Abstract
Background: Cervical cancer is a significant health burden, especially in less developed countries with limited access to HPV vaccines and screening. Dysregulation of immune cells, interleukin-6 (IL-6), and proinflammatory mediators have been implicated in cancer progression. SNPs in the IL-6 gene are thought to influence cervical cancer. A meta-analysis investigated the relationship between the IL-6 rs1800797 polymorphism and cervical cancer risk.
Methods: We conducted data mining on the PubMed database to identify relevant studies meeting specific criteria, including genotype data for IL-6 rs1800797, publication between 2015 and 2023, and reporting covariate risk factors. The meta-analysis comprised six publications focusing on the polymorphism at rs1800797 in IL-6 associated with cervical cancer.
Results: None of the five genetic models studied proved a significant link between the IL-6 rs1800797 polymorphism and cervical cancer risk. Because it included data from many ethnic groups, some racial groups may not experience the same consequences as others, based on this meta-analysis. The research revealed substantial heterogeneity. Egger's test and sensitivity analysis showed no evidence of publication bias.
Conclusion: Based on this comprehensive meta-analysis, we find no evidence that the IL-6 rs1800797 polymorphism contributes substantially to cervical cancer risk. However, further study is needed to investigate possible connections with additional IL-6 polymorphisms and the interplay between genetic and environmental variables in the development of cervical cancer. Identifying reliable tumor markers for cancer therapy remains an important area of investigation.
Introduction
Women worldwide have cervical cancer at a rate three times greater than that of breast and colorectal cancers, with 569,000 new cases annually. It occurs in the cells of the cervix and is predominantly caused by an infection with the Human Papillomavirus (HPV), with a higher incidence rate in less developed countries, likely due to the high cost of HPV vaccines and less accessible screening1. It has been reported that low- and middle-income countries have an 18-fold increase in deaths compared to high-income countries2. The adherence of rural women to cervical cancer screening highly depends on their knowledge of the condition and the screening process. Limited awareness about the disease or misconceptions about preventative measures may hinder screening uptake3. The study examined the factors affecting rural women's commitment to cervical cancer screening in South Chennai4. All women need to be informed about cervical cancer and its prevention. A recent study recommends that awareness among women regarding cervical cancer and ways to prevent it should be heightened5. Cancer progression is often associated with the dysregulation of various immune cells; moreover, cytokines and chemokines can induce inflammation, including Interleukin-6, an essential cytokine whose dysfunction can lead to chronic inflammation, autoimmunity, and cancers6.
Interleukin-6 (IL-6) is a bioactive peptide located on chromosome 7 (7p21-14), with a length of 5 kb, consisting of four introns and five exons7. It modulates several cellular mechanisms such as cell proliferation, differentiation, immune response, invasion, metastasis, and tumorigenesis8. Factors such as Single Nucleotide Polymorphism (SNP) may also influence gene expressions; hence, polymorphisms in specific genotypes of IL-6 have a higher chance of developing cervical cancer9. The SNP is possibly due to differences in the production of cytokines in the regulatory regions, and genetic polymorphisms result from modifications in the function of proteins10. The polymorphism at the 5' flanking regions affects expressions based on the IL-6 promoter (rs1800795, rs1800796, and rs1800797)11. Although several hereditary predispositions to cervical cancer have been reported, the results have been inconsistent due to varying sample sizes, genders, and ethnicities. Hence, a meta-analysis is conducted to determine the connection between the rs1800797 variant and cervical cancer.
Methods
Literature Search
After data mining the PubMed database, we identified several relevant studies on cervical cancer/cervical carcinoma and genetic variations (polymorphisms/single nucleotide polymorphisms, SNP/SNV/Mutation). To be included in the meta-analysis, studies had to meet certain conditions. These included reporting covariate risk factors such as HPV status, smoking status, oral contraceptive use, age at menarche, age at menopause, age at first pregnancy, and parity. They also had to provide case-control genotype data for the polymorphic variant, complete research articles, and be published between 2015 and 2023. Only polymorphisms referred to in two or more papers were considered.
Data Extraction and Quality Assessment
Six papers were chosen for further meta-analysis, including data on various factors. When citing a study, it is essential to include critical details such as the first author's name, year of publication, median age (including standard deviation), gender distribution, smoking and HPV statuses, ages at menarche and menopause, ages at marriage and first pregnancies, number of children, use of oral contraceptives, genetic variations, and specific data on case-control groups. The selected papers specifically focused on one gene and one genetic polymorphism. The Newcastle-Ottawa Scale was created to evaluate the quality of case-control studies that are deemed acceptable12. This scale comprises three domains: selection, exposure, and comparison, each assessed based on five parameters, including the Hardy-Weinberg equilibrium (HWE) index, the number of cases and controls, the method of association assessment, and the genotyping method. A maximum score of 8 indicates high quality in the evaluation process.
Statistical Analysis
Individual analyses were conducted for gene polymorphisms with at least two available studies, estimating Odds Ratios (OR) and their corresponding confidence intervals (CI). These were derived from the primary data obtained during the review. We estimated odds ratios (ORs) for each genetic polymorphism and conducted heterogeneity tests using Cochran's Q to assess variations within and between studies. The null hypothesis for these tests suggests that any observed variability is due to chance alone, indicating no significant differences in associations among studies. The alternative hypothesis proposes the presence of heterogeneity in the associations between studies. To quantify the level of heterogeneity, we calculated I2 based on Cochran's Q. The I2 value indicates the degree of heterogeneity, with low heterogeneity (I2: 25-50%), moderate heterogeneity (I2: 50-75%), and high heterogeneity (I2 > 75%)13, 14. If I2 is less than 50%, we used a fixed-effects model; if I2 is more than 50%, random effects were chosen to interpret the results.
Heterogeneity indicates notable variations among the studies included in the meta-analysis. Delving deeper into the origins of this heterogeneity could offer valuable insights. Potential sources of heterogeneity may stem from differences in study design, characteristics of the study populations, or methods of measurement. Exploring these factors could elucidate why certain studies diverge in their findings and enhance the understanding of the overall results.
The Cochran's Q test was employed to determine whether to use the random or fixed-effects models for pooled odds ratio estimation for each polymorphism. The random-effects model was utilized if the two measures showed significance with a significance level (α) of 0.05 (corresponding to at least moderate I2). If both tests were not statistically significant (equivalent to low I2), fixed-effects models were employed. We performed sensitivity analyses on polymorphisms with more than four studies. To ensure consistency of results, this was done by removing studies iteratively and recalculating the pooled odds ratio (OR) each time. Additionally, the Egger Test was used to assess publication bias for polymorphisms with more than five studies, regardless of whether a significant association was observed15, 16.
The significance level for all analyses was set at 0.05, a commonly used value in the field's original studies. This choice was made to address the limitation of statistical power in the meta-analysis and minimize the risk of Type II errors. Despite this, all reported p-values are provided for transparency, allowing readers to interpret the results critically, regardless of whether the associations were significant17, 18.
| Study Name | Year | Country | Source of DNA | No of Cases | No of Control | NOS Score | Genotyping Method |
| Agne et al. | 2021 | Lithuania | Blood | 89 | 84 | 6 | Real-time PCR |
| Kushwah et al. | 2020 | India | Venous Blood | 246 | 246 | 6 | PCR-RFLP |
| Maneesh et al. | 2015 | India | Peripheral Blood | 100 | 100 | 7 | PCR-RFLP |
| Monishita et al. | 2023 | Bangladesh | Venous Blood | 126 | 120 | 8 | PCR-RFLP |
| Sabrina et al. | 2016 | Tunisia | Blood | 112 | 164 | 8 | Real-time PCR |
| Sayma et al. | 2020 | Bangladesh | Blood | 252 | 228 | 7 | ARMS-PCR |
| Author | Genotype | Allele | X2 | HWE | ||||||||
| Case | Control | Case | Control | |||||||||
| GG | GA | AA | GG | GA | AA | G | A | G | A | |||
| Agne et al. | 25 | 41 | 23 | 27 | 44 | 13 | 98 | 70 | 238 | 0.5 | 0.47 | |
| Kushwah et al. | 86 | 101 | 59 | 182 | 47 | 17 | 411 | 81 | 445 | 573 | 22.9 | 0.000002 |
| Maneesh e t al. | 35 | 40 | 25 | 77 | 20 | 3 | 174 | 26 | 180 | 226 | 1.34 | 0.24 |
| Monishita et al. | 8 | 68 | 50 | 4 | 19 | 97 | 27 | 213 | 221 | 453 | 5.14 | 0.02 |
| Sabrina et al. | 4 | 34 | 74 | 15 | 33 | 116 | 63 | 265 | 295 | 593 | 20.2 | 0.000007 |
| Sayma et al. | 14 | 150 | 88 | 3 | 39 | 186 | 45 | 411 | 417 | 867 | 0.33 | 0.56 |
Results
Study Characteristics
The study selection and assessment process were thoroughly illustrated in a PRISMA flow diagram (Figure 1); this refers to the rigorous criteria for inclusion and exclusion. Initially, overall, 541 publications were gathered and evaluated for eligibility. Afterward, the selected studies underwent thorough evaluation using the HWE and NOS scales19, which led to the inclusion of nine studies in this meta-analysis. The characteristics of each study, including their NOS scores, were presented in Table 1. Detailed information on the genotype distribution, allelic frequency, and HWE/Chi-square values of the selected polymorphisms was also provided in Table 2.
Association between IL-6 Variant and Cervical Cancer Risk
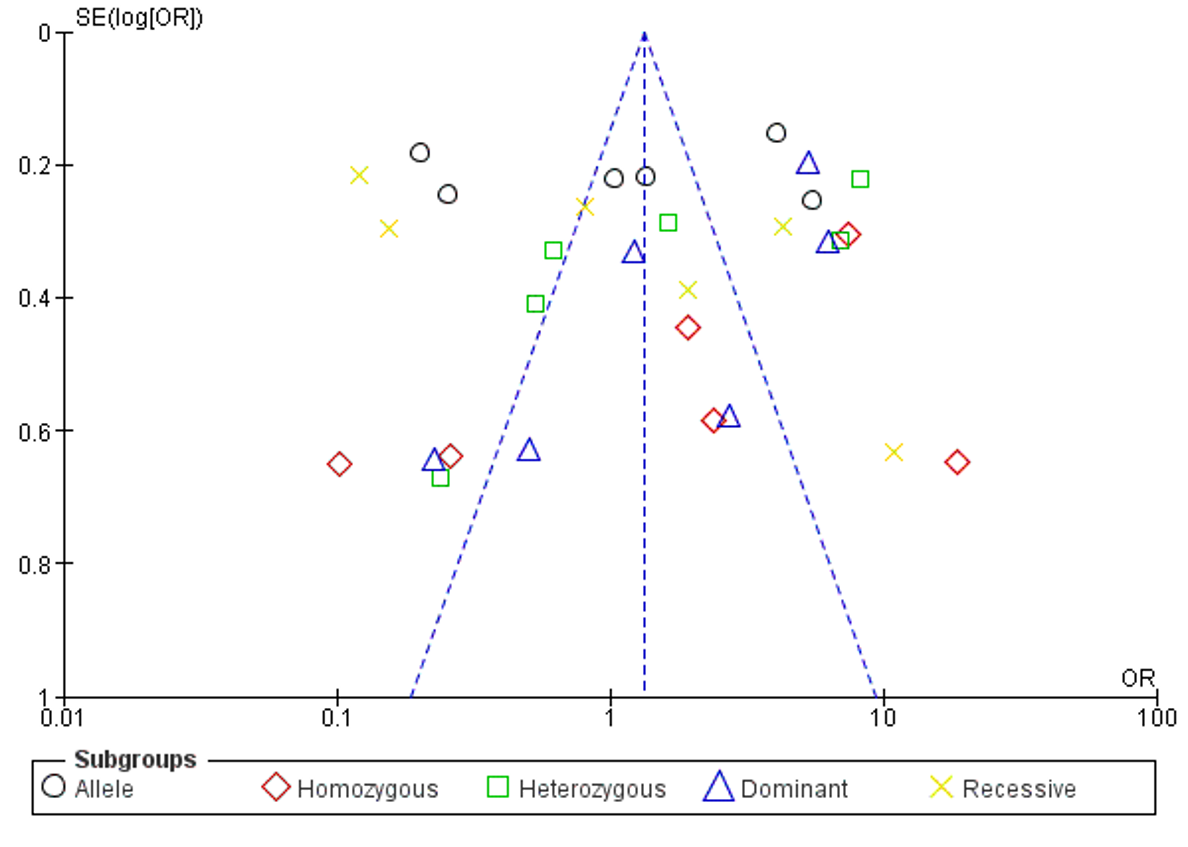
None of the five genetic models showed any significant associations. Random effects were implemented due to high heterogeneity, indicated by I2 values greater than 50%. In the allelic model, I2 was 98%, OR = 1.08, 95% CI [0.35-3.34], p = 0.90; in the homozygous model, I2 was 92%, OR = 1.64, 95% CI [0.40-6.71], p = 0.49; in the heterozygous model, I2 was 94%, OR = 1.48, 95% CI [0.49-4.42], p = 0.48; in the dominant model, I2 was 89%, OR = 1.69, 95% CI [0.61-4.26], p = 0.26; in the recessive model, I2 was 97%, OR = 1.00, 95% CI [0.26-3.88], p = 1.00. Forest plots were used to display all the results obtained. A thorough analysis was conducted to determine the sensitivity of interleukin-6 (rs1800797) polymorphisms, which included the execution of Begg's funnel plot and Egger's test. The results showed no publication bias for all five genetic models, as illustrated in Figure 2 and Figure 3.
Discussion
Our study examined a possible relationship between cervical cancer risk and specific IL-6 rs1800797 gene variants. Our results indicate there is insufficient evidence to connect the IL-6 rs1800797 polymorphism to an elevated risk of cervical cancer. Zidi's research on the Tunisian population, however, yielded opposite results. There was no discernible variation in the genotype distribution of IL-6 SNPs, including IL-6 rs1800797, between the cervical cancer cases and the control groups. The researchers concluded that more study is required to establish if these genetic variations are indeed related to cervical cancer20. Contrary to our results, Shi's analysis showed that the IL-6 rs1800797 polymorphism is associated with a greater prevalence of cervical cancer in the Chinese population21.
According to Gupta et al., individuals who have the IL-6-597A/G 597 G allele had up to a 6.2 times increased chance of developing cervical cancer (p < 0.001)22. However, the quality of the data and our capacity to evaluate the relationships between these genetic changes and cervical cancer susceptibility may be constrained by the paucity of studies and the small sample sizes. The existence of ethnically diverse groups may factor into the variations in findings across research. Our research found no statistically significant link between the IL-6 rs1800797 polymorphism and vulnerability to cervical cancer, in contrast to the results of Gupta et al. and Shi et al.
There is no evidence that the genotype and allele frequencies of the IL-6 rs1800797 gene polymorphism influence tumor growth in patients with cervical cancer. However, confirming any links has proven difficult in further studies. Results from Peng's research indicate that rs1800797 is more closely related to cancer risk in Caucasians than in Asians. Furthermore, IL-6 promoter polymorphisms are closely linked to cancer prognosis. These encouraging results imply that IL-6 promoters, particularly rs1800795 and rs1800797, might be beneficial as tumor indicators in cancer treatment23.
In the past, studies analyzing individual cases (as opposed to large groups) found no significant connection between the IL-6 rs1800797 polymorphism and the likelihood of developing cancer24. However, a recent study showed a meaningful relationship—not just a correlation—between this genetic variation and an increased risk of cervical cancer. Furthermore, there is a strong connection between the rs1800797 polymorphism and a higher cancer risk among African ethnic groups. The results of a previous meta-analysis examined each subgroup analysis finding by ethnicity25. Using meta-analyses is a great way to gain a more thorough and reliable understanding of how a genetic variant (rs1800797) relates to disease. With the small sample sizes in this study, these analyses can uncover potential genetic predispositions or correlations that may not have been noticeable otherwise. However, it's essential to confirm these discoveries and conduct additional research to explore the molecular pathways involved in this relationship. Our investigation did not examine how hereditary and environmental variables combine to cause cervical cancer.
Limitations of the study include the relatively small sample size and the lack of ethnic diversity among the included studies, which potentially limits the generalizability of the findings beyond the populations studied. Despite efforts to assess publication bias and heterogeneity, these factors could still influence the reliability of the pooled estimates. Additionally, variations in the quality of included studies, potential confounding variables not accounted for, and incomplete reporting of crucial data could introduce biases and affect the robustness of the results. Future research should aim for larger, more diverse samples, prioritize high-quality studies with comprehensive reporting, and consider potential confounders to strengthen the evidence base and provide more reliable insights into the relationship between the IL-6 rs1800797 variant and cervical cancer risk.
Conclusions
The research investigated the relationship between the IL-6 rs1800797 variant and the risk of developing cervical cancer. According to the findings, the IL-6 rs1800797 variant may not play a significant role in the risk of cervical cancer. However, larger sample sizes and participants from diverse ethnic origins are needed to corroborate these findings. Furthermore, studying the combination of genetic and environmental variables in the development of cervical cancer might reveal more information about the disease's underlying processes.
In addition to exploring genetic factors like the IL-6 rs1800797 variant, future research on cervical cancer development should also investigate potential environmental factors that may interact with genetic predispositions. Environmental factors such as lifestyle choices, exposure to certain chemicals or toxins, socioeconomic status, and access to healthcare services could play a significant role in cervical cancer risk, especially in conjunction with genetic variations. For instance, studies could examine how factors like smoking, diet, reproductive history, and HPV infection interact with genetic polymorphisms like IL-6 rs1800797 to influence cervical cancer susceptibility. Understanding these interactions could provide valuable insights into the underlying mechanisms of cervical cancer development and help identify high-risk populations for targeted interventions and preventive strategies. Moreover, investigating gene-environment interactions in cervical cancer could lead to the development of personalized prevention and treatment approaches. By identifying individuals who are genetically predisposed to cervical cancer and understanding how their genetic makeup interacts with environmental factors, healthcare providers can offer tailored screening protocols, lifestyle interventions, and therapeutic strategies to reduce cancer risk and improve outcomes. In conclusion, while the current study found no significant association between the IL-6 rs1800797 variant and cervical cancer risk, further research into the interplay between genetic and environmental factors is warranted. By elucidating these complex interactions, we can enhance our understanding of cervical cancer etiology and pave the way for more effective prevention and treatment strategies in the future.
Abbreviations
CI: Confidence Interval, HPV: Human Papillomavirus, HWE: Hardy-Weinberg Equilibrium, IL-6: Interleukin-6, NOS: Newcastle-Ottawa Scale, OR: Odds Ratio, PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SNP: Single Nucleotide Polymorphism, SNV: Single Nucleotide Variation
Acknowledgments
The authors thank the Chettinad Academy of Research and Education for the constant support and encouragement.
Author’s contributions
AGP, IBK and RV has collected the data and written the contents of this manuscript IBK edited the figures and tables of this manuscript RV designed the study, corrected and approved the manuscript for submission. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not Applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Johnson
C.A.,
James
D.,
Marzan
A.,
Armaos
M.,
Cervical cancer: an overview of pathophysiology and management. In: Seminars in oncology nursing.
2019;
2019
:
166-74
.
View Article Google Scholar -
Vu
M.,
Yu
J.,
Awolude
O.A.,
Chuang
L.,
Cervical cancer worldwide. Current Problems in Cancer.
2018;
42
(5)
:
457-65
.
View Article PubMed Google Scholar -
Anorlu
R.I.,
Cervical cancer: the sub-Saharan African perspective. Reproductive Health Matters.
2008;
16
(32)
:
41-9
.
View Article PubMed Google Scholar -
R. Narendranath,
A. Rathibala,
R. Gowthamkarthic,
A. Soumya,
Determinants of cervical cancer screening adherence among rural women of Chengalpattu district. International Journal Of Community Medicine And Public Health.
2023;
10
(8)
:
2854-2858
.
View Article Google Scholar -
Gunasundari
J.,
Rathiga
A.,
Knowledge on Cervical Cancer and It's Prevention among Women Psychology. IOSR Journal of Pharmacy and Biological Sciences.
2015;
10
(2)
:
47-49
.
-
Abaurrea
A.,
Araujo
A.M.,
Caffarel
M.M.,
The role of the il-6 cytokine family in epithelial-mesenchymal plasticity in cancer progression. International Journal of Molecular Sciences.
2021;
22
(15)
:
8334
.
View Article PubMed Google Scholar -
Zhou
L.,
Zheng
Y.,
Tian
T.,
Liu
K.,
Wang
M.,
Lin
S.,
Associations of interleukin-6 gene polymorphisms with cancer risk: evidence based on 49,408 cancer cases and 61,790 controls. Gene.
2018;
670
:
136-47
.
View Article PubMed Google Scholar -
Duan
H.X.,
Chen
Y.Y.,
Shi
J.Z.,
Ren
N.N.,
Li
X.J.,
Association of IL-6 -174G>C (rs1800795) polymorphism with cervical cancer susceptibility. Bioscience Reports.
2018;
38
(5)
.
View Article PubMed Google Scholar -
Liu
H.,
Lyu
D.,
Zhang
Y.,
Sheng
L.,
Tang
N.,
Association between the IL-6 rs1800795 polymorphism and the risk of cervical cancer: a meta-analysis of 1210 cases and 1525 controls. Technology in Cancer Research & Treatment.
2017;
16
(5)
:
662-7
.
View Article PubMed Google Scholar -
Falahi
S.,
Zamanian
M.H.,
Feizollahi
P.,
Rezaiemanesh
A.,
Salari
F.,
Mahmoudi
Z.,
Evaluation of the relationship between IL-6 gene single nucleotide polymorphisms and the severity of COVID-19 in an Iranian population. Cytokine.
2022;
154
.
View Article PubMed Google Scholar -
Peng
X.,
Shi
J.,
Sun
W.,
Ruan
X.,
Guo
Y.,
Zhao
L.,
Genetic polymorphisms of IL-6 promoter in cancer susceptibility and prognosis: a meta-analysis. Oncotarget.
2018;
9
(15)
:
12351-64
.
View Article PubMed Google Scholar -
Stang
A.,
Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology.
2010;
25
(9)
:
603-5
.
View Article PubMed Google Scholar -
Huedo-Medina
T.B.,
Sánchez-Meca
J.,
Marín-Martínez
F.,
Botella
J.,
Assessing heterogeneity in meta-analysis: Q statistic or I2 index?. Psychological Methods.
2006;
11
(2)
:
193-206
.
View Article PubMed Google Scholar -
Bedi
U.,
Singh
M.,
Singh
P.,
Molnar
J.,
Khosla
S.,
Arora
R.,
Effects of statins on progression of coronary artery disease as measured by intravascular ultrasound. Journal of Clinical Hypertension (Greenwich, Conn.).
2011;
13
(7)
:
492-6
.
View Article PubMed Google Scholar -
Egger
M.,
Davey Smith
G.,
Schneider
M.,
Minder
C.,
Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.).
1997;
315
(7109)
:
629-34
.
View Article PubMed Google Scholar -
Rothstein
H.R.,
Sutton
A.J.,
Borenstein
M.,
Publication bias in meta‐analysis. Publication bias in meta‐analysis: Prevention, assessment and adjustments.
2005;
2005
:
1-7
.
-
Hedges
L.V.,
Pigott
T.D.,
The power of statistical tests in meta-analysis. Psychological Methods.
2001;
6
(3)
:
203-17
.
View Article PubMed Google Scholar -
Gelman
A.,
P values and statistical practice. Epidemiology (Cambridge, Mass.).
2013;
24
(1)
:
69-72
.
View Article PubMed Google Scholar -
Varghese
S.,
Kumar
S.G.,
Association between genetic variants of NOS3, TGF-β and susceptibility of diabetic nephropathy: a meta-analysis. Meta Gene.
2019;
21
.
View Article Google Scholar -
Zidi
S.,
Stayoussef
M.,
Alsaleh
B.L.,
Gazouani
E.,
Mezlini
A.,
Ebrahim
B.H.,
Relationships between common and novel interleukin-6 gene polymorphisms and risk of cervical cancer: a case-control study. Pathology Oncology Research.
2017;
23
(2)
:
385-92
.
View Article PubMed Google Scholar -
Shi
W.J.,
Liu
H.,
Wu
D.,
Tang
Z.H.,
Shen
Y.C.,
Guo
L.,
Stratification analysis and case-control study of relationships between interleukin-6 gene polymorphisms and cervical cancer risk in a Chinese population. Asian Pacific Journal of Cancer Prevention.
2014;
15
(17)
:
7357-62
.
View Article PubMed Google Scholar -
Gupta
M.K.,
Renu Singh
R.,
Banerjee
M.,
Cytokine gene polymorphisms and their association with cervical cancer: A North Indian study. The Egyptian Journal of Medical Human Genetics.
2016;
17
(2)
:
155-63
.
View Article Google Scholar -
Peng
X.,
Shi
J.,
Sun
W.,
Ruan
X.,
Guo
Y.,
Zhao
L.,
Genetic polymorphisms of IL-6 promoter in cancer susceptibility and prognosis: a meta-analysis. Oncotarget.
2018;
9
(15)
:
12351-64
.
View Article PubMed Google Scholar -
Qian
D.,
Yan
S.,
Pan
X.,
Association of IL-6-597 G/A polymorphism with cancer risk: evidence from a meta-analysis. Critical Reviews™ in Eukaryotic Gene Expression.
2017;
27
(3)
:
211-217
.
View Article Google Scholar -
Zhou
L.,
Zheng
Y.,
Tian
T.,
Liu
K.,
Wang
M.,
Lin
S.,
Associations of interleukin-6 gene polymorphisms with cancer risk: evidence based on 49,408 cancer cases and 61,790 controls. Gene.
2018;
670
:
136-47
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 3 (2024)
Page No.: 6268-6275
Published on: 2024-03-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3342 times
- PDF downloaded - 1084 times
- XML downloaded - 117 times
 Biomedpress
Biomedpress




