Abstract
Background: Hypothyroidism, marked by a deficiency in thyroid hormone levels, is a common condition with varying clinical presentations. The diagnosis primarily relies on statistical reference ranges of biochemical parameters, which remains a topic of contention. While manageable, untreated cases can lead to serious consequences. MicroRNAs, small non-coding RNAs, have been implicated in hypothyroidism and other diseases, underscoring their significant role in pathophysiology.
Methods: The study describes the bioinformatics techniques utilized to identify miR-148a-5p and to determine its secondary structure in hypothyroidism using databases such as NCBI, miRbase, TargetScan, and RNAfold. For clinical validation, blood samples from individuals with hypothyroidism and normal controls were collected, RNA was extracted, and reverse transcription was performed. The expression levels of RNA and IL-6 were quantified, and statistical analysis was conducted.
Results: Examination of the secondary structure reveals that hsa-miR-148a-5p has a minimum free energy of -27.90 kcal/mol. Consequently, our findings indicate dysregulation of miR-148a-5p and IL-6 expression in patients with hypothyroidism. There is upregulation of IL-6 and downregulation of miR-148a-5p, suggesting potential roles for these molecules in the pathogenesis of hypothyroidism.
Conclusion: The computational analysis suggests that miR-148a-5p is a promising diagnostic, prognostic, and therapeutic target for hypothyroidism. Based on the data presented in the study, the decreased expression of miR-148a-5p and elevated levels of IL-6 in patients with hypothyroidism suggest potential roles for these molecules in the pathogenesis of hypothyroidism. Further research is warranted to comprehensively understand dysregulation at the level of miR-148a-5p and its impact on IL-6 pathways in hypothyroidism.
Introduction
The thyroid gland is a small, butterfly-shaped organ located at the front of the neck, playing a pivotal role in controlling various physiological processes. Hypothyroidism is a condition characterized by the insufficient production of thyroid hormones T4 and T3, which often results in elevated levels of thyroid-stimulating hormone (TSH). Globally, iodine deficiency is recognized as the primary cause of thyroid disorders1. Symptoms of hypothyroidism are diverse and include irregular menstrual cycles, impaired cognitive development in children, fatigue, weight gain, etc. Conventional diagnostic methods, such as laboratory tests measuring T3, T4, and TSH levels, are instrumental in assessing thyroid function2. Levothyroxine, a synthetic form of the thyroid hormone, is the first-line treatment for hypothyroidism and is widely prescribed. Following its conversion from thyroxine (T4) to its active form, triiodothyronine (T3), levothyroxine modulates gene expression through intricate signaling pathways3. This process is intricately controlled by tissue-specific and cell-specific thyroid hormone transporters, various receptor isoforms, and interactions with corepressors and coactivators. The thyroid gland consists of follicles that enable the synthesis of thyroid hormones through the iodination of tyrosine residues in the glycoprotein thyroglobulin. TSH, produced by the anterior pituitary gland and responsive to circulating thyroid hormones, directly impacts thyroid follicular cells by binding to the TSH receptor located on the basolateral membrane4. However, the early diagnosis and prognosis of hypothyroidism continue to be a challenge.
MicroRNAs (miRNAs) are small, non-coding RNAs that intricately regulate the translation of specific protein-coding genes. Numerous studies have linked alterations in miRNA expression to the development of various diseases, including several types of cancer5. miRNAs, typically containing 19-24 nucleotides, can bind to complementary sequences of target mRNAs, causing target destruction or translation repression, which can be used to mute gene expression. MicroRNAs regulate about one-third of all mammalian genes5, 6. Recent research reveals that miRNAs are detectable in plasma and exhibit remarkable stability, resisting endogenous ribonuclease activity by binding to specific plasma proteins or by being packaged into secretory vesicles such as exosomes. Some miRNAs are actively secreted, serving as intercellular messengers. Consequently, numerous up-regulated blood-based miRNAs have been identified, showing promise for cancer detection, predicting prognosis, and monitoring tumor dynamics7. RNA sequencing analyses reveal that miR-148a-5p is downregulated across various cancer types, including gastric cancer (GC), associated with poorer survival outcomes. In non-small-cell lung cancer (NSCLC), decreased miR-148a-3p expression suggests its role in disease progression and treatment8, 9. Several studies have investigated miRNA expression in various types of thyroid tumors, revealing deregulation of miRNAs in cancerous tissues compared to their normal counterparts, with miRNA expression profiles varying significantly among different histological types of thyroid cancer10. Growing evidence suggests that microRNAs, such as miR-148, miR-128, and miR-30, play a crucial role in regulating plasma LDL-C levels and controlling VLDL secretion, proposing that microRNAs could have functional and therapeutic potential in managing dyslipidemia development in patients with hypothyroidism11.
Notably, interleukin-6 (IL-6) is shown to be a key pathophysiological mediator in a wide range of diseases. Numerous cell types, including cancer cells, T and B lymphocytes, macrophages, fibroblasts, and endothelial cells, have been shown to release IL-6, playing a pivotal role in modulating inflammatory and immune responses. As a proinflammatory cytokine, IL-6 is a key contributor to inflammatory conditions and may serve as a valuable marker for distinguishing different thyroid diseases12. Recent studies indicate a significant elevation in serum IL-6 levels among hypothyroid patients13, 14. Patients with hypothyroidism generally have significantly higher levels of IL-6 and TNF-α compared to euthyroid controls, consistent with previous studies that show chronic inflammation in hypothyroid patients, leading to other risks such as heart disease14. In the present study, we identified a new microRNA involved in hypothyroidism and analyzed the expression levels of microRNA and its target in the blood samples of hypothyroidism patients. Our primary objective is to investigate the expression levels of miR-148a-5p and IL-6 in patients with hypothyroidism, comparing them statistically with those of a control group comprising healthy individuals. By focusing on these molecular components, we aimed to gain insights into the underlying mechanisms of this prevalent metabolic disorder.
Methods
Obtaining Hypothyroidism-Specific Sequences and the miRNAs
Data from the Human Genome Sequence were accessed through the web portal of the National Center for Biotechnology Information (NCBI) for the International Nucleotide Sequence Database Consortium. The search keyword "hypothyroidism genome sequences of Homo sapiens" was used to obtain hypothyroidism-specific sequences. After removing duplicates and low-quality sequences, we created a local nucleotide database. Pre-miRNA (38,589 as of 2022) and mature miRNA (48,885 as of 2023) of humans were retrieved from miRBase and used as reference sequences (http://www.mirbase.org/). Ultimately, we searched for homologous sequences in the hypothyroidism database15.
Identification of Precursor-miRNAs
Mature miRNA sequences served as the initial query for finding homologous sequences in hypothyroidism nucleotide databases. BLAST (Basic Local Alignment Search Tool) version 2.2.2+ was used, and homology searches were conducted with a threshold E-value of 0.01. Additionally, BLAST searches against the NCBI protein database were performed to validate sequences, allowing for a maximum of 3 mismatches. Subsequently, aligned portions were identified and considered as candidates for pre-microRNA sequences15.
Validation of Candidate Pre-miRNA and Their Targets
To validate candidate pre-miRNA sequences, RNAfold was used to predict their secondary structures within the hypothyroidism genome sequence. Several criteria were assessed: 1) confirmation of suitable stem-loop hairpin structures, 2) localization of mature miRNA on one side of the hairpin, 3) the presence of <7 mismatches of opposite microRNA present in the other arm, and 4) maintenance of a secondary structure with an A+U content between 40-70% and higher negative energy15. Additionally, target prediction was conducted using TargetScan to identify potential miRNA targets.
Collection of Samples
The study received approval from the institutional ethical committee, and all sample collection adhered to the principles outlined in the Helsinki Declaration. Twenty blood samples were collected from hypothyroidism patients and healthy individuals with informed consent from the Department of Medicine, at Saveetha Medical College and Hospitals. The diagnosis of hypothyroidism was confirmed by the Department of Biochemistry at the same institution. Following collection, the samples underwent centrifugation, and the plasma was stored at -20°C for further analysis.
Inclusion and Exclusion Criteria
Participants meeting the following requirements: >18 years of age, capacity to provide informed consent were eligible for recruitment. Individuals with secondary complications like hypertension were excluded from the study.
RNA Extraction and Quantification
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was added to plasma samples for extraction, as per the instructions in the manual. The quantity and quality of isolated RNA were assessed using the NanoDrop 2000 Lite spectrophotometer (Thermo Fisher Scientific, Waltham, US). Following this step, the RNA samples were stored at -20°C to maintain their integrity for subsequent analysis16, 17.
Reverse Transcription
Reverse transcription was performed by adding oligo-dT18 primer (Promega, 50µM), deoxyribonucleotide triphosphate (dNTP), and nuclease-free water to the RNA sample. The mixture was then incubated at 65°C for 5 minutes, followed by immediate cooling to achieve a total volume of 10µl. Subsequently, the 10µl template RNA primer mixture was added to reverse transcriptase, murine RNase inhibitor, and 5x prime buffer. Nuclease-free water was used to make up to 20µl. The reaction mixture underwent thermal cycling on a PCR (MiniAmp Plus Thermal Cycler, Thermo Fisher) at 30°C for 10 mins, 42°C for 30 mins, 95°C for 5 mins, followed by a final incubation at 40°C. The resulting cDNA was quantified using a NanoDrop Lite spectrophotometer and stored at -20°C for further processing16, 17.
Expression Using qRT-PCR
The cDNA samples were utilized for expression analysis using Sybr Green (Takara, Japan) targeting the IL-6 gene and miR-148a-5p. GAPDH served as the internal control for IL-6, while U6 was used for miR-148a-5p normalization. All the required primers were obtained from Origene. The CFX96 Real-Time System (Bio-Rad) was used for the expression studies. The PCR cycling process started with an initial denaturation step at 95°C for 30 seconds to separate the DNA strands. Then, for 40 cycles, denaturation occurred at 95°C for 5 seconds, followed by annealing at specific temperatures for 30 seconds. Each cycle allowed the DNA to denature, primer binding, and extension by DNA polymerase. Finally, a melt curve analysis was performed to assess specificity by gradually increasing the temperature. These tests were performed as duplicates, and the expression of the genes was calculated using the equation 2-ΔΔCq 16, 17.
Statistical Analysis
The mean values obtained from duplicate experiments, along with their standard error of the mean (SEM), were included in the data presentation. Statistical analysis was conducted using the Student's t-test, utilizing the Microsoft Excel 365 program, to determine the presence of significant differences between various experimental groups. A p-value of ≤ 0.05 was considered indicative of statistical significance (*).
Results
Identification of pre-miRNA and Its Secondary Structure
MicroRNAs play an important role in the regulation of gene expression, impacting disease regression and progression. Thus, the identification of miRNAs associated with hypothyroidism could facilitate early diagnosis and treatment. Using a computational approach, we retrieved hypothyroidism human genome sequences from the NCBI database and precursor miRNAs from miRBase. Following the collection of these sequences, we carefully assessed their secondary structures. One miRNA identified, hsa-miR-148a-5p, was found within the hypothyroidism genome sequence. The mature sequence of hsa-miR-148a-5p was determined using RNAfold, possessing a minimum free energy of -27.90 kcal/mol.
Table 1 illustrates the stem-loop and the mature sequences of miR-148a-5p, while Figure 1 depicts its secondary structure. Table 2 provides more details, including the mature sequence, minimum free energy, pre-miRNA length, A+U% content of hsa-miR-148a-5p, and match extent.
| No | Structure | Sequence |
|---|---|---|
| 1 | Stem-loop | GAGGCAAAGUUCUGAGACACUCCGACUC UGAGUAUGAUAGAAGUCAGUGCACU ACAGAACUUUGUCUC |
| 2 | Mature miRNA | AAAGUUCUGAGACACUCCGACU |
| Source miRNA | Source organism | Pre-miRNA length | Minimum Free Energy | Mature Sequence | Match Extent | Strand | A+U% |
|---|---|---|---|---|---|---|---|
| miR-148a-5p | Homo sapiens | 68 | -27.90 kcal | AAAGUUCUGAGA CACUCCGACU | 22/22 | 5p | 54.41 |
| No | Target Protein | Molecular function | Biological process |
|---|---|---|---|
| 1 | Interleukin 6 | Multifunctional cytokine | Cellular function |
| 2 | Cyclin 1 | Phosphorylates targets | Mitotic cell cycle |
| 3 | Vasohibin 2 | Actin binding | Metastasis |
| 4 | Cell division cycle 73 | Transcription | Parafibromin formation |
| 5 | Transferrin receptor | Erythrocyte development | T and B cell proliferation |
Finding the Targets
A TargetScan analysis was conducted to ascertain the targets of this specific miRNA. The analysis revealed various significant transcripts targeted by miR-148a-5p, including IL-6, Cyclin D1, Vasohibin 2, Transferrin Receptor, among others. Table 3 provides an overview of these target genes in hsa-miR-148a-5p and their respective biological processes and molecular functions.
Gene Expression Analysis of miR148a-5p and IL-6
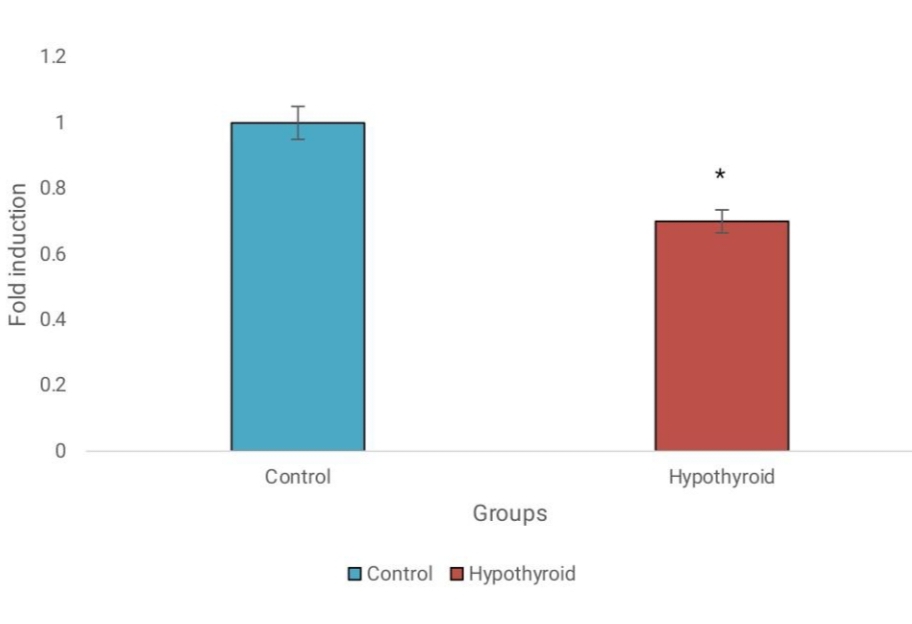
The analysis of gene expression via qRT-PCR indicated differential expression levels of miR-148a-5p and IL-6 in hypothyroid patient blood samples compared to normal samples. Specifically, miR-148a-5p expression was significantly downregulated in hypothyroidism, while IL-6 exhibited markedly elevated expression levels in hypothyroid patients. These findings suggest potential roles for miR-148a-5p and IL-6 in the pathogenesis of hypothyroidism. Refer to Figure 2 and Figure 3 for a graphical representation of the gene expression profiles of miR-148a-5p and IL-6 in hypothyroid and normal blood samples, respectively.
Discussion
Hypothyroidism, a prevalent endocrine disorder, arises from insufficient production of thyroid hormone, often leading to various metabolic disorders. Despite its widespread occurrence, diagnosing hypothyroidism poses significant challenges due to its nonspecific symptoms. Treatment typically involves levothyroxine replacement therapy, aiming to restore thyroid hormone levels to normal2. However, achieving optimal dosing and symptom management can be complex, requiring careful monitoring and adjustment over time. Thyroid hormones are integral to regulating numerous metabolic processes, and are also involved in non-alcoholic fatty liver disease (NAFLD). Individuals diagnosed with hypothyroidism may have a higher risk of developing NAFLD compared to those with normal thyroid function18.
Identification of miRNAs associated with hypothyroidism holds significant promise for the early diagnosis and treatment of the disease. Initially, computational analysis is required to identify the novel miRNA biomarker, miR-148a-5p, within the hypothyroidism genome sequences, highlighting its potential role in disease control. These miRNAs also show downregulation in hypothyroidism and gastric cancer, and upregulation in liver fibrosis8. Additionally, target scan analysis reveals several genes targeted by this miRNA, including IL-6, cyclin D1, etc. From this study, IL-6 is highly expressed in hypothyroidism and in advanced-stage tumors. IL-6 has potential tumorigenicity and anti-inflammatory functions, so it is expressed more in diseased conditions. Dysregulation of miR-148a and IL-6 may correlate with disease severity in hypothyroidism. Our study did not investigate the functions or associations of these molecules; instead, it focused exclusively on their expression levels. Gene expression analysis using qRT-PCR demonstrates miR-148a-5p downregulation and elevated IL-6 in hypothyroid patients compared to normal samples, indicating their involvement in disease pathology. To fully understand how miR-148a-5p and IL-6 regulate the onset and progression of hypothyroidism, further research is needed. Moreover, as this study is a pilot study, larger and more diverse populations should be included in further investigation. Hence, to gain a greater understanding of hypothyroidism and its link between miR-148a-5p and the IL-6 gene, more research is required to explore potential connections between miR-148a-5p, IL-6, and other genes or biological pathways linked to this condition.
Conclusions
Overall, our study primarily focused on the expression levels of miR-148a-5p and IL-6 in patients with hypothyroidism. We statistically compared those values with those of normal individuals. Decreased levels of miR-148a-5p contribute to hypothyroidism by disrupting gene expression crucial for thyroid hormone synthesis and metabolism, exacerbating the condition. Elevated IL-6 levels indicate an inflammatory response, common in hypothyroidism, leading to various complications. A feedback loop likely exists between miR-148a-5p and IL-6 levels, perpetuating inflammation and worsening hypothyroidism. Understanding this interplay offers potential therapeutic avenues for managing hypothyroidism and its complications. Conclusively, a better understanding of those molecular components may lead to the discovery of innovative pharmaceutical strategies to better treat those with common metabolic disorders and assist patients suffering from hypothyroid complications in order to reduce morbidity and mortality. These findings shed light on the molecular mechanism underlying hypothyroidism and provide a basis for further investigation into potential therapeutic targets and diagnostic biomarkers.
Abbreviations
A+U - Adenine + Uracil (nucleotide bases), BLAST - Basic Local Alignment Search Tool, Cq - Quantification Cycle (threshold cycle in PCR), GC - Gastric Cancer, GAPDH - Glyceraldehyde 3-Phosphate Dehydrogenase (internal control), Homo sapiens - The scientific name for humans, IL-6 - Interleukin-6, LDL-C - Low-Density Lipoprotein Cholesterol, miRbase - MicroRNA database, NAFLD - Non-Alcoholic Fatty Liver Disease, NCBI - National Center for Biotechnology Information, NSCLC - Non-Small Cell Lung Cancer, PCR - Polymerase Chain Reaction, qRT-PCR - Quantitative Reverse Transcription Polymerase Chain Reaction, RNA - Ribonucleic Acid, SEM - Standard Error of the Mean, SIMATS - Saveetha Institute of Medical and Technical Sciences, T3 - Triiodothyronine, T4 - Thyroxine, TNF-α - Tumor Necrosis Factor alpha, TRIzol - A reagent used for RNA extraction, TSH - Thyroid-Stimulating Hormone, U6 - U6 snRNA (small nuclear RNA, serves as a normalization control), VLDL - Very Low-Density Lipoprotein
Acknowledgments
None.
Author’s contributions
Ponthankam. S collected the related articles and original draft writing, Ashikha Shirin Usman PP reviewed and edited the manuscript, Ameya K P did the visualisation and performed experiment, Durairaj Sekar initiated the study and finalised manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Brent
G.A.,
Mechanisms of thyroid hormone action. The Journal of Clinical Investigation.
2012;
122
(9)
:
3035-43
.
View Article PubMed Google Scholar -
Rahman
W.,
Stefan
S.,
Ahmed
A.,
Siddiqi
M.,
Malek
R.,
Lamos
E.M.,
The effects of calcitonin on quality of life in hypothyroid patients - A pilot study. Endocrine and Metabolic Science.
2023;
11
:
100125
.
View Article Google Scholar -
Colucci
P.,
Yue
C.S.,
Ducharme
M.,
Benvenga
S.,
A Review of the Pharmacokinetics of Levothyroxine for the Treatment of Hypothyroidism. European Endocrinology.
2013;
9
(1)
:
40-7
.
PubMed Google Scholar -
Nussey
S.,
Whitehead
S.,
Endocrinology: An Integrated Approach, Chapter 3, The thyroid gland. Oxford: BIOS Scientific Publishers; 2001. 2001
.
-
Komatsu
S.,
Imamura
T.,
Kiuchi
J.,
Takashima
Y.,
Kamiya
H.,
Ohashi
T.,
Depletion of tumor suppressor miRNA-148a in plasma relates to tumor progression and poor outcomes in gastric cancer. American Journal of Cancer Research.
2021;
11
(12)
:
6133-46
.
PubMed Google Scholar -
Ling
H.,
Fabbri
M.,
Calin
G.A.,
MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nature Reviews. Drug Discovery.
2013;
12
(11)
:
847-65
.
View Article PubMed Google Scholar -
Pozniak
T.,
Shcharbin
D.,
Bryszewska
M.,
Circulating microRNAs in Medicine. International Journal of Molecular Sciences.
2022;
23
(7)
:
23
.
View Article PubMed Google Scholar -
Kawagoe
K.,
Wada
M.,
Idichi
T.,
Okada
R.,
Yamada
Y.,
Moriya
S.,
Regulation of aberrantly expressed SERPINH1 by antitumor miR-148a-5p inhibits cancer cell aggressiveness in gastric cancer. Journal of Human Genetics.
2020;
65
(8)
:
647-56
.
View Article PubMed Google Scholar -
Su
H.,
Fan
G.,
Huang
J.,
Qiu
X.,
YBX1 regulated by Runx3-miR-148a-3p axis facilitates non-small-cell lung cancer progression. Cellular Signalling.
2021;
85
:
110049
.
View Article PubMed Google Scholar -
Leonardi
G.C.,
Candido
S.,
Carbone
M.,
Colaianni
V.,
Garozzo
S.F.,
Cinà
D.,
microRNAs and thyroid cancer: biological and clinical significance (Review). International Journal of Molecular Medicine.
2012;
30
(5)
:
991-9
.
View Article PubMed Google Scholar -
Su
X.,
Chen
X.,
Peng
H.,
Song
J.,
Wang
B.,
Wu
X.,
New insights into the pathological development of dyslipidemia in patients with hypothyroidism. Bosnian Journal of Basic Medical Sciences.
2021;
22
(3)
:
326-39
.
View Article Google Scholar -
Kobawala
T.P.,
Trivedi
T.I.,
Gajjar
K.K.,
Patel
D.H.,
Patel
G.H.,
Ghosh
N.R.,
Significance of Interleukin-6 in Papillary Thyroid Carcinoma. Journal of Thyroid Research.
2016;
2016
:
6178921
.
View Article PubMed Google Scholar -
Taddei
S.,
Caraccio
N.,
Virdis
A.,
Dardano
A.,
Versari
D.,
Ghiadoni
L.,
Low-grade systemic inflammation causes endothelial dysfunction in patients with Hashimoto's thyroiditis. The Journal of Clinical Endocrinology and Metabolism.
2006;
91
(12)
:
5076-82
.
View Article PubMed Google Scholar -
Goyal
S.,
Dixit
A.,
Vaney
N.,
Madhu
S.,
Serum Levels of Inflammatory Markers in Newly Diagnosed Hypothyroid Patients Before and After Levothyroxine Therapy. Journal of Clinical and Diagnostic Research.
2022;
16
(9)
:
CC09-CC12
.
View Article Google Scholar -
Rajkumar
K.V.,
Lakshmanan
G.,
Sekar
D.,
Identification of miR-802-5p and its involvement in type 2 diabetes mellitus. World Journal of Diabetes.
2020;
11
(12)
:
567-71
.
View Article PubMed Google Scholar -
Reddy
C.S. Shreya,
Usman
P.P.,
Ganapathy
D.M.,
K.P. Ameya,
D. Sekar,
MicroRNA-21 as a biomarker in terminal stage oral squamous cell carcinoma (OSCC) in the South Indian population. Oral Oncology Reports.
2024;
9
:
100139
.
View Article Google Scholar -
A. Ganesh,
P.A. Usman,
K.P. Ameya,
P. Thomas,
D.M. Ganapathy,
D. Sekar,
Expression analysis of transforming growth factor beta (TGF-β) in oral squamous cell carcinoma. Oral Oncology Reports.
2024;
9
:
100195
.
View Article Google Scholar -
Elshinshawy
S.,
Elhaddad
H.,
Abdel Alem
S.,
Shaker
O.,
Salam
R.,
Yosry
A.,
The Interrelation Between Hypothyroidism and Non-alcoholic Fatty Liver Disease, a Cross-sectional Study. Journal of Clinical and Experimental Hepatology.
2023;
13
(4)
:
638-48
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 5 (2024)
Page No.: 6379-6386
Published on: 2024-05-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2479 times
- PDF downloaded - 942 times
- XML downloaded - 110 times
 Biomedpress
Biomedpress




