Abstract
Introduction: Cisplatin-induced acute nephrotoxicity is typically manifested by a decrease in kidney function, electrolyte imbalances, and acute kidney injury (AKI). Most therapies for AKI are costly and have serious side effects. Utilizing phytotherapy for the prevention of kidney disease has the potential to reduce both treatment expenses and the occurrence of associated side effects. Benincasa cerifera (BC) offers extensive advantages owing to its anti-inflammatory, antioxidant, and anti-diabetic characteristics. This study aims to demonstrate the protective effects of BC on the inflammatory mediators and regulatory pathways in a cisplatin-induced acute kidney injury (AKI) model.
Methods: Fifteen 6–8-week-old female BALB/c mice (weighing 20-25 g) were divided into three groups as follows: (I) Control, (II) Cisplatin, (III) Cisplatin + BC. The disease was induced with a single dose (15 mg/kg) of cisplatin on day 0, followed by treatment with an ethanolic extract of BC (500 mg/kg). On day 7, the sacrificed mice were used to study the histopathological, biochemical, immunological, and inflammatory parameters.
Results: Treatment with BC successfully restored the clonogenic potential of the kidney, blood, spleen, and bone marrow cells. Flow cytometry showed that cisplatin-induced abnormalities in cellular circulation and the migration of CD45+B220+ B cells, CD4+, CD8+ T lymphocytes, and F4/80+ cells in the blood were significantly recovered with the treatment of BC. This study also found that BC inhibited the upregulation of different inflammatory cytokines, which were involved in the Th1, Th2, and Th17 response.
Conclusion: Phytochemical-based novel treatment strategies developed from this work will provide safe options for patients and assist in reducing the disease burden of AKI.
Introduction
Acute Kidney Injury (AKI), characterized by a sudden decline in kidney function, arises from a variety of causes including urinary tract blockages, glomerulonephritis, sepsis, and reduced blood flow due to organ failure or significant blood loss. It is an urgent health concern, particularly in hospitalized children and critically ill pediatric patients, where it affects around 5-10% and exhibits higher incidence rates respectively, leading to increased morbidity and mortality1, 2. A notable cause of AKI, particularly relevant in cancer treatment, is nephrotoxicity induced by cisplatin—a widespread chemotherapeutic agent. While cisplatin is efficacious in treating various cancers, its associated nephrotoxic effects, which manifest in 21–31.5% of treated patients, severely limit its clinical application, necessitating dose adjustments or discontinuation to mitigate renal damage3, 4, 5. Cisplatin-induced AKI, marked by tubular cell death, inflammation, and oxidative stress, underscores the need for innovative therapeutic approaches and deeper understanding of its pathology6. However, the progression from acute cisplatin nephrotoxicity to chronic kidney disease in mouse models remains underexplored due to the absence of standard protocols7.
In West Bengal, the surge in kidney diseases has been attributed to increased prevalence of hypertension8. Standard AKI treatments involve managing hypovolemia—a critical reduction in blood plasma volume—using isotonic saline, albumin, and tailored fluid therapy to prevent rapid heartbeat, weakness, confusion, or unconsciousness. Vasopressors such as Noradrenaline, Vasopressin, and Terlipressin offer further support; however, these treatments can be expensive and yield severe side effects9. Ultimately, for both acute and chronic kidney diseases, dialysis or kidney transplantation are the primary interventions, each carrying substantial impacts on patient quality of life and potential risks, including immunological rejection and ethical concerns such as organ trafficking.
Given these challenges, there's growing interest in alternative treatments like those offered by Ayurveda, including phytochemical extracts known for their regenerative properties and minimal side effects. Notably, extracts from Benincasa sp., commonly known as winter melon, are gaining attention for their antioxidant properties and potential in treating kidney diseases10, 11, 12, 13, 14, 15. This study aims to explore the effects of Benincasa sp. extract on the inflammation and regulatory pathways involved in cisplatin-induced AKI, using a mouse model treated with cisplatin followed by oral administration of the extract. Through this research, we hope to uncover novel therapeutic pathways and validate the efficacy of traditional medicines in the context of modern-day ailments.
| Gene | Primer sequence (5’-3’) | Tm | |
| GAPDH | F | GAGGGGCCATCCACAGTCTTC | 62.8˚C |
| R | CATCACCATCTTCCAGGAGCG | ||
| IFNγ | F | AGCGGCTGACTGAACTCAGATTGTAG | 62.9˚C |
| R | GTCACAGTTTTCAGCTGTATAGGG | ||
| TNFα | F | GGCAGGTCTACTTTGGAGTCATTGC | 64.6˚C |
| R | ACATTCGAGGCTCCAGTGAATTCGG | ||
| IL1β | F | TCATGGGATGATGATGATAACCTGCT | 61.5˚C |
| R | CCCATACTTTAGGAAGACACGGATT | ||
| IL-4 | F | ACCTTGCTGTCACCCTGTTC | 58.3˚C |
| R | TTGTGAGCGTGGACTCATTC | ||
| IL-13 | F | CGGCAGCATGGTATGGAGTG | 60.4˚C |
| R | ATTGCAATTGGAGATGTTGGTCAG | ||
| IL-10 | F | ATTTGAATTCCCTGGGTGAGAAG | 54.1˚C |
| R | CACAGGGGAGAAATCGATGACA | ||
Methods
Ethical approval
Every experiment was conducted in compliance with the guidelines established by the animal ethics committee of the University of Calcutta (No- ERB/ZOO/2023/III, Dated 02.08.2023). The mice were housed in the University of Calcutta's Department of Zoology animal facility under specific pathogen-free conditions.
Mice
Female BALB/c mice aged 6-8 weeks (weighing 20-25 g) were divided into three groups, each comprising n = 5: (I) Control, (II) CIS, and (III) CIS+BEN.
Collection and Preparation of Benincasa cerifera (BC)
BC was collected from the Indian Market in Kolkata during the summer. The rind was peeled, and the fruit was cut into small pieces, dried in a freeze-dryer, and then ground using an electric blender. The powder was then dissolved in 70% v/v ethanol and shaken for an entire day. A gas dryer was used to evaporate and dry the mixture. To remove water and yield a solid powder, the evaporated solution was placed in a freeze-dryer again. The resultant powder was extracted in water and administered orally (Figure 1).
Induction of Acute Kidney Injury (AKI) and treatment with Benincasa cerifera
Cisplatin (Cisplatin injection, Cizcan) was administered intraperitoneally once. For the diseased and treatment groups, an intraperitoneal injection of cisplatin was given on day 0 (15 mg/kg body weight). The control group received normal saline. For the treatment group, Benincasa sp. extract (500 mg/kg body weight) dissolved in water was administered orally on day 0 (after 6 hours of cisplatin induction), and then on days 2, 4, 6. Animals were euthanized on day 7 (Figure 2).
Sacrifice and collection of tissues
On day 7, mice were euthanized by cervical dislocation, and the following tissues were collected. 1 ml of peripheral blood (PB) was collected via cardiac puncture into tubes containing EDTA as an anticoagulant. A smear was made on a microscope slide for a differential cell count.
For assays
The kidney, spleen, and liver were placed in a Petri dish and finely chopped. The kidney was then treated with a 1X cocktail of collagenase/hyaluronidase (Stem Cell Technology) overnight at 37°C. Once a single-cell suspension was achieved, it was filtered through a no. 60 sieve (Sigma Aldrich).
For gene expression
One kidney was taken whole, washed with PBS, and stored in RNAlater solution (Ambion, Inc) at -80°C.
For protein expression
One kidney was taken whole, washed with PBS, and stored in PBS at -80°C.
For histology
One kidney was collected whole in 10% buffered formalin.
Total cell (TC) count
The TC count of PB, kidney, bone marrow, spleen, and liver cells was performed according to Mitra et al., 202216.
Differential cell (DC) count
The DC count of PB was conducted according to Mitra et al., 202216.
BUN and Serum Creatinine Level Test
PB from each group of mice was collected in uncoated tubes and allowed to stand for at least 30 minutes. The samples were then centrifuged at 10,000 rpm for 10 min at 4°C, and the supernatant was collected in fresh 1.5 ml tubes. The serum was stored at -20°C for later analysis. The BUN and serum creatinine levels were evaluated using the Autospan Urea Test kit and Autospan Liquid Gold Creatinine, both from ARKRAY Healthcare Pvt. Ltd, respectively.
CFU-c assay
To assess the clonogenic capability of the tissue cells, a colony-forming unit-cell assay was performed using methylcellulose semisolid media (Himedia, India), following a protocol standardized in the lab of the University of Calcutta.
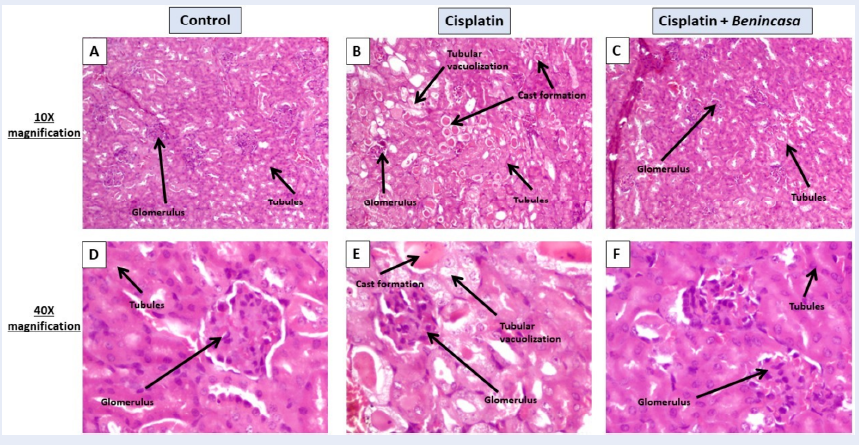
Histology
Kidney tissues were fixed in 10% formalin, dehydrated, and then embedded in molten paraffin to form blocks. The tissues were then sectioned into 5 µm slices. Hematoxylin and eosin-stained kidney sections showed changes in the glomeruli and renal tubules. A light microscope (Dewinter Fluorex LED, camera- DIGIEYE-510CCD) was used to capture images of the H&E-stained kidney sections. A slightly modified version of the previously disclosed semi-quantitative pathological scoring system was applied. A grading scale (0, 1, 2, 3) was used to determine the degree of kidney damage, with detailed criteria for each grade. For the cortex and outer stripe of the outer medulla in each kidney section, ten fields (40x) were counted. The sum of all scores from 100 fields, with a maximum score of 300, was used to determine the overall score for each kidney. The percentage of cast formation was calculated from 20 fields for the cortex and medulla in each kidney section.
Flow cytometry
Cells from the blood, kidney, bone marrow, and spleen were stained with suitable antibodies and analyzed using flow cytometry on a BD FACSVerse (BD Biosciences, USA), with results processed using the FACSSuite (BD Biosciences, USA) software. The antibodies used for cell surface staining included: CD45-PerCPCy5.5 (BioLegend), CD4-V450 (BD Biosciences, USA), CD3e-PE (BD Biosciences, USA), B220-FITC (BD Biosciences, USA), CD8a-Alexa Fluor 488 (BD Biosciences, USA), GR-1-FITC (MACS), and F4/80-PE (eBiosciences). Among the CD45+ hematopoietic cells, CD45+B220+ are B cells, and CD45+CD3+ are T cells, with subsets identified as helper T (TH) cells (CD3+CD4+) and cytotoxic T (TC) cells (CD3+CD8+), while neutrophils and macrophages are identified as CD45+Gr1+ and CD45+F4/80+, respectively.
Gene expression Study
Total RNA was extracted from kidney tissues using TRIzol solution according to the manufacturer's instructions (Life Technologies, USA). The expression of TNFα, IFNγ, IL1β, IL13, IL4, and IL10 was evaluated using GAPDH as the housekeeping gene. To visualize the expression of inflammatory genes, PCR products were run on 1% agarose gels, and band intensities were quantified using ImageJ software following visualization under UV light in a gel-doc system (BioRad).
Protein expression by Western blot
Kidneys were homogenized, and total cellular protein was extracted in RIPA buffer solution. Western blotting was performed using antibodies against GAPDH, TGFβ, AQP1, and AQP5, followed by detection with HRP-conjugated secondary antibodies. The blots were developed using BioRad Western ECL substrate and visualized in a chemidoc system (BioRad). The band intensities were calculated using ImageJ software.
Results
Total cell counts of blood, kidney, spleen, and bone marrow
Reduction of total cell (TC) count in blood and bone marrow is an indication of cisplatin-induced toxicity. The TC of bone marrow and PB decreased by 23.11-fold (p < 0.05) and 3.48-fold (p < 0.05) after treatment with cisplatin, compared to control, and increased by 12.29-fold (p < 0.05) and 1.89-fold (p < 0.05), respectively, with Benincasa treatment (Figure 3A, C). The TC of spleen and kidney increased by 5.42-fold (p < 0.05) and 3.57-fold (p < 0.05), respectively, with cisplatin treatment, and reduced by 1.79-fold (p < 0.05) and 1.91-fold (p < 0.05), respectively, with Benincasa extract (Figure 3 B, D).
Differential count of peripheral blood
Cisplatin treatment led to an increase in the count of both neutrophils and lymphocytes in the blood by 1.41-fold (p < 0.05) and 1.11-fold (p < 0.05), respectively. Benincasa therapy successfully reduced both counts by 1.09-fold (p < 0.05) and 1.04-fold, respectively (Figure 4 A, B). The count of eosinophils, basophils, and monocytes decreased by 17.5-fold, 9-fold (p < 0.05), and 18.3-fold (p < 0.05), respectively, with cisplatin treatment and increased by 4-fold (p < 0.05), 4.5-fold, and 3.5-fold, respectively, with Benincasa extract (Figure 4 C, D, E).
Estimation of kidney weight, serum creatinine level, and blood urea nitrogen (BUN) level
An increase in kidney weight, serum creatinine level, and BUN are indications of cisplatin-induced toxicity. The kidney weight increased by 1.18-fold (p < 0.05) after treatment with cisplatin, compared to control, and decreased by 1.10-fold with Benincasa treatment (Figure 5 A). Levels of serum creatinine and BUN increased by 7.09-fold (p < 0.05) and 2.04-fold (p < 0.05), respectively, with cisplatin treatment and were successfully reduced by 2.16-fold (p < 0.05) and 4.92-fold (p < 0.05), respectively, with Benincasa extract (Figure 5 B, C).
Estimation of clonogenic potential (assessed by the CFU-c assay) of cells
Loss of the ability to proliferate to their full potential of cells is an indication of inflammation or degeneration. Cisplatin treatment led to a decrease in the clonogenic potential of blood (Figure 6 A, B) and kidney (Figure 6 C, D) by 1.65-fold and 25.5-fold (p < 0.05), respectively. Benincasa extract successfully restored the clonogenic potential by 1.38-fold (p < 0.05) and 4.80-fold (p < 0.05), respectively.
Hematoxylin-eosin staining of kidney sections
Representative images (Figure 7) of the renal pathology (H&E staining, magnification 10x, 40x) on day 7 after a single administration of cisplatin showed renal damages. Control groups showed normal renal structure. In the case of cisplatin-treated kidney sections, kidney injury scores, and the percentage of cast formation increased by 19.66-fold (p < 0.05) and 11.14-fold (p < 0.05), respectively, with cisplatin treatment. Benincasa extract significantly reduced the damage by 2.80-fold (p < 0.05) and 5.2-fold (p < 0.05), respectively (Figure 8 A, B).
Immunophenotyping and migration pattern of immune cells in the bone marrow, spleen, blood, and kidney
Cisplatin treatment increased the CD45+B220+ B cell populations by 2.42-fold (p < 0.05) and 1.94-fold (p < 0.05) in the spleen and blood, respectively, and decreased by 2.38-fold (p < 0.05) and 1.52-fold (p < 0.05) in bone marrow and kidney, respectively. Benincasa therapy successfully reduced the B cell population by 9.24-fold (p < 0.05) and 1.09-fold (p < 0.05) in spleen and blood, respectively (Figure 9 B, C), and increased by 1.69-fold (p < 0.05) and 0.6-fold (p < 0.05) in bone marrow and kidney, respectively (Figure 9 A, D).
Cisplatin treatment increased the CD45+CD3+CD8+ TC cell populations by 1.57-fold (p < 0.05), 6-fold (p < 0.05) and 1.51-fold (p < 0.05) in bone marrow, spleen and blood respectively. Benincasa therapy successfully reduced the population by 2.5-fold (p < 0.05), 11.5-fold (p < 0.05) and 1.5-fold (p < 0.05) in bone marrow, spleen and blood respectively (Figure 10 A, B, C). In cisplatin induced mice, the CD45+CD3+CD4+ TH cell populations were increased by 3.75-fold (p < 0.05), 5.17-fold (p < 0.05) and 2.27-fold (p < 0.05) in kidney, spleen and blood respectively and decreased by 2.22-fold (p < 0.05) in bone marrow. Benincasa therapy successfully reduced the TH cell population by 1.36-fold (p < 0.05), 16.2-fold (p < 0.05) and 1.51 fold (p < 0.05) in kidney, spleen and blood respectively (Figure 10 B, C, D) and increased by 1.42 fold (p < 0.05) in bone marrow (Figure 10 A).
Cisplatin treatment increased the CD45+F480+ macrophage population by 1.23-fold (p < 0.05) and 2.25-fold (p < 0.05) in spleen and blood respectively and decreased by 2.48-fold (p < 0.05) and 1.53-fold (p < 0.05) in bone marrow and kidney respectively. Benincasa therapy successfully reduced the macrophages by 2.2-fold (p < 0.05) and 1.95-fold (p < 0.05) in spleen and blood respectively (Figure 11 B, C) and increased by 1.52-fold (p < 0.05) and 1.04-fold (p < 0.05) in bone marrow and kidney respectively (Figure 11 A, D). Cisplatin treatment increased the CD45+GR1+ neutrophil cell populations by 1.26-fold (p < 0.05) and 1.91-fold (p < 0.05) in spleen and blood respectively and decreased by 1.55-fold (p < 0.05) and 1.63-fold (p < 0.05) in bone marrow and kidney respectively. Benincasa therapy successfully reduced the neutrophil populations by 2.12-fold (p < 0.05) and 2.39-fold (p < 0.05) in spleen and blood respectively (Figure 11 B, C) and increased by 1.27-fold (p < 0.05) in kidney (Figure 11 A, D).
Estimation of the expression of proinflammatory cytokines and signaling molecules at the gene level
Using RT-PCR, this study evaluated the gene expression of signaling molecules and pro-inflammatory cytokines such as TNFα, IFNγ, IL1β, IL13, IL4, and IL10. The treatment in the study had no effect on the expression of GAPDH, which served as a housekeeping gene (Figure 12 A and E). However, the intensity of the bands on the gels showed that the expression of the genes of interest significantly rose after cisplatin treatment compared to control. With cisplatin treatment (lanes marked "Cis" in Figure 12 A, E), the expression of IFNγ, TNFα, IL1β, IL4, IL13, and IL10 increased by 1.72-fold (p < 0.05) (Figure 12 B), 1.77-fold (p < 0.05) (Figure 12 C), 7.18-fold (p < 0.05) (Figure 12 D), 1.60-fold (p < 0.05) (Figure 12 F), 3.88-fold (p < 0.05) (Figure 12 G), and 3.19-fold (p < 0.05) (Figure 12 H), respectively, compared to control (lanes marked "Control" in Figure 12 A, E). With Benincasa extract treatment (lanes marked "Cis+Ben" in Figure 12 A, E), the expression of IFNγ, TNFα, IL1β, IL4, IL13, and IL10 decreased by 1.23-fold (p < 0.05) (Figure 12 B), 7.76-fold (p < 0.05) (Figure 12 C), 3.64-fold (p < 0.05) (Figure 12 D), 2.22-fold (p < 0.05) (Figure 12 F), 1.26-fold (p < 0.05) (Figure 12 G), and 2.01-fold (p < 0.05) (Figure 12 H), respectively, compared to the diseased group. These results imply that the expression of inflammatory molecules has been downregulated.
Estimation of the expression of signaling molecules and transmembrane channels at the protein level
This study also evaluated the protein expression of signaling molecules and transmembrane channels like TGFβ (molecular weight 44 kDa), AQP1 (molecular weight 34 kDa), and AQP5 (molecular weight 35 kDa). The treatment in the study had no effect on the expression of GAPDH, which served as a housekeeping protein (Figure 13 A). However, the intensity of the bands on the blots showed that the expression of the study proteins significantly changed following cisplatin treatment when compared to control. With cisplatin treatment (lanes marked "Cis" in Figure 13 A), the expression of TGFβ increased by 73.99-fold (p < 0.05) (Figure 13 B), compared to control (lanes marked "Control" in Figure 13 A). With Benincasa extract treatment (lanes marked "Cis+Ben" in Figure 13 A), the intensity of TGFβ decreased by 13.45-fold (p < 0.05) (Figure 13 B). With cisplatin treatment (lanes marked "Cis" in Figure 13 A), the expression of AQP1 and AQP5 decreased by 0.14-fold (p < 0.05) and 0.14-fold (p < 0.05), respectively (Figure 13 C, D), compared to control (lanes marked "Control" in Figure 13 A). With Benincasa extract treatment (lanes marked "Cis+Ben" in Figure 13 A), the intensities of AQP1 and AQP5 increased by 0.26-fold (p < 0.05) and 0.24-fold (p < 0.05), respectively (Figure 13 C, D). These results imply that the expression of signaling molecules and transmembrane channels has been restored with the use of Benincasa therapy.
Discussion
Cisplatin-induced acute kidney injury (AKI) is a significant complication of chemotherapy, characterized by tubular cell injury, inflammation, and oxidative stress. Cisplatin, a widely used chemotherapeutic agent, exerts nephrotoxic effects primarily by accumulating in renal tubular cells, leading to cellular apoptosis and necrosis. This nephrotoxicity often limits the clinical utility of cisplatin, necessitating dose reductions or discontinuation of treatment, which can compromise the efficacy of cancer therapy17, 18, 19, 20, 21. Effective management of cisplatin-induced AKI remains a critical challenge, highlighting the need for novel protective strategies to mitigate renal damage without impeding the anti-cancer efficacy of cisplatin21. Few investigations have clarified the roles of oxidative stress injury, apoptosis, and inflammation as causes of nephrotoxicity, and the mechanism by which cisplatin causes kidney injury is still poorly understood. Renal replacement therapy, kidney transplantation, and dialysis are associated with several risk factors. The synthetic drugs for AKI are expensive and have side effects on other organ functions. Therefore, scientists are interested in natural compounds that have beneficial antioxidant properties. This study used Benincasa, a widely used vegetable and fruit in India, which contains beneficial constituents like triterpenoids, vitamins, amino acids, and uronic acids. Benincasa sp. has been successfully used for renal failure in the human body, but the mechanism is still unknown. There is no in vivo animal AKI model to investigate the immune profile of this therapy. This study aimed to investigate whether the peel extract of Benincasa sp. showed a protective role against renal cell injury in the case of cisplatin-induced AKI. Benincasa sp. has been used at a dose of 500 mg/kg/day in previous investigations, and therapy with Benincasa sp. extract produced good ameliorative effects on renal ischemia and diabetes15. The identical dosage of 500 mg/kg/day of Benincasa sp. in an aqueous solution was utilized in this investigation. Furthermore, Benincasa sp. was given orally rather than intraperitoneally, intravenously, or intratracheally as had been done in other investigations. Oral medication operates systemically on the disease and offers a more convenient route of treatment.
Most people with kidney disease develop anemia. In AKI patients, the occurrence of anemia is frequent and rapid22. A crucial hormone called erythropoietin (EPO) is produced by the kidneys. Production of red blood cells is regulated by EPO. In renal disease, the kidneys are unable to produce enough EPO, leading to a drop in the total blood cell count and subsequent anemia. Treatment with cisplatin led to a 3.48-fold decrease in the total cell count of the blood compared to the untreated control (p < 0.05), indicating that the kidneys cannot make enough EPO to produce sufficient blood cells. Oral treatment with Benincasa extract led to a slight increase in the total cell count of blood, showing its ability to significantly increase blood cell production. The total cell counts at the site of inflammation (i.e., the kidney) increased 3.57-fold after treatment with cisplatin, indicating the presence of inflammation. Benincasa extract significantly reduced the cell count (p < 0.05), indicating that it is significantly successful in reducing inflammation when applied orally. Cisplatin had a significant toxic effect on both cellular and vascular components of the spleen, increasing the total cell (TC) count in the spleen as an indication of cisplatin-induced toxicity. The TC count of the spleen decreased after treatment with Benincasa extract. In the bone marrow, Benincasa extract successfully reduced inflammation (Figure 14, Figure 15, Figure 3). Both the number of neutrophils and lymphocytes in the blood increased after receiving the chemotherapeutic drug cisplatin. Neutrophil and lymphocyte counts were successfully reduced by Benincasa therapy. With cisplatin treatment, the count of eosinophils, basophils, and monocytes decreased. The number of lymphocytes was successfully boosted by Benincasa extract (Figure 4, Figure 14, Figure 15). The amount of nitrogen in the blood that originates from the waste product urea is measured by a BUN test. When protein is broken down by the body, urea is produced. The body excretes urea through urine, which is generated in the liver. A BUN test is performed to assess how effectively the kidneys are functioning. A rise in BUN level indicates the inability of kidneys to regularly eliminate urea from the blood. Kidneys also remove creatinine from the body. Thus, a rise in the level of creatinine in the blood indicates renal malfunction. Increases in serum creatinine, BUN, and kidney weight are signs of cisplatin-induced kidney damage. The toxicity was successfully decreased by the Benincasa extract (Figure 5, Figure 14, Figure 15). Clonogenic potential measures a cell's capacity to grow into colonies. Stressed cells typically lose some of their capacity for clonogenic reproduction. The clonogenic potential of kidney cells and blood cells from mice given cisplatin decreased after 6 days of incubation as compared to controls. All of these cell types had their clonogenic potential successfully recovered after treatment with Benincasa extract (Figure 6, Figure 14). The formation of monoclonal immunoglobulin free light chains (FLCs), which coprecipitate with Tamm-Horsfall glycoprotein (THP) in the lumen of the distal nephron and block tubular fluid flow, is directly associated with renal lesions (casts). Additionally, FLCs that evade tubular reabsorption are delivered to the distal nephron where they, under the right circumstances, create intraluminal casts that block tubular fluid flow. AKI and progressive renal failure are two clinical symptoms of this condition, also known as cast nephropathy23. Urinary casts are a helpful predictor of AKI in acute heart failure, and AKI is associated with a poor prognosis among patients with acute heart failure (AHF)24. When compared to the control group in this investigation, the cisplatin-injected kidneys displayed glomerular damage, collapsing Bowman's space, cast formation, epithelial necrosis, and vacuolization in the proximal tubules. Treatment with Benincasa extract significantly reduced these alterations (Figure 7, Figure 8, Figure 14).
By making cell suspensions and tissue sections from lymphoid and nonlymphoid organs, it has been possible to evaluate the frequency and distribution of B lymphocyte subsets in numerous human tissues and fluids. B cells may be a component of a circulating system that helps kidney disease patients' immune systems by acting as antigen-presenting cells and a source of cytokines that encourage T-cell proliferation. Therefore, in this cisplatin-induced AKI, the number of matured B cells is enhanced in the blood and spleen and decreased in the kidney and bone marrow (Figure 9). The dynamics of CD45+B220+ B cell populations in the bone marrow, spleen, blood, and kidney were successfully restored by Benincasa therapy (Figure 9, Figure 14). The relationships between the various lymphocyte subsets vary in most tissues and fluids. Between B and T lymphocytes, there is more compositional heterogeneity than between CD3+CD4+ and CD3+CD8+ T lymphocytes. Peripheral blood and the spleen have fewer CD3+ T lymphocytes in a healthy person than they do in renal disease. It has been established that T lymphocytes have a role in ischemia AKI25. Compared to T cell replete mice, the T cell deficient mice experienced less renal dysfunction and tubular damage and fared better in terms of survival21. It was found that CD3+CD4+ T cells and, to a lesser extent, CD3+CD8+ T cells were the main mediators of the negative effects of T cells. T cells are thought to play a role in the delayed cell-mediated immune response according to conventional immunology theories26. Benincasa therapy dramatically decreased the infiltration of T cell populations in the spleen, blood, and kidney in cisplatin-induced mice, whereas the cell populations of CD3+CD4+ and CD3+CD8+ T lymphocytes increased in the blood, spleen, and kidney (Figure 10, Figure 14, Figure 15). After being activated, naive CD3+CD4+ T cells in cisplatin-induced AKI develop into T-helper type 1 (Th1) and type 2 (Th2), which generate cytokines such as interferons like IFNγ and TNFα and interleukins like IL4, IL13, and IL10, respectively. When exposed to infections, CD3+CD8+ T cells can develop into cytotoxic effector cells that produce tumor necrosis factor (TNFα) and interferon (IFNγ) and migrate to eradicate the infection. Antigen-presenting cells in the lymph nodes and spleen prime CD3+CD8+ T cells during an infection26, 27, 28, 29. Regulatory T cells (Tregs), Th1, Th2, and Th17 can all be functionally differentiated from CD4+ T cells, and they can also activate the TGF signaling pathway. IFNγ and TNFα, two significant pro-inflammatory cytokines produced by Th1 cells, and IL4, IL10, and IL13, anti-inflammatory cytokines produced by Th2 cells, are proinflammatory cytokines. Anti-inflammatory cytokines like IL4, IL10, and IL13 showed higher levels of expression in cisplatin-induced animals, which may have activated STAT6-mediated Th2 differentiation. The expression of the IL4, IL10, and IL13 genes was dramatically decreased by Benincasa therapy (Figure 12, Figure 14). According to previous research, the pattern of Th2 inflammatory cells is protective in a kidney disease model, while the pattern of Th1 inflammatory cells is pathogenic and causes more damage26, 27, 28, 29. Th1 differentiation is mediated by the signal transducer and activator of transcription (STAT4) gene, whereas Th2 differentiation requires STAT6. Studies employing STAT4- and STAT6-knockout mice in an ischemia AKI model demonstrated that STAT6 deficiency resulted in decreased renal function and tubular damage due to impaired Th2 cell development. In STAT6-deficient mice, IFNγ expression was shown to be upregulated, whereas IL4 expression was downregulated. According to studies by scientists26, 27, 28, 29, ischemia-reperfusion injury (IRI) in STAT4-deficient mice led to a slight improvement in renal function. IFNγ, TNFα, and TGFβ gene expression levels rose in cisplatin-induced mice, possibly activating the TGFβ signaling pathway or STAT4-mediated Th1 differentiation. IFNγ, TNFα, and TGFβ expression at the gene and protein levels were all dramatically reduced by Benincasa therapy (Figure 12, Figure 13). In the pathogenesis of AKI, Th17 cells have more recently become important participants28. Different studies have shown that the kidney-infiltrating lymphocytes known as Th17 are the most prevalent after AKI in mice. Researchers have found that after developing AKI due to ureteral obstruction, resident dendritic cells and tubular epithelial cells (TEC) release IL-1 (α and β of IL-1), IL-23, and IL-6 to encourage intrarenal IL-17 migration and activation26, 27, 28, 29. In cisplatin-induced kidneys, increased levels of IL-1 gene expression may trigger Th17 differentiation, which was significantly suppressed by Benincasa therapy (Figure 12, Figure 14, Figure 15, Figure 16). During the first few days of AKI, this study looked at the dynamics of leukocyte populations in the bone marrow, spleen, blood, and kidney. As previously mentioned, extravascular kidney leukocytes were identified by administering a CD45+ antibody just before organ harvest. A dispersed mononuclear phagocyte cellular system (MPS) made up of tissue macrophages helps the body react to physiologic changes and infectious threats. As macrophages are flexible cells, they can quickly change their gene expression patterns and functionalities to match the changing renal microenvironment. The pathogenesis of ischemic AKI has been linked to macrophages. Their contribution to cisplatin nephrotoxicity is unclear, though. After cisplatin administration, researchers observed a 2-fold increase in CD11+ macrophages in the kidney and F480+ macrophages in the blood and spleen. Through their functional characteristics, neutrophils, the most prevalent leukocyte population in circulation, are important effectors of the inflammatory cascade. There is debate concerning neutrophils' contribution to acute renal failure. There is no doubt that the absence of neutrophils can result in acute renal failure. According to scientists25, neutrophil retention is influenced by the level of neutrophil activation and the length of renal ischemia. It is possible that this relationship explains why acute renal failure occurs so frequently26, 27, 28, 29. Neutrophil (CD45+GR1+) counts decreased in bone marrow after cisplatin-induced AKI (Figure 11 A), and a reciprocal increase was seen in blood and spleen (Figure 11 B, C). According to studies, the quantity of neutrophils in the blood is positively connected with the severity of AKI. The dynamics of leukocyte populations (CD45+F480+ and CD45+GR1+ cells) in the bone marrow, spleen, blood, and kidney were dramatically restored by Benincasa therapy (Figure 12, Figure 14, Figure 16). The elevated incidence of acute renal failure may be explained by this interaction. As a result of cisplatin-induced AKI, neutrophil (CD45+GR1+ cells) counts decreased in bone marrow (Figure 11 A), and a corresponding rise in blood and spleen (Figure 11 B, C) numbers was observed. According to scientists27, 28, 29, there is a positive correlation between the blood's neutrophil count and the severity of AKI. In the bone marrow, spleen, blood, and kidney, Benincasa therapy greatly improved the dynamics of leukocyte populations (CD45+F480+ and CD45+GR1+ cells) (Figure 12, Figure 14, Figure 16, Figure 17). A family of highly selective transmembrane channels called aquaporins (AQPs) primarily transports water across cells while also facilitating the passage of some low-molecular-weight solutes. The apical and basolateral plasma membrane of the proximal tubule, the descending thin limbs of Henle, and the descending vasa recta all contain the first water channel, known as AQP1, which mediates water reabsorption. A very selective water-permeable channel is AQP1. Polyuria in AQP1-deficient mice demonstrates this protein's critical function in the development of hypertonicity. AQP5 is said to be present in type B intercalated cells of collecting ducts, according to research published a few years ago30. In this investigation, it was found that cisplatin-induced mice had lower levels of AQP1 and AQP5 protein expression, and Benincasa therapy significantly corrected the deficiency in these two transmembrane channels (Figure 13, Figure 14). With the worldwide increase in kidney disease, the high cost of therapy, and the side effects of current therapeutic strategies, our study of Benincasa extract in a pre-clinical mouse model may provide a starting point for the development of a safer, more efficient, and more cost-effective treatment for acute kidney disease.
Conclusions
Recent findings from a preclinical study involving mouse models suggest that oral Benincasa extract, delivered in an aqueous solution, offers a cost-effective and safe intervention for the early stages of acute kidney disease, primarily during its acute inflammatory phase. These findings, supported by colony-forming unit (CFU) data, indicate that Benincasa extract holds promise as a therapeutic agent for kidney regeneration and the treatment of anemia induced by acute kidney injury (AKI).
The mechanism by which Benincasa extract exerts its therapeutic effects appears to involve the inhibition of several pathological processes. Notably, the extract prevents the synthesis and migration of CD3+CD4+ T helper cells and mitigates increases in blood urea nitrogen (BUN) and serum creatinine levels, which are critical markers of kidney function. Moreover, it inhibits the activation of key inflammatory mediators, including TGFβ, IFNγ, and TNFα, along with Th2 and Th17 cytokines, during the critical acute phase of kidney disease. Additionally, in cases of acute renal illness triggered by cisplatin, Benincasa extract has been shown to counteract the loss of aquaporin transmembrane water channels, as evidenced by Figure 14 through Figure 17.
Overall, this study strongly suggests that Benincasa extract could be a valuable therapeutic option for addressing the early stages of acute kidney illness, offering a new avenue for the treatment of this condition and its complications, including AKI-related anemia.
Abbreviations
AQP1: Aquaporin 1, AQP5: Aquaporin 5, AHF: Acute Heart Failure, AKI: Acute Kidney Injury, BC: Benincasa cerifera, BUN: Blood Urea Nitrogen, CD: Cluster of Differentiation, CFU-c: Colony-Forming Unit-Cell, DC: Differential Cell, ECL: Enhanced Chemiluminescence, EDTA: Ethylenediaminetetraacetic Acid, EPO: Erythropoietin, FLC: Free Light Chain, GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase, H&E: Hematoxylin and Eosin, HRP: Horseradish Peroxidase, IFNγ: Interferon Gamma, IL10: Interleukin 10, IL13: Interleukin 13, IL1β: Interleukin 1 Beta, IL4: Interleukin 4, MPS: Mononuclear Phagocyte System, PB: Peripheral Blood, PBS: Phosphate-Buffered Saline, RT-PCR: Reverse Transcription Polymerase Chain Reaction, STAT4: Signal Transducer and Activator of Transcription 4, STAT6: Signal Transducer and Activator of Transcription 6, TC: Total Cell, TGFβ: Transforming Growth Factor Beta, Th1: T Helper 1, Th2: T Helper 2, Th17: T Helper 17, THP: Tamm-Horsfall Glycoprotein, TNFα: Tumor Necrosis Factor Alpha, Tregs: Regulatory T Cells
Acknowledgments
We would like to acknowledge the University Grants Commission (UGC), New Delhi, for awarding Payal Pal a fellowship. We also thank the Central instrument facilities of Department of Zoology, University of Calcutta, Kolkata, India.
Author’s contributions
Ms. Payal Pal performed the immunological experiments, assays, analyzed data and drafted manuscript. Prof. Ena Ray Banerjee initiated the project, designed the whole experiments, analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
A project proposal no ERB/ZOO/2023/III submitted by Ms. Payal Pal has been approved/recommended by the Institutional Animal Ethics Committee of the Department of Zoology, University of Calcutta on 02.08.2023 using BALB/c mouse, to support the work done in this manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Krishnamurthy
S.,
Narayanan
P.,
Prabha
S.,
Mondal
N.,
Mahadevan
S.,
Biswal
N.,
Clinical profile of acute kidney injury in a pediatric intensive care unit from Southern India: A prospective observational study. Indian Journal of Critical Care Medicine : Peer-Reviewed, Official Publication of Indian Society of Critical Care Medicine.
2013;
17
(4)
:
207-13
.
View Article PubMed Google Scholar -
Lio
F.,
Pascual
J.,
Madrid
T.,
Bajo
M.A.,
Sicilia
L.S.,
Paz
L.,
Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney international.
1996;
50
(3)
:
811-8
.
View Article Google Scholar -
Sato
K.,
Watanabe
S.,
Ohtsubo
A.,
Shoji
S.,
Ishikawa
D.,
Tanaka
T.,
Nephrotoxicity of cisplatin combination chemotherapy in thoracic malignancy patients with CKD risk factors. BMC Cancer.
2016;
16
(1)
:
222
.
View Article PubMed Google Scholar -
Latcha
S.,
Jaimes
E.A.,
Patil
S.,
Glezerman
I.G.,
Mehta
S.,
Flombaum
C.D.,
Long-term renal outcomes after cisplatin treatment. Clinical Journal of the American Society of Nephrology ; CJASN.
2016;
11
(7)
:
1173-9
.
View Article PubMed Google Scholar -
Ozkok
A.,
Ravichandran
K.,
Wang
Q.,
Ljubanovic
D.,
Edelstein
C.L.,
NF-κB transcriptional inhibition ameliorates cisplatin-induced acute kidney injury (AKI). Toxicology Letters.
2016;
240
(1)
:
105-13
.
View Article PubMed Google Scholar -
Katagiri
D.,
Hamasaki
Y.,
Doi
K.,
Negishi
K.,
Sugaya
T.,
Nangaku
M.,
Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney International.
2016;
89
(2)
:
374-85
.
View Article PubMed Google Scholar -
Sharp
C.N.,
Siskind
L.J.,
St
S.H.,
Developing better mouse models to study cisplatin-induced kidney injury. American Journal of Physiology. Renal Physiology.
2017;
313
(4)
:
835-41
.
View Article PubMed Google Scholar -
John
O.,
Gummudi
B.,
Jha
A.,
Gopalakrishnan
N.,
Kalra
O.P.,
Kaur
P.,
Chronic Kidney Disease of Unknown Etiology in India: What Do We Know and Where We Need to Go. Kidney International Reports.
2021;
6
(11)
:
2743-51
.
View Article PubMed Google Scholar -
Al-Husinat
L.,
Alsabbah
A.,
Hmaid
A.A.,
Athamneh
R.,
Adwan
M.,
Hourani
M.N.,
Norepinephrine May Exacerbate Septic Acute Kidney Injury: A Narrative Review. Journal of Clinical Medicine.
2023;
12
(4)
:
1373
.
View Article Google Scholar -
Bhalodia
Y.,
Kanzariya
N.,
Patel
R.,
Patel
N.,
Vaghasiya
J.,
Jivani
N.,
Renoprotective activity of Benincasa cerifera fruit extract on ischemia/reperfusion-induced renal damage in rat. Iranian Journal of Kidney Diseases .
2009;
3
(2)
:
80-85
.
-
Al-Snafi
A.E.,
The pharmacological importance of Benincasa hispida. A review. Int Journal of Pharma Sciences and Research.
2013;
4
(12)
:
165-70
.
-
Kabra
A.,
Parashar
B.,
Badhani
S.,
Kainth
A.,
Evaluation of Antioxidant Activity of Benincasa hispida Fruit Extracts. American Journal of PharmTech Research.
2013;
3
(2)
:
335-45
.
-
Wang
Q.,
Yang
X.,
Zhu
C.,
Liu
G.,
Han
W.,
Sun
Y.,
Valorization of Polysaccharides From Benincasa hispida: Physicochemical, Moisturizing, and Antioxidant Skincare Properties. Frontiers in Pharmacology.
2022;
13
:
912382
.
View Article PubMed Google Scholar -
Mishra
M.K.,
Barik
B. Bhusan,
Antidiabetic activity of Benincasa hispida (Thumb.) Cogn. fruit peels on alloxan induced diabetic rats. Indian Drugs.
2009;
46
(10)
:
66-9
.
-
Hegazy
G.A.,
Mustafa
H.N.,
Altalhi
R.M.,
Yousef
J.M.,
The ameliorative potential of dexmedetomidine and Benincasa cerifera extract in renal ischemia/reperfusion injury in a streptozotocin-induced diabetic model. Biomedical & Pharmacology Journal.
2018;
11
(1)
:
285-303
.
View Article Google Scholar -
Mitra
S.,
Ghosh
N.,
Paul
P.,
Banerjee
E.R.,
Orally Administered Fisetin Reduces the Symptoms of Acute Allergic Asthma in a Preclinical Mouse Model. Biomedical Research and Therapy.
2022;
9
(3)
:
4953-70
.
View Article Google Scholar -
Panesso
M.C.,
Shi
M.,
Cho
H.J.,
Paek
J.,
Ye
J.,
Moe
O.W.,
Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney International.
2014;
85
(4)
:
855-70
.
View Article PubMed Google Scholar -
Kim
A.J.,
Ro
H.,
Kim
H.,
Chang
J.H.,
Lee
H.H.,
Chung
W.,
Klotho and S100A8/A9 as discriminative markers between pre-renal and intrinsic acute kidney injury. PLoS One.
2016;
11
(1)
:
e0147255
.
View Article PubMed Google Scholar -
Karagozian
R.,
Bhardwaj
G.,
Wakefield
D.B.,
Verna
E.C.,
Acute kidney injury is associated with higher mortality and healthcare costs in hospitalized patients with cirrhosis. Annals of Hepatology.
2019;
18
(5)
:
730-5
.
View Article PubMed Google Scholar -
Chen
Y.T.,
Jenq
C.C.,
Hsu
C.K.,
Yu
Y.C.,
Chang
C.H.,
Fan
P.C.,
Acute kidney disease and acute kidney injury biomarkers in coronary care unit patients. BMC Nephrology.
2020;
21
(1)
:
207
.
View Article PubMed Google Scholar -
Miller
R.P.,
Tadagavadi
R.K.,
Ramesh
G.,
Reeves
W.B.,
Mechanisms of Cisplatin nephrotoxicity. Toxins.
2010;
2
(11)
:
2490-518
.
View Article PubMed Google Scholar -
Kellum
J.A.,
Lameire
N.,
Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical care.
2013;
2013
(17)
:
1-5
.
View Article Google Scholar -
Ying
W.Z.,
Allen
C.E.,
Curtis
L.M.,
Aaron
K.J.,
Sanders
P.W.,
Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. The Journal of Clinical Investigation.
2012;
122
(5)
:
1777-85
.
View Article PubMed Google Scholar -
Higuchi
S.,
Kabeya
Y.,
Matsushita
K.,
Yamasaki
S.,
Ohnishi
H.,
Yoshino
H.,
Urinary cast is a useful predictor of acute kidney injury in acute heart failure. Scientific Reports.
2019;
9
(1)
:
4352
.
View Article PubMed Google Scholar -
Westermann
J.,
Pabst
R.,
Glinical Investigator Distribution of lymphocyte subsets and natural killer cells in the human body. Vol. 70. The Clinical Investigator.
1992;
70
(7)
:
539-544
.
View Article Google Scholar -
Fathabad
S. Gharaie,
Kurzhagen
J.T.,
Sadasivam
M.,
Noel
S.,
Bush
E.,
Hamad
A.R.,
Lymphocytes in Acute Kidney Injury and Repair. InSeminars in Nephrology.
2020;
40
(2)
:
114-25
.
View Article Google Scholar -
Imig
J.D.,
Ryan
M.J.,
Immune and inflammatory role in renal disease. Comprehensive Physiology.
2013;
3
(2)
:
957-76
.
View Article PubMed Google Scholar -
Dellepiane
S.,
Leventhal
J.S.,
Cravedi
P.,
T Cells and Acute Kidney Injury: A Two-Way Relationship. Frontiers in Immunology.
2020;
11
:
1546
.
View Article Google Scholar -
Inaba
A.,
Tuong
Z.K.,
Riding
A.M.,
Mathews
R.J.,
Martin
J.L.,
Saeb-Parsy
K.,
B Lymphocyte-Derived CCL7 Augments Neutrophil and Monocyte Recruitment, Exacerbating Acute Kidney Injury. The Journal of Immunology : Official Journal of the American Association of Immunologists.
2020;
205
(5)
:
1376-84
.
View Article PubMed Google Scholar -
He
J.,
Yang
B.,
Aquaporins in renal diseases. International journal of molecular sciences.
2019;
20
(2)
:
366
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 6 (2024)
Page No.: 6494-6510
Published on: 2024-06-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 2765 times
- PDF downloaded - 965 times
- XML downloaded - 109 times
 Biomedpress
Biomedpress



















