Abstract
Objective: Plasma cell myeloma (PCM) is an incurable clonal neoplasm of plasma cells, which typically presents a poor prognosis. This study aimed to determine the clinical profile of newly diagnosed plasma cell myeloma cases in two tertiary care centers in Malaysia and evaluate the association of aberrant immunophenotypic expression with prognostic factors and clinical stages.
Methods: A four-year retrospective study of 78 newly diagnosed PCM cases was conducted at Hospital Kuala Lumpur (HKL) and Hospital Universiti Sains Malaysia (HUSM). The study retrieved data from medical records, including socio-demographic characteristics, hematological and biochemical parameters, cytogenetic and molecular abnormalities, immunophenotypic expression profile, and clinical stages of PCM cases. All data were statistically analyzed using SPSS 26.0.
Results: The mean age of PCM patients was 60 years, with 73.1% of cases showing a normal white blood cell (WBC) count. A total of 65.4% and 24.4% of cases had anemia and mild to severe anemia, respectively. Cases were associated with thrombocytopenia (24.4%), normal platelet counts (75.6%), a bone marrow plasma cell percentage >10% (93.6%), and elevated erythrocyte sedimentation rate (ESR) (63.3%). Additionally, 66.7% of cases demonstrated hypoalbuminemia and elevated lactate dehydrogenase (LDH), calcium, and creatinine levels. All cases indicated hyperproteinemia (56.4%), hypoproteinemia (6.4%), normal serum total protein (37.2%), elevated serum paraprotein (69.2%), and blood beta-2 microglobulin (B2M) (62.5%) levels, as well as aberrant cytogenetic abnormalities (16.7%). The cases were grouped into Stage III (39.7%), Stage II (24.4%), and Stage I (5.1%). CD38 and CD138 demonstrated 100% expression, with every case exhibiting expression of more than one aberrant antigen, including CD19 (28.2%), CD45 (23.1%), CD56 (83.3%), CD117 (25.6%), kappa (60.3%), and lambda light chain (38.5%). However, only CD19 markers and serum creatinine levels exhibited a statistically significant association (p = 0.036).
Conclusion: Immunophenotyping by multiparametric flow cytometry is powerful in distinguishing PCM from normal cells. The aberrant antigens expressed in this study displayed a heterogeneous immunophenotypic profile unique to our population. However, to enhance outcome and robustness of this study, it is recommended to engage additional centers purposefully to increase the sample size.
Introduction
Plasma cell myeloma (PCM) is described as an aberrant proliferation of neoplastic plasma cells in the bone marrow, resulting in multiple health complications such as bone pain, fractures, hypercalcemia, osteolytic lesions, anemia, recurrent infections, and renal insufficiency1. PCM is the second most common hematological malignancy2, more prevalent in males in the sixth decade of life, and lacks a definitive cure3. Currently, in Malaysia, the incidence rate of PCM is still one of the lowest at 0.75 per 100,000 populations, similar to South Korea (0.54/100,000), the Philippines (0.86/100,000), and China (0.92/100,000). Additionally, New Zealand possesses the highest PCM incidence rate at 5.3/100,000, followed by Australia at 5.0/100,000, the U.K. at 4.3/100,000, and Israel and Norway, both at 4.2/100,0004. However, Malaysia is experiencing a rise in population growth and is projected to become an aging country by 2030. Statistics indicate a rise in the elderly population aged 60 and above, accompanied by a decline in the percentage of individuals aged 14 and below over time5. Almost all PCM cases experience relapse and refractory phases after achieving higher rates of complete remission, which appear as the most challenging part of treating PSM disease and consume a higher financial burden6.
Immunophenotyping is a powerful and robust approach to identify and characterize the cell surface and intracellular markers on plasma cells, providing valuable insights into the disease's biology and informing treatment strategies7. Immunophenotypic markers are essential in risk stratification systems for PCM by classifying patients into risk groups according to the probability of disease advancement and overall prognosis. Incorporating immunophenotypic markers into risk stratification systems offers valuable insights into the features of the malignant plasma cell population8. A panel of combination antigens, including CD38, CD138, CD19, CD45, CD56, CD117, cytoplasmic lambda, and kappa, can be utilized to discriminate neoplastic plasma cells in a single tube via the presence of aberrant expression of immunophenotypic markers and clonality of light chains9. Specifically, CD38 is commonly utilized as a gating marker due to its nonspecific nature for plasma cells and its presence on both B- and T-lymphocytes. Moreover, it is also present in PCM cells. Utilizing CD38 for gating may present challenges because of its widespread expression. However, CD138 is the preferred marker for the initial identification of plasma cells in this study due to its exclusive expression by plasma cells in the bone marrow, which may enhance the selection for a more homogeneous population selection10. Additionally, CD138 serves as a distinctive indicator for plasma cells, playing a vital role in the identification and isolation of this cell subset for subsequent FCM assessment. Hence, it offers a more accurate identification of PCM cells in comparison to CD38 gating, resulting in a more uniform selection of neoplastic plasma cells11. Furthermore, CD45 was utilized alongside CD38 and CD138 to differentiate between normal/reactive plasma cells and PCM cells in minimal residual disease (MRD) monitoring. It aids in distinguishing clonal plasma cells from normal/reactive plasma cells in the bone marrow12. CD19 was used as an aberrant marker because PCM cells are associated with losing the expression of surface CD19, and aberrant expression of CD19 can be indicative of PCM transformation and disease progression11. CD56 is another aberrant marker used in this study because PCM cells are often characterized by being CD56+ or CD56-, and relatively low CD38 expression. The expression of CD56 is associated with the dissemination of PCM cells within the bone marrow environment10. Moreover, CD117, which is expressed in PCM cells along with other markers like CD28, has been linked to different risk categories in myeloma patients. Evaluating the expression of CD117 helps in stratifying patients based on prognosis and potential response to therapy13.
A recent study examined the immunophenotypic profile of newly diagnosed PCM cases at a tertiary care center in India. In their study, it was demonstrated that CD19 was the most sensitive marker and CD81 was the most specific marker for distinguishing aberrant plasma cell morphology from normal plasma cells in their patient cohort. Therefore, it was suggested that FCM be regularly included in PCM cases at the time of diagnosis due to its prognostic importance7. Currently, there is no single marker that offers sufficient specificity to clearly distinguish between clonal plasma cells and normal plasma cells. Flores-Montero et al. reported that an analysis comparing single normal plasma cells with PCM bone marrow suggests that monitoring minimal residual disease in PCM patients may be best achieved by using a combination of CD138, CD38, CD45, CD19, CD56, CD27, CD81, and CD117. In addition, evaluating clonality through the inclusion of cytoplasmic immunoglobulin helps to distinguish between normal/reactive and clonal small suspicious PC populations with high sensitivity12.
Numerous studies have been conducted to establish an immunophenotypic profile of neoplastic plasma cells. However, only a limited number of studies have investigated the prognostic significance of immunophenotypic markers in PCM. Nevertheless, the outcomes were varied, as there were frequent inconsistencies noted concerning the prognostic significance of the expression of immunophenotypic markers in PCM. Although various studies have highlighted the significance of aberrant markers' expression on plasma cells in predicting disease prognosis and clinical outcomes, it is noteworthy that none of the parameters related to aberrant antigen expression are incorporated in the risk stratification system, including the International Staging System (ISS) and Revised International Staging System (R-ISS). Therefore, the clinical and prognostic significance of the immunophenotypic markers expression in PCM is still uncertain14.
There is a lack of indigenous data on the expression of immunophenotypic markers in newly diagnosed PCM cases among the Malaysian population. The expression of immunophenotypic markers was subsequently correlated with associated factors, including clinical and laboratory parameters that are reported to have prognostic significance. Thus, we conducted a four-year retrospective study in two tertiary care centers in Malaysia; Hospital Universiti Sains Malaysia (HUSM) and Hospital Kuala Lumpur (HKL). Primarily, this study aimed to examine the immunophenotypic markers of plasma cells in newly diagnosed PCM patients and their correlation with prognostic markers and staging. The specific immunophenotypic markers proportions among patients with PCM were determined based on the results from flow cytometry analysis. Subsequently, we investigated the relationship between immunophenotypic marker expressions and various clinical and laboratory parameters in patients with PCM, including demographic characteristics, hematological parameters, biochemical parameters, and cytogenetic abnormalities. We established the correlation between immunophenotypic marker expressions and disease clinical stages upon completion of the study.
Methods
Study Design and Patient Recruitment
A four-year retrospective study was conducted at HKL and HUSM from June 2016 to June 2019. Both study sites were selected as primary centers for diagnosing PCM disease in Malaysia. Patient data were retrieved from the medical records unit and the hematology laboratory's laboratory information system in both hospitals. This study was authorized by the USM Human Research Committee (USM/JEPeM/19040237) and the Medical Research & Ethics Committee (NMRR-18-3904-45526 (IIR)) and complied with the Declaration of Helsinki. Patient medical information was kept confidential, and each patient's records were assigned a unique identical number. In 2021, statistics from the Global Cancer Observatory (GLOBALCAN) demonstrated that PCM is a rare disease in Malaysia. Given its infrequency and limited occurrence, we calculated the sample size using the guidelines outlined in Conroy (2015)15, which suggest that a suitable maximum sample size is typically 10% of the total identified cases. However, we recruited a larger sample size in this study (n=78) to conduct a retrospective study in two tertiary care centers. Newly diagnosed PCM cases were defined based on the updated Classification of Tumors of Hematopoietic and Lymphoid Tissue 201716. PCM cases were diagnosed with the existence of a clonal bone marrow plasma cell percentage equal to or greater than 10%, or a biopsy-proven plasmacytoma, and associated with one or more of the myeloma-defining events genuinely diagnosed in HUSM and HKL. The inclusion criteria encompassed all cases that had been diagnosed with PCM, as determined by the above criteria. Cases in which immunophenotyping assays were not performed, cases referred to HKL and HUSM for outsourced PCM, and relapsed cases were excluded.
FCM Immunophenotyping
BM aspirate samples were collected in EDTA anticoagulant tubes and processed within 12 hours post-collection. The samples were analyzed with the six-color flow cytometer BD FACSCanto II (BD Biosciences, Erembodegem, Belgium) using the myeloma panel antibodies. The gating markers (CD38, CD138, CD45), aberrant markers (CD19, CD56, CD117), and clonality evaluation (cytoplasmic and light chain Ig) were utilized. Briefly, 100 μl of count-adjusted anticoagulated bone marrow samples were treated with monoclonal antibodies in the dark at room temperature for 15 minutes. Then, the red blood cells were lysed and washed twice with PBS. The supernatant was discarded, and the cell pellet was resuspended in PBS. After discarding the supernatant, the cell pellet was then resuspended in PBS. Analyzed stained cells were swiftly identified with the BD FACSCanto II flow cytometer. Non-binding mouse isotype antibodies were utilized as a control experiment.
For the gating strategy, singlet exclusion was acquired by gating on forward scatter area (FSC-A) vs. forward scatter height (FSC-H) to exclude doublets. A live cell gate was used to gate on a live cell population and excluding debris and dead cells based on forward scatter (FSC) and side scatter (SSC) properties. A CD38 vs. CD138 plot was created by plotting CD38 (x-axis) vs. CD138 (y-axis) to identify PCM cells, followed by CD45 exclusion to exclude any remaining contaminating cells by gating out CD45-positive cells, as PCM cells are typically CD45-negative.
Besides, the aberrant marker analysis was detected by CD19, CD56, and CD117 expression via creating individual histograms or scatter plots for each aberrant marker to identify the presence or absence of these markers in the PCM cell population. The PCM cell clonality evaluation was assessed by Ig light chain analysis, in which cytoplasmic and surface immunoglobulin light chain stains were used to evaluate the clonality of the PCM cells. Then, histograms or scatter plots for each light chain (kappa and lambda) were created to assess the expression pattern. FACSDivaTM software was used to generate summary statistics for each population and marker. The graphs and plots were created for better visualization of the results. Internal controls of positive and negative controls were included to validate the staining and gating strategy. Compensation controls were used to ensure proper compensation by using single-stained controls for each fluorochrome. Neoplastic plasma cells were defined as CD38-positive and CD138-positive light-chain-restricted. Cells were considered positive when more than 20% of cells expressed the antigen profile of that marker. The percentage of positive cells was provided by the percentage of events above the cursor defined on the negative control. The cells were acquired by flow cytometer with the total acquisition events being about 20,000 to 30,000 nucleated cells for diagnosis assessment.
Prognostic Factors and Clinical Stages
An additional parameter was used to examine the relationship between the immunophenotyping profile, prognostic variables, and clinical stages. Demographics (age, gender, and ethnicity), hematological parameters (FBC, ESR, and bone marrow plasma cell percentage), biochemical parameters (serum albumin, B2M, calcium, LDH, creatinine, paraprotein, and total protein), and molecular and cytogenetic assessment were examined. The three clinical stages including Stage I, Stage II, and Stage III were defined by the Multiple Myeloma ISS.
Statistical Analysis
The statistical analysis was performed using the SPSS software version 26.0. Descriptive statistics were utilized to present the demographic characteristics, and the mean value along with the standard deviation (mean±SD) was used to express the numerical data, whereas the frequency and percentage (%) were employed to express the categorical data. The study examined the correlation between the manifestation of immunophenotypic markers and their hematological, biochemical, and cytogenetic anomalies, along with clinical stages via the Pearson Chi-square/Fisher Exact test. A p-value below 0.05 was used to determine statistical significance.
Results
Patients' Demographic Characteristics
A total of 78 newly diagnosed PCM cases were recruited from HKL and HUSM between June 2016 and June 2019. Males (56.4%) outnumbered females (43.6%), resulting in a male-to-female ratio of 1.29:1. Malays constituted 73.6% of the cases, followed by Chinese (12.8%) and Indian (11.5%) ethnicities. The average age of PCM cases was 60 years, with the highest prevalence in the 61-70 age range.
Hematological Parameters of Newly Diagnosed PCM Patients
Most of the study cases (73.1%) were associated with a normal WBC count (4-11x109/L). A total of 10.3% and 16.7% of cases had leucopenia and leukocytosis, respectively. The study demonstrated that only 10.3% of cases had Hb >12.0, while 65.4% had anemia (Hb <10 g/dL), and 24.4% had mild to severe anemia (10.0 – 11.9 g/dL). Additionally, 7.7% and 16.7% of cases had thrombocytopenia (platelet count <100 x109/L and 100 – 149 x109/L, respectively), whereas 75.6% had normal platelet counts (>150 x109/L). Our study found that 93.6% of cases had a bone marrow plasma cell percentage >10%, whereas 6.4% had <10%. More than half of the cases studied (63.3%) had elevated ESR (>100 mm/hour).
Clinical Stages of Newly Diagnosed PCM Patients
More than half of the cases (n=54, 69.2%) were successfully staged using the Multiple Myeloma ISS. Most cases were staged as Stage III (39.7%), followed by Stages II (24.4%) and Stage I (5.1%). Due to the lack of serum B2M, 24 cases (30.8%) were classified as unknown stages. Table 1 shows the demographics, MM ISS-based clinical staging, and hematological data of newly diagnosed PCM patients.
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 44 (56.4) |
| Female | 34 (43.6) |
| Age Group | |
| <30 | 1 (1.3) |
| 31-40 | 2 (2.6) |
| 41-50 | 10 (12.8) |
| 51-60 | 28 (35.9) |
| 61-70 | 29 (37.2) |
| >70 | 8 (10.3) |
| Staging, ISS | |
| I | 4 (5.1) |
| II | 19 (24.4) |
| III | 31 (39.7) |
| Unknown | 24 (30.8) |
| Parameters | |
| White Blood Cell (x109/L) (n=78) | |
| < 4.0 | 8 (10.3) |
| 4.0- 11.0 | 57 (73.1) |
| ≥ 11.1 | 13 (16.7) |
| Hemoglobin (gm/dL) (n=78) | |
| <10.0 | 51 (65.4) |
| 10.0-11.9 | 19 (24.4) |
| ≥12.0 | 8 (10.3) |
| Platelet (x 109/L) (n=78) | |
| <100 | 6 (7.7) |
| 100-149 | 13 (16.7) |
| ≥150 | 59 (75.6) |
| Bone marrow plasma cell % (n=78) | |
| <10 | 5 (6.4) |
| 10-59 | 47 (60.3) |
| ≥60 | 26 (33.3) |
| *ESR (mm/hour) (n=49) | |
| ≤50 | 10 (20.4) |
| 51-100 | 8 (16.3) |
| >100 | 31 (63.3) |
| Parameters | n (%) |
|---|---|
| Albumin (gm/l) | |
| < 35 | 52 (66.7) |
| ≥ 35 | 26 (33.3) |
| Corrected Calcium (mmol/L) | |
| > 2.75 ≤ 2.75 | 50 (64.1) 28 (35.9) |
| LDH (U/L) | |
| < 300 | 26 (33.3) |
| ≥ 300 | 52 (66.7) |
| Creatinine (μmol/L) | |
| < 177 | 20 (25.6) |
| ≥ 177 | 58 (74.4) |
| Total protein (g/L) | |
| < 60 | 5 (6.4) |
| 61 – 89 | 29 (37.2) |
| ≥ 90 | 44 (56.4) |
| Serum paraprotein (g/L) | |
| < 30 | 24 (30.8) |
| ≥ 30 | 54 (69.2) |
| Serum B2M (mg/L) | |
| <3.5 | 5 (8.9) |
| 3.5-5.4 | 16 (28.6) |
| ≥5.5 | 35 (62.5) |
| Cytogenetic abnormalities | n (%) |
|---|---|
| Absent | 25 (32.0) |
| Present | 13 (16.7) |
| Hypodiploid | 6 |
| Hyperdiploid | 2 |
| Translocation (4,14) | 1 |
| Deletion chromosome 13,17 and translocation (14,16) | 1 |
| Monosomy 13 | 1 |
| Abnormal karyotype with additional material of unknown origin attached to 14a32 and 19p13.3 | 1 |
| Abnormal male near triploid karyotype with gain X | 1 |
| Unknown | 40 (51.3) |
| i) No metaphase | 11 (27.5) |
| ii) No result available | 29 (72.5) |
| Parameters | CD19 | ||||
|---|---|---|---|---|---|
| Negative n (%) | Positive n (%) | χ2 (df)* | p-value | ||
| White cell count (x10 9 /L) | 78 | - | 0.839b | ||
| < 4.0 | 5 (62.5) | 3 (37.5) | |||
| 4.0 – 11.0 | 41 (71.9) | 16 (28.1) | |||
| ≥ 11.1 | 10 (76.9) | 3 (23.1) | |||
| Hemoglobin (gm/dl) | 78 | 2.534(2) | 0.282a | ||
| < 10.0 | 39 (76.5) | 12 (23.5) | |||
| 10.0 – 11.9 | 13 (68.4) | 6 (31.6) | |||
| ≥ 12.0 | 4 (50.0) | 4 (50.0) | |||
| Platelet (x10 9 /L) | 78 | - | 0.832b | ||
| < 100 | 4 (66.7) | 2 (33.3) | |||
| 100 – 149 | 9 (69.2) | 4 (30.8) | |||
| ≥ 150 | 43 (72.9) | 6 (27.1) | |||
| Bone marrow plasma cell percentage (%) | 78 | - | 0.172b | ||
| < 10 | 3 (60.0) | 2 (40.0) | |||
| 10 - 59 | 31 (66.0) | 16 (34.0) | |||
| ≥ 60 | 22 (84.6) | 4 (15.4) | |||
| Albumin (g/dL) | 78 | 0.791(1) | 0.374a | ||
| < 35 | 39 (75.0) | 13(25.0) | |||
| ≥ 35 | 17 (65.4) | 9 (34.6) | |||
| Corrected calcium (mmol/L) | 78 | 0.003(1) | 0.957a | ||
| > 2.75 | 36 (72.0) | 14 (28.0) | |||
| ≤ 2.75 | 20 (71.4) | 8 (28.6) | |||
| LDH (U/L) | 78 | 3.830(1) | 0.050a | ||
| < 300 | 15 (57.7) | 11 (42.3) | |||
| ≥ 300 | 41 (78.8) | 11 (21.2) | |||
| Creatinine (µmol/L) | 78 | 4.402(1) | 0.036a | ||
| < 177 | 18 (90.0) | 2 (10.0) | |||
| ≥ 177 | 38 (65.5) | 20 (34.5) | |||
| Serum total protein (g/L) | 78 | - | 0.104b | ||
| < 60 | 2 (40.0) | 3 (60.0) | |||
| 61 – 89 | 19 (65.5) | 10 (34.5) | |||
| ≥ 90 | 35 (79.5) | 9 (20.5) | |||
| Serum paraprotein (g/L) | 78 | 0.016(1) | 0.900a | ||
| < 30 | 17 (70.8) | 7 (29.2) | |||
| ≥ 30 | 39 (72.2) | 15 (27.8) | |||
| Serum B2M (mg/L) | 56 | 2.667(2) | 0.359a | ||
| <3.5 | 5 (100.0) | 0 (0.0) | |||
| 3.5-5.4 | 11 (64.7) | 6 (35.3) | |||
| ≥5.5 | 26 (76.5) | 8 (23.5) | |||
| Types of light chain | 78 | - | 0.717b | ||
| Kappa | 35 (74.5) | 12 (25.5) | |||
| Lambda | 20 (66.7) | 10 (33.3) | |||
| Inconclusive | 1 (100.0) | 0 (0.0) | |||
| Cytogenic abnormalities | 39 | 0.253(1) | 0.719a | ||
| Present | 10 (76.9) | 3 (23.1) | |||
| Absent | 18 (69.2) | 8 (30.8) | |||
| Immunophenotypic markers | Staging | χ2 (df)* | p-value | ||
| I (n/%) | II (n/%) | III (n/%) | |||
| CD138 | - | - | |||
| Negative | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Positive | 4 (7.4) | 19 (35.2) | 31 (57.4) | ||
| CD38 | - | - | |||
| Negative | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Positive | 4 (7.4) | 19 (35.2) | 31 (57.4) | ||
| CD56 | 2.430(2) | 0.311a | |||
| Negative | 2 (18.2) | 2 (18.2) | 6 (54.5) | ||
| Positive | 2 (4.7) | 16 (37.2) | 25 (58.1) | ||
| CD117 | 1.963(2) | 0.432a | |||
| Negative | 0 (0.0) | 8 (38.1) | 13 (61.9) | ||
| Positive | 1 (8.3) | 5 (41.7) | 6 (50.0) | ||
| CD45 | 2.859(2) | 0.210a | |||
| Negative | 2 (5.1) | 12 (30.8) | 25 (64.1) | ||
| Positive | 2 (13.3) | 7 (46.7) | 6 (40.0) | ||
| CD19 | 1.557(2) | 0.703a | |||
| Negative | 4 (10.0) | 14 (35.0) | 22 (55.0) | ||
| Positive | 0 (0.0) | 5 (35.7) | 9 (64.3) | ||
Biochemical Parameters of Newly Diagnosed PCM Patients
All biochemical markers of cases showed an increasing trend, except for albumin. A proportion of 66.7% of cases had hypoalbuminemia (serum albumin <35g/l), high LDH (> 300U/L), high calcium (>2.75mmol/L), and substantially increased creatinine (>177 μmol/L). Hyperproteinemia (>90g/L) was seen in 56.4% of patients, while 6.4% had hypoproteinemia (60g/L). Serum total protein was normal in 37.2% of cases. Additionally, 69.2% of cases were associated with serum paraprotein levels more than 30 g/L, whereas 30.8% had values below 30g/L. Our study revealed that 62.5% of cases had a higher blood B2M level (>5.5mg/L), whereas 28.6% and 8.9% had a lower level (3.5-5.5mg/L and <3.5mg/L, respectively), as illustrated in Table 2.
Immunophenotypic Markers Expression in Newly Diagnosed PCM Patients
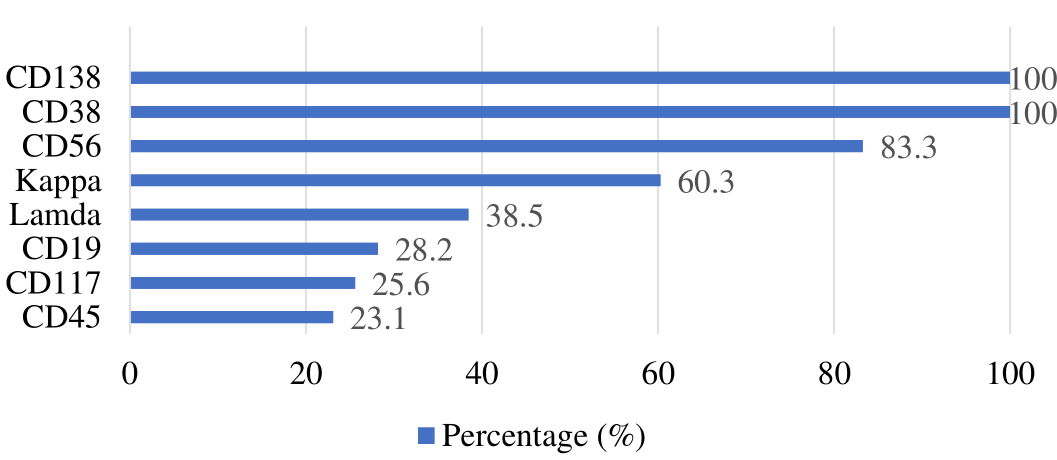
Flow cytometry was used to assess immunophenotypic marker positivity in 78 cases. Gated at CD138 and CD38 demonstrated that all patients' plasma cells were positive, whereas other immunophenotypic markers expressed differently. Neoplastic plasma cells expressed CD19, CD45, CD56, and CD117 at 28.2%, 23.1%, 83.3%, and 25.6%, respectively. Each case had abnormal marker expression, and 60.3% had kappa light chain restriction, and 38.5% had lambda light chain restriction, indicating clonality, as illustrated in Figure 1. In our study, only one case possessed inconclusive light chain expression.
Cytogenetic Abnormalities in Newly Diagnosed PCM Patients
The cytogenetic anomalies revealed a total of 38 cases completed standard cytogenetic testing, and the status for 40 cases was unknown, perhaps owing to metaphase acquisition issues or unavailability of results from medical records. Table 3 shows the results of cytogenetic abnormalities and their types in our PCM cases. A total of 6 out of 13 cases had hypodiploidy, representing the most common cytogenetic abnormality. Specifically, out of 13 (16.7%) of 38 cases had aberrant cytogenetic abnormalities, whereas 25 (32%) had normal or no abnormalities identified, as shown in Table 3.
Association between Immunophenotypic Markers Expression and Associated Factors among Newly Diagnosed PCM Patients
CD19 with Associated Factors
Table 4 presents a comparison of the association between CD19 positive and negative results in cases of PCM at the time of diagnosis, in addition to their respective associated factors. A statistically significant association was observed between CD19 and serum creatinine level (p = 0.036), where a significant proportion of cases (90.0%) who were CD19 negative exhibited lower levels of creatinine (<177 μmol/L) compared to their CD19 positive counterparts (10.0%). Conversely, individuals with a creatinine level of ≥177 μmol/L exhibited a greater correlation with CD19 negative (65.5%) in contrast to CD19 positive (34.5%). However, no significant association was found between CD45, CD56, and CD117 expression and associated factors in newly diagnosed PCM cases (Supplementary data Table S1- S3).
Association between Immunophenotypic Markers Expression and Clinical Stages among Newly Diagnosed PCM Cases According to ISS
The statistical analysis of the association between the expression of immunophenotypic markers and stages of PCM based on the ISS was conducted using the linear-by-linear association test, and the results are presented in Table 5. The cross-tabulation of CD38 and CD138 was not feasible as there was only one variable available for comparison among the immunophenotypic markers.The statistical insignificance of the myeloma stage and the expression of CD19, CD45, CD56, and CD117 immunophenotypic markers was observed. The findings indicate a greater incidence of negativity in markers CD19, CD45, and CD117 in Stage III, with a prevalence of 55.0%, 64.1%, and 61.7%, respectively, except for CD56. The prevalence of CD56 positive was found to be higher in Stage III (58.1%) compared to CD56 negative (54.4%). However, none of the markers exhibited statistically significant differences between the groups, as indicated by a p-value greater than 0.05.
Discussion
In this study, we observed an increase in PCM cases with age; the mean age was reported as 60 years, which is close to the mean age of the Libyan population (61.8 years)17 and higher than that of the Indian population (55 years)18. Another study reported incidence ages of 61–7019 and 50–6020. Interestingly, we found that 2 cases (2.6%) of PCM occurred in patients under 40 years old, while a single case was under 30 years old. This is consistent with a previous study, which also reported that 2% of cases occurred in individuals under 40 years old21, and unusual cases were reported under 30 years old22. In Malaysia, where Malays are the predominant ethnic group, cases involving Malays (75.6%) dominated this study, followed by Chinese and Indian cases. The study showed a male predominance (56.4%), similar to the previous study conducted by Kyle et al. 23, while another study reported a female predominance17. Our study demonstrated a male-to-female ratio of 1.29:1, in agreement with Madu et al. 24; a slightly higher ratio of 1.5:1 has been previously reported25.
A proportion of 65.4% of cases exhibited hemoglobin levels less than 10g/dl, suggesting a strong association between low hemoglobin levels and concurrent anemia in our PCM cases. Anemia in PCM is often multifactorial, arising from factors such as impaired erythropoiesis, bone marrow infiltration, and cytokine-mediated effects26. Anemia in patients with PCM may occur due to malignant plasma cells overtaking the bone marrow or reduced levels of erythropoietin. The presence of PCM cells in the bone marrow disrupts erythropoiesis, resulting in decreased hemoglobin levels and subsequent anemia27.
Additionally, leukopenia and thrombocytopenia were rare symptoms of newly diagnosed PCM, with our study reporting occurrences at 10.3% and 7.7%, respectively. However, another study reported higher proportions of both parameters, 19.3% and 14.2%, respectively23. Leukopenia may result from myelosuppression, leading to reduced production of white blood cells by the bone marrow. This condition is part of the wider range of hematopoietic suppression observed in PCM28, 29. Research indicates that thrombocytopenia may be a predictor of poor outcomes in these patients, potentially linked to the influence of tumor cells on the bone marrow microenvironment30. Our study indicated that 63.3% of PCM cases possessed higher erythrocyte sedimentation rates (ESR >100 mm/hour), which contradicted a previous study17. The ESR demonstrated a linear increase with concentrations of fibrinogen or gamma globulin (IgG) surpassing normal thresholds. Increasing concentrations of albumin led to a slight decrease in the ESR. Albumin exhibited a synergistic effect on the ESR when combined with gamma-globulin, but not when combined with fibrinogen31, 32.
Normal bone marrow contains 1% plasma cells, but depending on severity, tumor burden may increase this number by up to 80%33. In our study, we found that 93.6% of cases correlated with 10% or more bone marrow plasma cells, which is nearly comparable to the 92% reported in a previous study34. However, Hussain et al. discovered that only 70% of cases had more than 10% bone marrow plasma cells17. A small proportion of our cases (6.4%) exhibited fewer than 10% bone marrow plasma cells, which might be explained by hemodilution or plasma cell dispersion. PCM cases with plasma cell infiltration percentages exceeding 50% in the bone marrow are associated with significantly lower median survival compared to those with more favorable characteristics35, 36.
Renal impairment frequently occurs in PCM due to the accumulation of monoclonal light chains in the kidneys, leading to cast nephropathy. Malignant plasma cells produce light chains that form obstructive casts in the renal tubules, impairing kidney function37. In this study, the majority of cases presented with renal impairment and elevated serum creatinine levels. In contrast, other studies reported lower proportions of 27.2%17 and 20.5%19. Moreover, our study reported a higher incidence of hypercalcemia (64.1%), compared to the 22.7%19 and 22%38 reported in previous studies. Hypercalcemia in PCM is caused by increased cytokine-induced osteoclastic bone resorption and renal impairment. In cases with large tumor burdens, neoplastic plasma cells induce bone resorption, causing calcium to efflux into the extracellular fluid. Renal dysfunction, with increased tubular calcium reabsorption, also contributes to hypercalcemia because the kidneys are unable to remove excess calcium from the circulation39. This may explain the higher prevalence of hypercalcemia secondary to renal impairment observed in our study. Thus, both renal failure and hypercalcemia are poor prognostic factors, as PCM cases with hypercalcemia are associated with features of advanced disease40.
Monoclonal light chain proteinuria and tubular injury, which were demonstrated by 74.4% of cases in our study, reflect the renal impairment with serum creatinine levels above 177 μmol/L. Conversely, previous studies reported much lower proportions of 27.2%17 and 20.5%19. Our study cases revealed a 64.1% incidence of hypercalcemia, which was higher compared to the 22.7% and 22% reported in previous studies19. Renal dysfunction caused by PCM-related hypercalcemia is an indicator of advanced PCM and poor prognosis40. Our study also demonstrated that a higher proportion (69.2%) of cases were linked with serum paraprotein levels ≥ 30g/L, while 30.8% were below.
PCM prognostic variables, including serum albumin, LDH, and B2M, can be monitored to represent disease progression and tumor load41. In our study, 66.7% of cases exhibited hypoalbuminemia and elevated LDH levels > 300 U/L, nearly matching the 66% reported in another study19. Most of the 56 serum samples evaluated for B2M were associated with significantly higher values (> 5.5 mg/L), and even with these few cases, this was substantial because serum B2M is a strong predictive factor for tumor load and renal impairment42. Since renal glomeruli filter B2M, high serum B2M levels may indicate renal failure, leading to renal insufficiency and B2M accumulation43. This underscores that PCM cases in our study were significantly associated with a substantial rate of renal failure, suggesting that further treatment alternatives should be considered to address this finding.
Early B cell differentiation is activated and regulated by CD19, an acquired biological marker. In PCM cells, fewer than 5% express CD1944. We found that 28.2% of cases were CD19-positive, which is significantly higher than the 8.8% observed among Koreans45 but lower than the 38.7% among Egyptians46. This indicates that such variations may reflect population genetic heterogeneity. A previous study reported that CD19 in PCM was associated with diagnostic value rather than prognostic significance47. In our study, 90% of CD19-negative cases involved PCM patients with serum creatinine levels below 177 μmol/L, suggesting a prevalence of less severe renal impairment among these subjects. Conversely, 65.5% of CD19-negative cases were associated with serum creatinine levels exceeding 177 μmol/L, indicating that a substantial proportion of CD19-negative patients also experienced more significant renal impairment. The dominance of CD19 negativity in cases with serum creatinine levels below 177 μmol/L suggests a potential association between CD19 negativity and less severe renal impairment in PCM. Additionally, the fact that a significant proportion (65.5%) of CD19-negative cases had serum creatinine levels above 177 μmol/L highlights the heterogeneity within the CD19-negative subgroup. This suggests that while CD19 negativity may generally correlate with less severe renal impairment, it does not uniformly predict renal function in our population. The findings suggest that CD19 status could serve as a potential marker for risk stratification in PCM patients based on renal function. CD19-negative cases may be stratified into subgroups with varying degrees of renal impairment, thus tailoring treatment approaches based on CD19 status and associated renal function could be considered. For instance, CD19-negative patients with less severe renal impairment might benefit from different therapeutic strategies than those with more significant renal complications. Further research is warranted to understand the underlying mechanisms contributing to the heterogeneity within the CD19-negative group. Exploring the molecular and clinical characteristics of CD19-negative cases with varying renal function could provide insights into disease pathogenesis48.
However, conducting a validation study in larger and more diverse patient cohorts will be necessary to confirm the robustness of the association between CD19 negativity and renal function. This will enhance the generalizability of the results to different populations. The association between CD19 negativity and serum creatinine levels in PCM patients is a significant finding with potential implications for risk stratification and treatment decisions. The dominance of CD19 negativity in cases with less severe renal impairment suggests its potential as a marker for a more favorable clinical presentation. However, the observed heterogeneity within the CD19-negative group emphasizes the need for further research and validation studies to refine our understanding of this association and its clinical implications in the context of PCM.
CD45 plays a crucial role in regulating antigen-mediated signaling and activation in lymphocytes, showing elevated levels during the initial phases of plasma cell maturation49. The persistent high expression of CD45 in clonal plasma cells from PCM patients indicates a greater cell proliferation rate. Hence, CD45 positivity in PCM cells could indicate negative prognostic implications50. Our study found a CD45-negative rate of 76.9%, which is higher than the rates reported in equivalent studies: 50%45, 30%44, and 22.6%46. This suggests that our PCM cases potentially have more positive prognostic implications. However, the clinical relevance of CD45 positivity or negativity in PCM remains uncertain51. Another study reported that CD45-negative expression was associated with higher serum B2M, elevated calcium levels, and advanced disease stage, suggesting an unfavorable prognosis46. In contrast, Mateo et al. (2008) reported no prognostic impact of CD45, which aligns with our findings44.
CD56 anchors plasma cells to the BM microenvironment, thereby preventing disease dissemination. It exhibits lower expression in normal plasma cells (<15%) and dramatically higher expression (75%) in PCM cells52. In agreement, the present study showed greater CD56 positive expression (83.3%) than previously reported45. CD56-positive cases possess higher B2M levels than CD56-negative cases46; however, our study demonstrated no relationship between the CD56 marker and related variables, similar to the findings of another study53.
Normal plasma cells lack the expression of CD117, a cell growth factor or tyrosine kinase, while neoplastic plasma cells display an expression rate of 30%52. Our PCM cases had a 25.6% expression rate of CD117, which was lower than that found in the studies by Shin et al. (39.7%)45 and Mateo et al. (36%)44, but higher than that reported by Lin et al. (17.8%)54. Another study found that being CD117-negative was strongly associated with increased serum creatinine46, although our findings did not support this association, likely due to our small sample size, with only 50 of 78 cases included for CD117 marker evaluation.
Several studies have also linked CD117 negativity to cytogenetic abnormalities in PCM cases13. CD117 negativity was associated with hypodiploidy, t(11;14), nonhyperdiploid DNA content, and 13q deletion, all of which are unfavorable prognostic markers in PCM13. However, our study found no such correlation, which was probably due to our limited sample size, where only 13 cases exhibited cytogenetic abnormalities and 26 had normal cytogenetics. All cases in our study exhibited at least one abnormal marker expression, such as negative or loss of CD19 or CD45, or positive or gain of CD56 or CD117 with cytoplasmic light chain clonality, which distinguishes malignant plasma cells from benign ones and detects residual disease in PCM. The absence of CD19 is diagnostically sensitive for PCM, whereas a gain of CD56 or CD117 may indicate a good prognostic factor in PCM55. CD117 expression was linked to favorable genetic mutations and improved outcomes56, reflecting the importance of this aberrant marker for PCM patient follow-up through minimum residual disease monitoring. PCM exhibited greater antigenic aberrancies than MGUS, and disease progression was linked to an increase in aberrancies, indicating clonal evolution55.
Our study found that only CD19 was statistically associated with serum creatinine (p = 0.036); other markers did not show statistical relationships with linked variables. Additionally, compared to other studies, the expressions of both CD19 and CD56 were higher than those of CD117 and CD45. These findings suggest genetic heterogeneity and varied immunophenotypic marker expression in neoplastic PCM cells, accurately representing our population and differing significantly from other studies.
PCM can be classified into ISS Stages I, II, or III. Stage III, associated with the poorest outcomes57, correlated with the majority of our cases (39.7%) and is consistent with other studies reporting 39%19 and 33.0%58. Notably, Kumar et al., 2006, reported a much higher prevalence of PCM at Stage III, at 81%18. The late presentation of the disease could be attributed to a low proliferative index of neoplastic plasma cells and a nonspecific clinical presentation in earlier stages59. Additionally, the absence of routine annual health evaluations in healthy adults might also contribute to the late detection and more advanced stage of the disease at diagnosis. Several studies have reported associations among immunophenotypic markers CD19, CD45, CD56, and CD117 with clinical stages. Negativity for both CD45 and CD117 was associated with advanced stages45. Consistent with previous findings, our study showed the highest rates of CD45 (n=25, 64.1%) and CD117 (n=13, 61.9%) negativity in Stage III patients. This discovery underscores the need for proactive early disease screening in our population to prevent worsening outcomes in PCM patients. Furthermore, positive expressions of both CD19 and CD20 were related to clinical stages of PCM and associated with worse disease stages60, and CD56 positivity indicated an advanced stage46. However, none of the immunophenotypic markers in our study showed a statistically significant association with the PCM stage.
Limitations and Directions for Future Research
Several limitations arose during this study. First, the sample size was small, with only a few newly diagnosed PCM cases at both recruitment sites, HKL and HUSM, from June 2016 to June 2019. Moreover, most newly identified PCM cases at HKL were referrals from other hospitals and were thus excluded from this study. Therefore, interpreting the findings with caution is crucial, as they may not accurately reflect the entire PCM population in Malaysia. We also found that many cases could not achieve complete cytogenetic results due to inadequate metaphase quality, and some cases lacked CD117 results because the reagents were unavailable at HKL during the study.
To improve the study's sample size and robustness, it is recommended to include additional centers with newly diagnosed PCM patients. Utilizing molecular techniques, such as incorporating a Fluorescence in situ hybridization (FISH) panel in all PCM patients, is highly recommended for testing underlying cytogenetic and molecular abnormalities due to its enhanced sensitivity and specificity. Moreover, conducting more extensive studies with a larger number of PCM patients to assess the impact of various immunophenotypic markers expressed by clonal plasma cells on patient outcomes and genetic abnormalities could be beneficial.
Conclusions
Our study demonstrated that a combination of antigens—including CD38, CD138, CD19, CD45, CD56, CD117, and cytoplasmic kappa and lambda light chains—used in a 6-color flow cytometry approach, can distinguish cancerous plasma cells from benign cells. This method was applied to assess clonal plasma cell immunophenotypic markers in newly diagnosed cases of PCM within our population, revealing significant variances in the expression of immunophenotypic markers. Our findings indicated a higher proportion of CD19 and CD56 positive markers and a lower prevalence of CD45 and CD117 positive markers in our population. These observations suggest variability in disease biology and genetic heterogeneity among our representative PCM cases. Consequently, kappa light chain restriction clonality and at least one aberrant marker expression were observed in our population. Additionally, our study revealed that severe disease manifestations are associated with a worse prognosis and poor clinical outcomes. Immunophenotyping is crucial for the early and accurate identification of PCM, as it helps to differentiate abnormal plasma cells from normal ones through their unique surface marker expressions, enabling clinicians to promptly initiate appropriate treatment strategies. We also recommend utilizing aberrant markers to monitor PCM treatment response through multiparametric flow cytometry for detecting minimal residual disease. The increased sensitivity of this method allows for more accurate monitoring of the patient's response to treatment, aiding in decisions regarding whether to continue, modify, or adjust therapeutic interventions. Furthermore, our study found that most of our newly diagnosed PCM cases are associated with Stage III disease, highlighting the need for proactive early disease screening and awareness in our population to prevent worsening outcomes in PCM patients.
Abbreviations
B2M: Beta 2 Microglobulin, CD: Cluster of differentiation, ESR: Erythrocyte sedimentation rate, HKL: Hospital Kuala Lumpur, HUSM: Hospital Universiti Sains Malaysia, IgG: Immunoglobulin G, ISS: International Staging System, LDH: Lactate dehydrogenase, MGUS: Monoclonal gammopathy of undetermined significance, PBS: Phosphate-buffered saline, PCM: Plasma cell myeloma, R-ISS: Revised International Staging System, SPSS: Statistical Package for the Social Sciences, WBC: White blood cell, WHO: World Health Organization
Acknowledgments
The authors express their sincere gratitude for the invaluable support rendered by supervisors and personnel from Hospital Universiti Sains Malaysia and the Ministry of Health Malaysia, particularly Hospital Kuala Lumpur and Hospital Ampang, throughout the training and data collection procedure.
Author’s contributions
Conceptualization, L.P.C.; S.M.Y.; and R.H.; methodology, L.P.C.; and S.M.Y.; investigation, L.P.C.; N.R.M.N.Y.; R.M.; R.Z.H.; writing original draft preparation, L.P.C.; S.M.Y.; and N.H.I.; writing review and editing, N.H.I.; S.M.Y.; and M.F.J.; supervision, M.F.J.; S.M.Y.; and R.H.; funding acquisition, S.M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The Human Research Ethics Committee, Universiti Sains Malaysia has approved the study in principle [USM/JEPeM/19040237] and the Medical Research & Ethics Committee [NMRR-18-3904-45526 (IIR)].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Abdullah
H.M.,
Ellithi
M.,
Waqas
Q.,
Cunningham
A.,
Oliver
T.,
Hypercalcaemia, renal dysfunction, anaemia and bone lesions (CRAB) do not always represent multiple myeloma: diffuse large B cell lymphoma presenting with CRAB symptoms in a 69-year-old man. BMJ Case Reports.
2019;
12
(8)
.
View Article PubMed Google Scholar -
Cowan
A.J.,
Allen
C.,
Barac
A.,
Basaleem
H.,
Bensenor
I.,
Curado
M.P.,
Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncology.
2018;
4
(9)
:
1221-7
.
View Article PubMed Google Scholar -
Bird
S.,
Cairns
D.,
Menzies
T.,
Boyd
K.,
Davies
F.,
Cook
G.,
Sex Differences in Multiple Myeloma Biology but not Clinical Outcomes: Results from 3894 Patients in the Myeloma XI Trial. Clinical Lymphoma, Myeloma {&}amp; Leukemia.
2021;
21
(10)
:
667-75
.
View Article PubMed Google Scholar -
Ludwig
H.,
Novis Durie
S.,
Meckl
A.,
Hinke
A.,
Durie
B.,
Multiple Myeloma Incidence and Mortality Around the Globe; Interrelations Between Health Access and Quality, Economic Resources, and Patient Empowerment. The Oncologist.
2020;
25
(9)
:
e1406-13
.
View Article PubMed Google Scholar -
Nor
N.,
Ghazali
S.,
Malaysia towards an ageing country. Geografia : Malaysian Journal of Society and Space.
2021;
17
(3)
:
234-45
.
-
Podar
K.,
Leleu
X.,
Relapsed/Refractory Multiple Myeloma in 2020/2021 and Beyond. Cancers (Basel).
2021;
13
(20)
:
5154
.
View Article PubMed Google Scholar -
Rath
A.,
Panda
T.,
Dass
J.,
Seth
T.,
Mahapatra
M.,
Tyagi
S.,
Immunophenotypic Profile of Multiple Myeloma: A Tertiary Care Centre Experience. Journal of Laboratory Physicians.
2023;
15
(3)
:
392-8
.
View Article PubMed Google Scholar -
Yanis
M.,
Rahali
M.,
Belakehal
S.,
Benfenatki
N.,
Ardjoune
F.,
Chaib
S.,
Plasma Cell Immunophenotyping Improve Prognostic Stratification of Multiple Myeloma Patients. International Journal of Cancer Management.
2018
.
-
Dold
S.M.,
Riebl
V.,
Wider
D.,
Follo
M.,
Pantic
M.,
Ihorst
G.,
Validated single-tube multiparameter flow cytometry approach for the assessment of minimal residual disease in multiple myeloma. Haematologica.
2020;
105
(10)
.
View Article PubMed Google Scholar -
Jeong
T.D.,
Park
C.J.,
Shim
H.,
Jang
S.,
Chi
H.S.,
Yoon
D.H.,
Simplified flow cytometric immunophenotyping panel for multiple myeloma, CD56/CD19/CD138(CD38)/CD45, to differentiate neoplastic myeloma cells from reactive plasma cells. The Korean Journal of Hematology.
2012;
47
(4)
:
260-6
.
View Article PubMed Google Scholar -
Robillard
N.,
Wuillème
S.,
Moreau
P.,
Béné
M.C.,
Immunophenotype of normal and myelomatous plasma-cell subsets. Frontiers in Immunology.
2014;
5
:
137
.
View Article PubMed Google Scholar -
Flores-Montero
J.,
de Tute
R.,
Paiva
B.,
Perez
J.J.,
Böttcher
S.,
Wind
H.,
Immunophenotype of normal vs. myeloma plasma cells: toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry. Part B, Clinical Cytometry.
2016;
90
(1)
:
61-72
.
View Article PubMed Google Scholar -
Chen
F.,
Hu
Y.,
Wang
X.,
Fu
S.,
Liu
Z.,
Zhang
J.,
Expression of CD81 and CD117 in plasma cell myeloma and the relationship to prognosis. Cancer Medicine.
2018;
7
(12)
:
5920-7
.
View Article PubMed Google Scholar -
Lebel
E.,
Nachmias
B.,
Pick
M.,
Gross Even-Zohar
N.,
Gatt
M.E.,
Understanding the Bioactivity and Prognostic Implication of Commonly Used Surface Antigens in Multiple Myeloma. Journal of Clinical Medicine.
2022;
11
(7)
:
1809
.
View Article PubMed Google Scholar -
Conroy, R. (2015). Sample size: A rough guide. Retreived from http://www. beaumontethics. ie/docs/application/samplesizecalculation. pdf..
.
-
Choi
S.M.,
O'Malley
D.P.,
Diagnostically relevant updates to the 2017 WHO classification of lymphoid neoplasms. Annals of Diagnostic Pathology.
2018;
37
:
67-74
.
View Article PubMed Google Scholar -
Hussain
A.,
Almenfi
H.F.,
Almehdewi
A.M.,
Hamza
M.S.,
Bhat
M.S.,
Vijayashankar
N.P.,
Laboratory Features of Newly Diagnosed Multiple Myeloma Patients. Cureus.
2019;
11
(5)
.
View Article PubMed Google Scholar -
Kumar
L.,
Vikram
P.,
Kochupillai
V.,
Recent advances in the mangement of multiple myeloma. The National Medical Journal of India.
2006;
19
(2)
:
80-9
.
PubMed Google Scholar -
A.
Kumar,
Anupam
Kumar V.,
Clinical profile of multiple myeloma in a tertiary care center from North East India. Journal of Evolution of Medical and Dental Sciences.
2016;
2016
:
5
.
-
Dash
N.,
Mohanty
B.,
Biochemical profile and electrophoretic pattern in multiple myeloma patients. Journal of Evidence Based Medicine and Healthcare..
2015;
2
(39)
:
6166-70
.
View Article Google Scholar -
Rajkumar
S.V.,
Updated Diagnostic Criteria and Staging System for Multiple Myeloma. American Society of Clinical Oncology Educational Book.
2016;
35
(36)
:
e418-23
.
View Article PubMed Google Scholar -
Crusoe
E.Q.,
da Silva
A.M.,
Agareno
J.,
Chauffaille
M.L.,
Bonfim
C.,
Moraes Hungria
V.T.,
Multiple myeloma: a rare case in an 8-year-old child. Clinical Lymphoma, Myeloma {&}amp; Leukemia.
2015;
15
(1)
:
e31-3
.
View Article PubMed Google Scholar -
Kyle
R.A.,
Gertz
M.A.,
Witzig
T.E.,
Lust
J.A.,
Lacy
M.Q.,
Dispenzieri
A.,
Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proceedings.
2003;
78
(1)
:
21-33
.
View Article PubMed Google Scholar -
Madu
A.J.,
Ocheni
S.,
Nwagha
T.A.,
Ibegbulam
O.G.,
Anike
U.S.,
Multiple myeloma in Nigeria: an insight to the clinical, laboratory features, and outcomes. Nigerian Journal of Clinical Practice.
2014;
17
(2)
:
212-7
.
View Article PubMed Google Scholar -
Bhat
G.,
Treatment outcome of multiple myeloma (MM) based on cytogenetic risk stratification: a single institute experience from south India. International Journal of Advances in Medicine..
2019;
6
(1)
:
170
.
View Article Google Scholar -
Liu
L.,
Yu
Z.,
Cheng
H.,
Mao
X.,
Sui
W.,
Deng
S.,
Multiple myeloma hinders erythropoiesis and causes anaemia owing to high levels of CCL3 in the bone marrow microenvironment. Scientific Reports.
2020;
10
(1)
:
20508
.
View Article PubMed Google Scholar -
Liu
L.,
Yu
Z.,
Cheng
H.,
Mao
X.,
Sui
W.,
Deng
S.,
Multiple myeloma hinders erythropoiesis and causes anaemia owing to high levels of CCL3 in the bone marrow microenvironment. Scientific Reports.
2020;
10
(1)
:
20508
.
View Article PubMed Google Scholar -
Bruns
I.,
Cadeddu
R.P.,
Brueckmann
I.,
Fröbel
J.,
Geyh
S.,
Büst
S.,
Multiple myeloma-related deregulation of bone marrow-derived CD34(+) hematopoietic stem and progenitor cells. Blood.
2012;
120
(13)
:
2620-30
.
View Article PubMed Google Scholar -
L.
Qiu,,
Z.
Yu,,
The bone marrow microenvironment of multiple myeloma promotes myeloma related anemia by suppressing the differentiation of hematopoietic stem cells. Clinical Lymphoma, Myeloma & Leukemia.
2019;
19
(10)
:
e92-3
.
View Article Google Scholar -
Al Saleh
A.S.,
Sidiqi
M.H.,
Dispenzieri
A.,
Kapoor
P.,
Muchtar
E.,
Buadi
F.K.,
Hematopoietic score predicts outcomes in newly diagnosed multiple myeloma patients. American Journal of Hematology.
2020;
95
(1)
:
4-9
.
View Article PubMed Google Scholar -
Silvestris
F.,
Cafforio
P.,
Tucci
M.,
Dammacco
F.,
Negative regulation of erythroblast maturation by Fas-L(+)/TRAIL(+) highly malignant plasma cells: a major pathogenetic mechanism of anemia in multiple myeloma. Blood.
2002;
99
(4)
:
1305-13
.
View Article PubMed Google Scholar -
Talstad
I.,
Haugen
H.F.,
The relationship between the erythrocyte sedimentation rate (ESR) and plasma proteins in clinical materials and models. Scandinavian Journal of Clinical and Laboratory Investigation.
1979;
39
(6)
:
519-24
.
View Article PubMed Google Scholar -
Hutchison
C.A.,
Cockwell
P.,
Cook
M.,
Diagnostic accuracy of monoclonal antibody based serum immunoglobulin free light chain immunoassays in myeloma cast nephropathy. BMC Clinical Pathology.
2012;
12
(1)
:
12
.
View Article PubMed Google Scholar -
Li
Y.,
Li
H.,
Li
W.,
Wang
L.,
Yan
Z.,
Yao
Y.,
Pretreatment neutrophil/lymphocyte ratio but not platelet/lymphocyte ratio has a prognostic impact in multiple myeloma. Journal of Clinical Laboratory Analysis.
2017;
31
(5)
.
View Article PubMed Google Scholar -
Subramanian
R.,
Basu
D.,
Dutta
T.K.,
Prognostic significance of bone marrow histology in multiple myeloma. Indian Journal of Cancer.
2009;
46
(1)
:
40-5
.
View Article PubMed Google Scholar -
Sbaraglia
M.,
Multiple Myeloma. 2020;
:
349-53
.
View Article Google Scholar -
Kundu
S.,
Jha
S.B.,
Rivera
A.P.,
Flores Monar
G.V.,
Islam
H.,
Puttagunta
S.M.,
Multiple Myeloma and Renal Failure: Mechanisms, Diagnosis, and Management. Cureus.
2022;
14
(2)
.
View Article PubMed Google Scholar -
Dancaster
C.P.,
Hussain
O.A.,
Jackson
W.P.,
Clinical features of multiple myeloma. A review of the clinical manifestations and laboratory investigations in 40 cases. Postgraduate Medical Journal.
1959;
35
(410)
:
662-7
.
View Article PubMed Google Scholar -
Bao
L.,
Wang
Y.,
Lu
M.,
Chu
B.,
Shi
L.,
Gao
S.,
Hypercalcemia caused by humoral effects and bone damage indicate poor outcomes in newly diagnosed multiple myeloma patients. Cancer Medicine.
2020;
9
(23)
:
8962-9
.
View Article PubMed Google Scholar -
Kastritis
E.,
Katodritou
E.,
Pouli
A.,
Hatzimichael
E.,
Delimpasi
S.,
Michalis
E.,
Frequency and Prognostic Significance of Hypercalcemia in Patients with Multiple Myeloma: An Analysis of the Database of the Greek Myeloma Study Group. Blood.
2011;
118
(21)
:
5083
.
View Article Google Scholar -
Hanbali
A.,
Hassanein
M.,
Rasheed
W.,
Aljurf
M.,
Alsharif
F.,
The Evolution of Prognostic Factors in Multiple Myeloma. Advances in Hematology.
2017;
2017
.
View Article PubMed Google Scholar -
Argyropoulos
C.P.,
Chen
S.S.,
Ng
Y.H.,
Roumelioti
M.E.,
Shaffi
K.,
Singh
P.P.,
Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Frontiers in Medicine.
2017;
4
:
73
.
View Article PubMed Google Scholar -
Sedighi
O.,
Abediankenari
S.,
Omranifar
B.,
Association between plasma Beta-2 microglobulin level and cardiac performance in patients with chronic kidney disease. Nephro-Urology Monthly.
2014;
7
(1)
.
View Article PubMed Google Scholar -
Mateo
G.,
Montalbán
M.A.,
Vidriales
M.B.,
Lahuerta
J.J.,
Mateos
M.V.,
Gutiérrez
N.,
Study Group
PETHEMA,
Study Group
GEM,
Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. Journal of Clinical Oncology.
2008;
26
(16)
:
2737-44
.
View Article PubMed Google Scholar -
Shin
S.Y.,
Lee
S.T.,
Kim
H.J.,
Kim
S.J.,
Kim
K.,
Kang
E.S.,
Antigen Expression Patterns of Plasma Cell Myeloma: An Association of Cytogenetic Abnormality and International Staging System (ISS) for Myeloma. Journal of Clinical Laboratory Analysis.
2015;
29
(6)
:
505-10
.
View Article PubMed Google Scholar -
Boshnak
N.H.,
A. E. Hashem,
Association between immunophenotypic markers and cytogenetic aberrations in Egyptian patients with plasma cell myeloma. The Egyptian Journal of Haematology.
2017;
42
(1)
:
1-8
.
-
Cannizzo
E.,
Carulli
G.,
Del Vecchio
L.,
Ottaviano
V.,
Bellio
E.,
Zenari
E.,
The role of CD19 and CD27 in the diagnosis of multiple myeloma by flow cytometry: a new statistical model. American Journal of Clinical Pathology.
2012;
137
(3)
:
377-86
.
View Article PubMed Google Scholar -
Pellat-Deceunynck
C.,
Bataille
R.,
Normal and malignant human plasma cells: proliferation, differentiation, and expansions in relation to CD45 expression. Blood Cells, Molecules & Diseases.
2004;
32
(2)
:
293-301
.
View Article PubMed Google Scholar -
Kumar
S.,
Rajkumar
S.V.,
Kimlinger
T.,
Greipp
P.R.,
Witzig
T.E.,
CD45 expression by bone marrow plasma cells in multiple myeloma: clinical and biological correlations. Leukemia.
2005;
19
(8)
:
1466-70
.
View Article PubMed Google Scholar -
Gonsalves
W.I.,
Timm
M.M.,
Rajkumar
S.V.,
Morice
W.G.,
Dispenzieri
A.,
Buadi
F.K.,
The prognostic significance of CD45 expression by clonal bone marrow plasma cells in patients with newly diagnosed multiple myeloma. Leukemia Research.
2016;
44
:
32-9
.
View Article PubMed Google Scholar -
Kumar
S.,
Rajkumar
S.,
Kimlinger
T.,
Greipp
P.,
Witzig
T.,
CD45 expression by bone marrow plasma cells in multiple myeloma: Clinical and biological correlations. Leukemia : official journal of the Leukemia Society of America. Leukemia Research Fund, UK..
2005;
19
:
1466-70
.
-
Rawstron
A.C.,
Orfao
A.,
Beksac
M.,
Bezdickova
L.,
Brooimans
R.A.,
Bumbea
H.,
European Myeloma Network
Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica.
2008;
93
(3)
:
431-8
.
View Article PubMed Google Scholar -
Kraj
M.,
Soko\lowska
U.,
Kopeć-Szlezak
J.,
Pog\lód
R.,
Kruk
B.,
Woźniak
J.,
Clinicopathological correlates of plasma cell CD56 (NCAM) expression in multiple myeloma. Leukemia {&}amp; Lymphoma.
2008;
49
(2)
:
298-305
.
View Article PubMed Google Scholar -
Lin
P.,
Owens
R.,
Tricot
G.,
Wilson
C.S.,
Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. American Journal of Clinical Pathology.
2004;
121
(4)
:
482-8
.
View Article PubMed Google Scholar -
Gupta
S.,
Karandikar
N.J.,
Ginader
T.,
Bellizzi
A.M.,
Holman
C.J.,
Flow cytometric aberrancies in plasma cell myeloma and MGUS - correlation with laboratory parameters. Cytometry. Part B, Clinical Cytometry.
2018;
94
(3)
:
500-8
.
View Article PubMed Google Scholar -
Bataille
R.,
Pellat-Deceunynck
C.,
Robillard
N.,
Avet-Loiseau
H.,
Harousseau
J.L.,
Moreau
P.,
CD117 (c-kit) is aberrantly expressed in a subset of MGUS and multiple myeloma with unexpectedly good prognosis. Leukemia Research.
2008;
32
(3)
:
379-82
.
View Article PubMed Google Scholar -
Gopalakrishnan
S.,
D\textquotesingleSouza
A.,
Scott
E.,
Fraser
R.,
Davila
O.,
Shah
N.,
Revised International Staging System Is Predictive and Prognostic for Early Relapse (<24 months) after Autologous Transplantation for Newly Diagnosed Multiple Myeloma. Biology of Blood and Marrow Transplantation.
2019;
25
(4)
:
683-688
.
-
Dimopoulos
M.,
Kyle
R.,
Fermand
J.P.,
Rajkumar
S.V.,
San Miguel
J.,
Chanan-Khan
A.,
International Myeloma Workshop Consensus Panel 3
Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood.
2011;
117
(18)
:
4701-5
.
View Article PubMed Google Scholar -
Tathineni
P.,
Cancarevic
I.,
Malik
B.H.,
Uncommon Presentations of Multiple Myeloma. Cureus.
2020;
12
(6)
.
PubMed Google Scholar -
Cui
Y.,
Zhang
F.,
Jiang
M.,
Pang
N.,
Li
Z.W.,
Zhu
Y.,
Expression of CD19 and CD20 in plasma cells are significantly different between Han and Uygur multiple myeloma patients. International Journal of Clinical and Experimental Medicine.
2016;
9
(7)
:
13041-6
.
Comments
Article Details
Volume & Issue : Vol 11 No 3 (2024)
Page No.: 6248-6261
Published on: 2024-03-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 2754 times
- PDF downloaded - 1014 times
- XML downloaded - 96 times
 Biomedpress
Biomedpress


