosteroids. However, complete clinical remission was achieved upon the addition of ketotifen, montelukast, and azathioprine, suggesting their efficacy in treatment25. Nonetheless, given its immunosuppressive properties, azathioprine requires careful administration in elderly patients due to the heightened risk of adverse events26. In another case report, Huynh et al. described a 57-year-old steroid-dependent Vietnamese patient who was administered azathioprine. Despite symptom management, the patient succumbed to SARS-CoV-2 infection three months after azathioprine initiation27. Consequently, a therapeutic regimen combining ketotifen and montelukast may be a safe and efficacious steroid-sparing treatment for patients diagnosed with eosinophilic gastroenteritis. Nonetheless, empirical evidence pertaining to the optimal duration and initiation timing of this regimen remains limited.
This case report suggests that EGID, particularly in patients with peripheral eosinophilia, is a potential etiology for chronic diarrhea. This finding underscores the importance of obtaining endoscopic gastrointestinal mucosa biopsies and subsequent eosinophil counts for histological assessment. This report also emphasizes the necessity of ruling out secondary causes of eosinophilia, notably prevalent parasitic infections in developing countries. While corticosteroid therapy is effective for EGID management, the associated infection risk necessitates the consideration of steroid-sparing therapies such as ketotifen and montelukast to mitigate long-term adverse effects and maintain clinical remission.
This case report, while providing valuable insights into the diagnosis and management of EGID, has several limitations. First, the findings are based on a single patient's experience, limiting the generalizability of the results to all EGID patients. Second, the report lacks long-term follow-up data, leaving questions about the durability of remission and the potential for relapse over time. Third, the absence of a comparison between the treatment regimen used in this case and other potential treatments makes it difficult to ascertain the relative effectiveness of the chosen strategy. Fourth, we were not able to investigate potential genetic, environmental, or lifestyle factors contributing to the condition. Fifth, the diagnosis and treatment decisions were based on available clinical, laboratory, and pathology data, potentially introducing diagnostic bias, as untested conditions could exhibit similar symptoms and pathological findings.
Conclusions
In summary, this report delineates a case of primary eosinophilic gastroenteritis and eosinophilic colitis characterized mainly by lower gastrointestinal manifestations. The patient showed a favorable response to initial steroid therapy and achieved sustained remission via a treatment regimen comprising ketotifen and montelukast. This case emphasizes the significance of considering eosinophilic gastroenteritis and colitis in the differential diagnosis of patients presenting with gastrointestinal symptoms and peripheral eosinophilia, especially when endoscopic assessment reveals mucosal abnormalities. Thorough investigations to rule out secondary causes of eosinophilia are imperative. Despite the potential side effects associated with systemic steroids, their efficacy in inducing remission has been substantiated. The adjunctive use of ketotifen and montelukast with steroids, maintained post-steroid treatment, presents an effective long-term remission strategy. Furthermore, dietary modifications based on radioallergosorbent test findings may offer additional advantages, albeit compliance may pose a challenge. The insights derived from this case underscore the necessity for prompt diagnosis and management of eosinophilic gastrointestinal disorders (EGIDs) and illuminate the role of innovative treatment approaches in improving patient outcomes. Accordingly, this case contributes to the expanding evidence base supporting the employment of combined pharmacological treatment modalities in the management of these uncommon diseases.
Abbreviations
CT - Computed Tomography, EGIDs - Eosinophilic Gastrointestinal Diseases, GI - Gastrointestinal, HPF - High Power Field, SARS-CoV-2 - Severe Acute Respiratory Syndrome Coronavirus 2
Acknowledgments
None.
Author’s contributions
Duc Trong Quach, Nhan Trung Phan: Conceptualization; writing—original draft; formal analysis; critical review
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Gonsalves
N.,
Eosinophilic gastrointestinal disorders. Clinical Reviews in Allergy & Immunology.
2019;
57
(2)
:
272-85
.
View Article PubMed Google Scholar -
Alfadda
A.A.,
Storr
M.A.,
Shaffer
E.A.,
Eosinophilic colitis: epidemiology, clinical features, and current management. Therapeutic Advances in Gastroenterology.
2011;
4
(5)
:
301-9
.
View Article PubMed Google Scholar -
Redd
W.D.,
Dellon
E.S.,
Eosinophilic gastrointestinal diseases beyond the esophagus: an evolving field and nomenclature. Gastroenterology {&}amp; Hepatology.
2022;
18
(9)
:
522-8
.
PubMed Google Scholar -
Hui
C.K.,
Hui
N.K.,
A prospective study on the prevalence, extent of disease and outcome of eosinophilic gastroenteritis in patients presenting with lower abdominal symptoms. Gut and Liver.
2018;
12
(3)
:
288-96
.
View Article PubMed Google Scholar -
Uppal
V.,
Kreiger
P.,
Kutsch
E.,
Eosinophilic gastroenteritis and colitis: a comprehensive review. Clinical Reviews in Allergy {&}amp; Immunology.
2016;
50
(2)
:
175-88
.
View Article PubMed Google Scholar -
Oh
H.E.,
Chetty
R.,
Eosinophilic gastroenteritis: a review. Journal of Gastroenterology.
2008;
43
(10)
:
741-50
.
View Article PubMed Google Scholar -
Klein
N.C.,
Hargrove
R.L.,
Sleisenger
M.H.,
Jeffries
G.H.,
Eosinophilic gastroenteritis. Medicine.
1970;
49
(4)
:
299-319
.
View Article PubMed Google Scholar -
Chehade
M.,
Kamboj
A.P.,
Atkins
D.,
Gehman
L.T.,
LT G. Diagnostic delay in patients with eosinophilic gastritis and/or duodenitis: a Population-based study. The Journal of Allergy and Clinical Immunology. In Practice.
2021;
9
(5)
:
2050-2059.e20
.
View Article PubMed Google Scholar -
Kelly
K.J.,
Eosinophilic gastroenteritis. Journal of Pediatric Gastroenterology and Nutrition.
2000;
30
:
28-35
.
View Article PubMed Google Scholar -
Kinoshita
Y.,
Furuta
K.,
Ishimaura
N.,
Ishihara
S.,
Sato
S.,
Maruyama
R.,
Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. Journal of Gastroenterology.
2013;
48
(3)
:
333-9
.
View Article PubMed Google Scholar -
Reed
C.,
Woosley
J.T.,
Dellon
E.S.,
Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver.
2015;
47
(3)
:
197-201
.
View Article PubMed Google Scholar -
Ko
H.M.,
Morotti
R.A.,
Yershov
O.,
Chehade
M.,
Eosinophilic gastritis in children: clinicopathological correlation, disease course, and response to therapy. The American Journal of Gastroenterology.
2014;
109
(8)
:
1277-85
.
View Article PubMed Google Scholar -
Zhang
L.,
Duan
L.,
Ding
S.,
Lu
J.,
Jin
Z.,
Cui
R.,
Eosinophilic gastroenteritis: clinical manifestations and morphological characteristics, a retrospective study of 42 patients. Scandinavian Journal of Gastroenterology.
2011;
46
(9)
:
1074-80
.
View Article PubMed Google Scholar -
Prussin
C.,
Eosinophilic gastroenteritis and related eosinophilic disorders. Gastroenterology Clinics of North America.
2014;
43
(2)
:
317-27
.
View Article PubMed Google Scholar -
Zhang
M.,
Li
Y.,
Eosinophilic gastroenteritis: A state-of-the-art review. Journal of Gastroenterology and Hepatology.
2017;
32
(1)
:
64-72
.
View Article PubMed Google Scholar -
Turner
K.O.,
Sinkre
R.A.,
Neumann
W.L.,
Genta
R.M.,
Primary colonic eosinophilia and eosinophilic colitis in adults. The American Journal of Surgical Pathology.
2017;
41
(2)
:
225-33
.
View Article PubMed Google Scholar -
Collins
M.H.,
Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterology clinics of North America.
2014;
43
(2)
:
257-68
.
View Article Google Scholar -
Dellon
E.S.,
Liacouras
C.A.,
Molina-Infante
J.,
Furuta
G.T.,
Spergel
J.M.,
Zevit
N.,
Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology.
2018;
155
(4)
:
1022-1033.e10
.
View Article PubMed Google Scholar -
Schreiner
P.,
Biedermann
L.,
Greuter
T.,
Wright
B.L.,
Straumann
A.,
How to approach adult patients with asymptomatic esophageal eosinophilia. Diseases of the Esophagus.
2021;
34
(1)
:
doaa105
.
View Article PubMed Google Scholar -
Chambrun
G. Pineton de,
Gonzalez
F.,
Canva
J.Y.,
Gonzalez
S.,
Houssin
L.,
Desreumaux
P.,
Natural history of eosinophilic gastroenteritis. Clinical gastroenterology and hepatology.
2011;
9
(11)
:
950-6.é
.
View Article PubMed Google Scholar -
Minai-Fleminger
Y.,
Levi-Schaffer
F.,
Mast cells and eosinophils: the two key effector cells in allergic inflammation. Inflammation research.
2009;
58
(10)
:
631-8
.
View Article PubMed Google Scholar -
Melamed
I.,
Feanny
S.J.,
Sherman
P.M.,
Benefit of ketotifen in patients with eosinophilic gastroenteritis. The American journal of medicine.
1991;
90
(3)
:
310-4
.
View Article Google Scholar -
Schwartz
D.A.,
Pardi
D.S.,
CASE REPORT: Use of Montelukast as Steroid-Sparing Agent for Recurrent Eosinophilic Gastroenteritis. Digestive diseases and sciences.
2001;
46
(8)
:
1787-90
.
View Article Google Scholar -
Quack
I.,
Sellin
L.,
Buchner
N.J.,
Theegarten
D.,
Rump
L.C.,
Henning
B.F.,
Eosinophilic gastroenteritis in a young girl-long term remission under Montelukast. BMC Gastroenterology.
2005;
5
(1)
:
24
.
View Article PubMed Google Scholar -
Nguyen
P.V.,
Quach
D.T.,
Bui
M.H.,
Dang
T.P.,
Talley
N.J.,
A Clinicopathologic Continuum of Eosinophilic Gastrointestinal Diseases in an Adult With Tuberculosis and Latent Hepatitis B Virus Infection. ACG Case Reports Journal.
2023;
10
(10)
:
e01176
.
View Article PubMed Google Scholar -
Kotlyar
D.S.,
Lewis
J.D.,
Beaugerie
L.,
Tierney
A.,
Brensinger
C.M.,
Gisbert
J.P.,
Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clinical gastroenterology and hepatology.
2015;
13
(5)
:
847-58.e4; quiz e48-50
.
View Article PubMed Google Scholar -
Huynh
T.N.D.,
Pham
T.Q.,
Pham
Q.T.T.,
Ho
P.T.,
Eosinophilic gastrointestinal disorders presenting with multiple gastric and colonic ulcerative lesions: a case report. MedPharmRes.
2022;
6
(s3)
:
s33-s7
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 6 (2024)
Page No.: 6520-6531
Published on: 2024-06-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3315 times
- PDF downloaded - 1082 times
- XML downloaded - 171 times
 Biomedpress
Biomedpress








