Abstract
Introduction: Twin-to-twin transfusion syndrome (TTTS) is a critical prenatal complication in monochorionic diamniotic twins with a high risk of mortality and neurological sequelae if left untreated. Of the various therapeutic approaches, fetoscopic laser surgery (FLS) has emerged as the predominant treatment modality worldwide. This study evaluates the outcomes and preoperative risks of TTTS treatment via FLS.
Methods: In this prospective cohort study, we analyzed 115 consecutive TTTS cases with gestational ages of 16 to 26 weeks, treated with FLS at Shariati Hospital, Tehran, Iran, from 2018 to 2022. The mean gestational ages at the time of laser surgery and delivery were 20.70 +/- 2.21 and 32.18 +/- 4.68 weeks, respectively. Postoperative survival was assessed with Doppler measurements post-laser treatment in all but one case, where amniotic sac rupture led to fetal loss.
Results: Postoperative survival rates at 30 days were 32.2% for both twins, 53% for either twin, and 85.2% for at least one twin. A notable correlation was observed between donor twin survival and a fetal weight discrepancy exceeding 25%. Additionally, a higher incidence of absent or reversed end-diastolic velocity in the donor umbilical artery was identified among survivors (p < 0.05), indicating a significant survival benefit with FLS.
Conclusions: FLS proved to be an effective intervention for TTTS prior to the 26th week of gestation, with outcomes in this Iranian cohort aligning with those reported in developed countries. This underscores FLS's viability as a primary treatment strategy for TTTS, highlighting the importance of early diagnosis and intervention.
Introduction
Monochorionic twin pregnancies fall into the category of high-risk pregnancies. These pregnancies are susceptible to developing twin-to-twin transfusion syndrome (TTTS) due to an imbalance in the blood flow between the twins through the vascular connections in the placenta1. TTTS impacts 8–10% of pregnancies involving monochorionic diamniotic twins2. If untreated, both fetuses are at risk of cardiovascular problems and death, with a fetal mortality rate of 90–100%1. While uneven sharing of the placenta can result in one twin experiencing selective intrauterine growth restriction, imbalanced blood flow through the placental anastomosis can lead to discrepancies in amniotic fluid or severe TTTS during the middle of the pregnancy term3. Perinatal death rates might approach 90% without intervention4. Over the past 10 years, fetoscopic laser coagulation (FLC) of placental anastomoses has become the preferred treatment for severe TTTS. This method has significantly increased survival rates and reduced the likelihood of neurological issues in survivors5, 6.
The Quintero staging system is utilized to assess the severity of TTTS, as follows:
Stage I: There is a mismatch in amniotic fluid volume, with the presence of polyhydramnios (the largest vertical sac exceeding 8 cm by 20 weeks of gestation and being over 10 cm post 20 weeks of gestation);
Stage II: The criteria of Stage I are met, but no urine is visible in the bladder;
Stage III: The criteria of Stage II are met, along with abnormal findings in the umbilical artery (UA) or umbilical vein during Doppler evaluation;
Stage IV: Ascites or hydrops is clearly present in the recipient fetus;
Stage V: Either of the fetuses has died7, 8.
Quintero ignores the echocardiographic results that have been reported in numerous investigations9, 10. The recipient fetus commonly exhibits cardiac functional abnormalities such as biventricular hypertrophy, systolic and diastolic dysfunction, regurgitation of the tricuspid and mitral valves, atresia or stenosis of the pulmonary valve, and progressive severe biventricular dysfunction and failure. These are presumably reactions to volume overload11. In TTTS, cardiovascular problems have a significant role in total morbidity and death. Changes in the donor include mild right ventricular diastolic dysfunction caused by increased placental resistance and inadequate renal perfusion11. Fetal echocardiography can assist in categorizing risk and tracking disease progression. Consequently, numerous studies have concentrated on the role of the heart and its prognostic value, finding that echocardiography can identify the escalating severity of cardiomyopathy related to the syndrome in the recipient twin12, 13, 14.The objective of the present study was to assess the preliminary outcomes of fetoscopic laser surgery (FLS) for TTTS. It aimed to ascertain the predictive potential of Doppler and cardiac information gathered pre- and post-laser treatment for TTTS in forecasting the outcomes for the twins. To our knowledge, this is the first study conducted in Iran to evaluate the effects of FLC in TTTS.
Materials and Methods
In this prospective cohort study, all successive monochorionic diamniotic twin pregnancies that were referred to Shariati Hospital in Tehran, Iran, from July 2018 to April 2022 underwent FLS as the primary treatment for TTTS. Patients who met the following requirements were eligible for intervention:
(1) monochorionic twin gestation;
(2) TTTS (Stages II, III, and IV) diagnosed before 26 weeks of gestation (16–26 weeks);
(3) polyhydramnios, or excess amniotic fluid, present in the amniotic sac of the recipient twin, characterized by a maximum vertical pocket exceeding 8 cm before 20 weeks of pregnancy and more than 10 cm between 21 and 25 weeks of pregnancy; and
(4) oligohydramnios in the amniotic sac of the donor twin less than 2 cm (stuck). Cases with a history of amniocentesis, early rupture of membrane, uterine contraction, fetal anomaly, and Stages I and V were all excluded.
A special questionnaire was prepared for each patient, and the demographic characteristics of the mother (age and body mass index), gestational age at laser treatment, sonographic findings including the stage of TTTS, maximal vertical pocket (MVP) per fetus, the weight of the fetus, the weight discordance between twins, hydrops, placental position (anterior or other), and cervical length were recorded.
Fetal echocardiography was performed to evaluate cardiac abnormalities and changes in cardiac function parameters following TTTS. All Doppler studies were recorded for both fetuses with three measurements, and their average was calculated. The Doppler parameters evaluated included the pulsatility index (PI) of the UA, the end diastolic flow (EDF), the PI of the middle cerebral artery (MCA-PI), MCA-PSV, and the umbilical vein and ductus venosus (DV).
Measurements were made at the site of separation of the umbilical cord from the fetal abdominal wall to assess UA Doppler, and cases where the fetus was immobile and the mother was not breathing were defined as normal or abnormal (since EDF was absent or reversed). MCA Doppler was measured at one-third of the artery near the site of separation from the Willis ring, and MCV-PSV was examined as multiple media. To measure DV Doppler, the transverse or sagittal section of the fetal abdomen was evaluated and classified as normal or abnormal (absent or reverse). UA Doppler was also performed in the abdomen of the fetus and was classified as normal (without pulse) and abnormal (venous pulsations).
Statistical analysis
The study parameters were compared using the Chi-square test or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. Tests were deemed significant if the probability value was less than 0.05 using two-tailed tests. Logistic regression analysis was employed to identify variables that significantly contributed to predicting postnatal twin survival. A univariate analysis was conducted to scrutinize the individual variables contributing to survival, assessing their odds ratios and 95% confidence intervals. Following this, a multivariate logistic regression analysis with backward stepwise elimination was performed to ascertain variables that made a significant independent contribution to the logistic model. The data analysis was performed using the IBM SPSS statistical software package, version 19.0 (IBM Corporation, Armonk, NY).
| Characteristics | All pregnancies | |
|---|---|---|
| Number of pregnancies | 115 | |
| Maternal age (years ± SD) | 29.57±5.36 | |
| GA at laser (weeks ± SD) | 20.70±2.21 | |
| Cervical length(mm) | 33.36 ± 5.91 | |
| EFWD ≥ 25% | 67 (58.3) | |
| Donor absent or reversed EDV | 59(51.3) | |
| Donor DV absent or reversed a-wave | 5 (4.3) | |
| Placenta location (n (%)) | Anterior | 53(46.1%) |
| Other | 62 (53.9%) | |
| Quintero stage (n(%)) | II | 43(37.4%) |
| III | 64 (55.7%) | |
| IV | 8 (6.9%) | |
| GA at delivery (weeks ± SD) | 32.18±4.68 | |
| GA at delivery among live births (weeks ±SD) 32.73±3.91 | ||
| Live birth | None (n (%)) | 7(6.1%) |
| One (n (%)) | 38 (33.0%) | |
| Two (n (%)) | 70 (60.9%) | |
| Donor (n (%)) | 89 (77.4%) | |
| Recipient (n (%)) | 88 (76.5%) | |
| At least one (n (%)) | 108 (93.9%) | |
| D30 survival | Number of twin pairs | |
| None (n (%)) | 16(14 . 8%) | |
| One (n (%)) | 37 (32.2%) | |
| Two (n (%)) | 61 (53%) | |
| Donor (n (%)) | 79 (68.7%) | |
| Recipient (n (%)) | 80 (69.6%) | |
| At least one (n (%)) | 98 (85.2%) | |
| Recipient twin maximum vertical pocket cm (mean) | 14.58±3.0 5 | |
| Minimum | 8 | |
| Maximum | 19.5 | |
| SIUGR at diagnosis (n(%)) | 65 (56.5%) | |
| SIUGR classification | Type I (n(%)) | 43 (66.2%) |
| Type II (n(%)) | 14 (21.5%) | |
| Type III (n(%)) | 8 (12.3%) | |
| Variable | Donor fetus | Recipient fetus | ||||
| Survived (n = 89) | Non-survived (n = 26) | p-value | Survived (n= 88) | Non-survived (n= 27) | p- value | |
| Obstetric factors | ||||||
| Gestational age at laser therapy, weeks (range) | 20.8 (18.6- 26) | 20.3 (17.3- 25) | 0.2 | 20.7 (18.3- 26) | 20.6 (18.3- 23.7) | 0.9 |
| Cervical length, mm (range) | 33.2 (17.4- 40) | 33.9 (31- 45) | 0.7 | 33 (17.4- 46) | 34.7 (20- 48) | 0.1 |
| Anterior placenta, n (%) | 41 (40.6) | 12 (46.15) | 0.3 | 39 (44.31) | 14 (51.85) | 0.3 |
| Fetal factors, n (%) | ||||||
| Estimated fetal weight discordance ≥25% | 45 (50.56) | 22 (84.61) | 0.001 | 54 (61.4) | 13 (48.1) | 0. 3 |
| Donor umbilical artery absent or reversed end-diastolic velocity | 36 (40.44) | 23(86.46) | <0.001 | 38(43.18) | 16(59.25) | 0.03 |
| Donor ductus venous absent or reversed a-wave | 4 (4.49%) | 1 (3.84) | 0.6 | 2 (2.27) | 3 (11.11) | 0.8 |
| Recipient umbilical artery absent or reversed end-diastolic velocity, | 5 (5.61%) | 1 (3.84) | 0.5 | 5 (5.68) | 1 (3.70) | 0.5 |
| Recipient ductus venous absent or reversed a-wave | 3 (3.37) | 1 (3.84) | 0.6 | 4 (4.54) | 0 (0) | 0.3 |
| Variable | Univariate Analysis | ||
| Odds ratio | 95% Confidence Interval | P-value | |
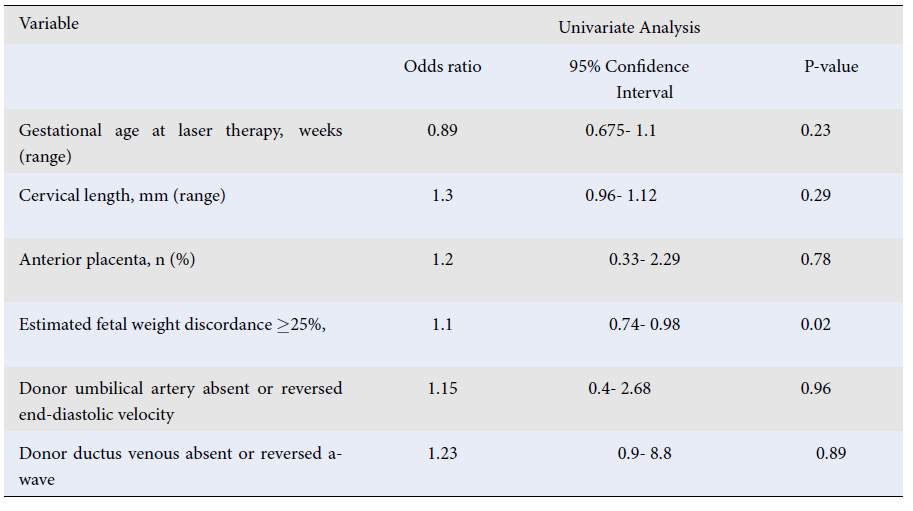
| Gestational age at laser therapy, weeks (range) | 0.89 | 0.675- 1.1 | 0.23 |
| Cervical length, mm (range) | 1.3 | 0.96- 1.12 | 0.29 |
| Anterior placenta, n (%) | 1.2 | 0.33- 2.29 | 0.78 |
| Estimated fetal weight discordance ≥25%, | 1.1 | 0.74- 0.98 | 0.02 |
| Donor umbilical artery absent or reversed end-diastolic velocity | 1.15 | 0.4- 2.68 | 0.96 |
| Donor ductus venous absent or reversed a-wave | 1.23 | 0.9- 8.8 | 0.89 |
Results
During the study period, our center performed FLC on 115 cases of TTTS (mean maternal age: 29.57 ± 5.36 years). Table 1 presents the primary obstetric and fetal traits of the study group. The mean gestational age at the time of laser treatment and the gestational age at delivery were 20.70 ± 2.21 weeks and 32.18 ± 4.68 weeks, respectively. Nineteen infants died after birth before 30 days. According to the Quintero classification, 43 patients had TTTS stage II (37.4%), 64 had TTTS stage III (55.7%), and eight had TTTS stage IV (6.9%).Doppler measurement was performed for all patients before and after the laser treatment except one due to rupture of the amniotic sac and abortion of the fetus after surgery. One case of twin anemia polycythemia sequence and one case of reverse TTTS were observed following FLS. Details of the pregnancy outcomes and neonatal survival demonstrated that the overall survival rate after 30 days with one, two, and at least one live birth was 32.2%, 53%, and 85.2%, respectively.Table 1 indicates that 59 patients, accounting for 51.3% of the cohort, exhibited preoperative absent or reversed end-diastolic velocity (EDV) of the donor twin, as determined by Doppler evaluation of the UA. Table 2 presents a comparison of preoperative obstetric findings in survivors versus instances of fetal or neonatal death. A significant relationship was observed between estimated fetal weight discordance ≥ 25% and survival of the donors (p < 0.001). Among the donor fetuses that did not survive, there was a notably higher occurrence of estimated fetal weight discrepancy of more than 25% with the co-twin. In addition, in non-surviving donor and recipient fetuses, the donor UA’s absent or reversed EDV was significantly higher (p < 0.05). Logistic regression analysis revealed a significant relationship between estimated fetal weight discordance with the co-twin > 25% (p < 0.05); see Table 3. In addition, no special neurological complications were observed after the 6-month follow-up of the patients.
Discussion
TTTS remains a major predictor of perinatal outcomes in monochorionic and diamniotic pregnancies. The best way to handle these pregnancies is yet to be determined15. Information on expectant management is derived from older literature sources with limited cases included; thus, the evidence’s quality is not high. In some countries, laser treatment is typically used for pregnancies from 16 to 26 weeks of gestation16.
In this study, we describe the preliminary outcomes of FLC of vascular anastomoses for severe TTTS. Perinatal survival rates in untreated severe TTTS have been found to vary between 20% and 37%17, 18, 19, 20. After laser treatment, an overall survival rate of 50–70% can be predicted, with the chance of aberrant neurodevelopmental results ranging between 4% and 18%21. In this research, the overall survival rate after 30 days with one, two, and at least one live birth was 32.2%, 53%, and 85.2%, respectively. These findings are consistent with those of other published studies. In a retrospective analysis of 340 cases with TTTS, Peeters et al. reported a survival rate of 86% and 59% for at least one twin and both twins, respectively22. Additionally, Anh et al. reported a total survival percentage of at least one infant of 84.85%23. In another study involving 181 women suffering from TTTS who were treated in Japanese clinics, the survival rates were reported to be 84% for at least one twin and 68% for both twins24. Delabaere et al.1 also reported that the overall Day 30 survival rate was 64.9%, with values of 51.9% with two infants, 26.0% with one infant, and 77.9% with at least one infant. The 30-day survival rate was 65.4% for donors and 64.4% for recipients, which aligns with the findings of the present study.
Several studies have evaluated the efficacy of FLP with various TTTS therapies. The Eurofetus trial showed that compared to serial amnioreduction, FLP treatment of Stage II or III TTTS-complicated pregnancies increased the odds that at least one twin would survive to 28 days and 6 months of age6. In a similar vein, a comprehensive review of 10 studies revealed that fetuses diagnosed with Stage II–III TTTS and treated with laser ablation had double the survival rate and an 80% decrease in neurological complications compared to those treated with serial amnioreduction. It is worth noting that TTTS was categorized using Quintero staging in eight of these studies25. TTTS is most common in the second trimester of pregnancy26. The mean gestational age at surgery was 20.70 weeks, with a standard deviation (SD) of 2.21 weeks. A comprehensive evaluation of 3868 pregnant women from 1995 to 2014 found that the mean gestational age at surgery was 20.9 weeks (SD: 1.9 weeks), consistent with our research and similar to earlier data. TTTS commonly emerges in the second trimester of pregnancy26. Anh et al. demonstrated that the mean gestational age at birth was 33.76 weeks, with an SD of 4.52 weeks23.
Neurological complications are another significant outcome of laser photocoagulation for TTTS that researchers report. Although treating TTTS with FPS enhances the survival rates of newborns, research indicates that neonatal outcomes may still be linked to neurological anomalies, including significant brain damage and neurodevelopmental disorders16. During neonatal follow-up observations up to 6 months postpartum, we found no neurological complications, which was consistent with the findings from a study conducted by Anh and colleagues23.
In cases of TTTS, the likelihood of preterm birth is invariably elevated due to factors such as multiple pregnancies, laparoscopic amniocentesis, and polyhydramnios, which increase the risk of premature delivery post-surgery. The average gestational age at birth in the present study was 32.18 weeks, with an SD of 4.68 weeks. This aligns with the gestational age of 32.4 weeks (± 1.3 weeks) reported in a study by Akkermans26. This holds significance as studies have indicated that the gestational age at the time of delivery can also forecast the risk of neonatal mortality24, 26, 27. However, the premature delivery rate for twins under 32 weeks is around 30% higher than for other uncomplicated twins. In the current study, 19 infant deaths occurred within 30 days of delivery; this high rate may be an indication of prematurity.
Doppler studies of fetal circulation can be used to measure cardiac function, and extremely abnormal venous flow velocity waveforms indicate congestive heart failure. We found a significant relationship between estimated fetal weight discordance ≥ 25% of the donors and survival (p < 0.001). In the case of donor fetuses that did not survive, there was a notably increased incidence of estimated fetal weight discrepancy of more than 25% with the co-twin, as well as abnormal umbilical Doppler readings. Weight discordance is a phenomenon that can be observed in all twin pregnancies and could be a natural occurrence or an adaptive response to the intrauterine environment. On the other hand, it could also be the result of pathological abnormalities in the fetus or the placenta. Twins experiencing severe growth discordance are at a heightened risk of perinatal mortality and morbidity28.
Furthermore, in the current study, a significantly higher donor UA absent or reversed EDV in non-surviving donor and recipient fetuses was noted. Some studies found that the presence or absence of EDV in the UA of the donor and reverse flow in the DV of the recipient was strongly related to donor survival24, 29, 30, 31, 32. Earlier studies have indicated that certain echocardiographic observations, including cardiac enlargement and dilation, irregularities in atrioventricular flow, an elevated myocardial performance index, and abnormal cardiac output, among others, can help identify a group of TTTS recipients who exhibit deteriorated cardiovascular function12, 33, 34. Stirnemann et al. found no significant difference between cardiac function outcomes and pregnancy outcomes13. Delabaere et al.1 found that UA-PI, cerebro-placental PI ratio, and UA EDF were preoperative predictors of infant survival for the recipient twin and donor right ventricular myocardial performance index (RV-MPI) and recipient UA EDF, and umbilical vein pulsations were postoperative indicators of donor survival. As can be observed, the published research reports conflicting results on the significance of an in-depth cardiovascular assessment in forecasting the survival of the recipient; hence, more research on this subject is needed13, 35, 36. However, Morris et al. found that gestational age was the only significant independent predictor of perinatal survival37.
Since the first FLS was performed 25 years ago, only a handful of institutions have specialized in minimally invasive fetal surgery. There is a broad consensus that fetoscopic laser photocoagulation is the optimal treatment for TTTS. Consequently, there is a need for an increase in the number of clinics offering fetoscopy. Despite the rarity of TTTS, several factors are crucial for achieving the best perinatal outcomes: the choice of surgical method, case selection, appropriate scheduling of operations, and sufficient postoperative follow-up. Furthermore, the establishment of dedicated units in tertiary institutions is required to maximize therapy for this condition38.
Most patients are admitted to this center for higher-stage treatment due to the distance and late diagnosis. Lack of data on long-term follow-up, especially due to the COVID-19 pandemic and the fact that laser treatment was performed at a single institution, might be a possible limitation of this study. Among the other limitations of this study, we can note that it was impossible to deliver all patients in specialist centers equipped to care for preterm newborns, which influences the patients’ outcomes.
Conclusions
This prospective study demonstrates that laser therapy improves survival rates and is an effective treatment option for TTTS. Its results underscore the effectiveness of FLS as a treatment modality for TTTS, particularly when the condition is diagnosed before the 26th week of pregnancy. The study also highlights the importance of Doppler measurements in monitoring the health of the fetuses post-surgery. Therefore, this approach could potentially guide clinicians in tailoring treatment strategies for TTTS. The higher incidence of absent or reversed EDV in the donor umbilical artery among survivors is another key finding that warrants further investigation and could potentially serve as a prognostic marker for clinicians.
Abbreviations
TTTS: Twin-to-Twin Transfusion Syndrome, FLS: Fetoscopic Laser Surgery, FLC: Fetoscopic Laser Coagulation, UA: Umbilical Artery, EDV: End-Diastolic Velocity, MCA: Middle Cerebral Artery, PI: Pulsatility Index, PSV: Peak Systolic Velocity, DV: Ductus Venosus, MVP: Maximal Vertical Pocket, SD: Standard Deviation, RV-MPI: Right Ventricular Myocardial Performance Index
Acknowledgments
None.
Author’s contributions
Conceptualization, N.M. and V.M.; methodology, N.M.; software, L.E.; validation, A.J. and M.Nu.; formal analysis, M.S. and N.M.; investigation, M.Na. and A.A.; resources, S.A.; data curation, A.A.; writing—original draft preparation, M.S. and A.A.; writing—review and editing, N.M., V.M., L.E. and A.J.; visualization, M.Nu. and M.Na; supervision, S.A.; project administration, N.M.; funding acquisition, N.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by Tehran University of Medical Sciences (Project code: 9711694009).
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The research design was approved by the ethical committee and institutional review board of Tehran University of Medical Sciences (IR.TUMS.MEDICINE>REC.1399.137).
Consent for publication
Informed consent was obtained from the parents of the children involved in this study.
Competing interests
The authors declare that they have no competing interests.
References
-
Delabaere
A.,
Leduc
F.,
Reboul
Q.,
Fuchs
F.,
Wavrant
S.,
Fouron
J.C.,
Prediction of neonatal outcome of TTTS by fetal heart and Doppler ultrasound parameters before and after laser treatment. Prenatal Diagnosis.
2016;
36
(13)
:
1199-205
.
View Article PubMed Google Scholar -
Simpson
L.L.,
Twin-twin transfusion syndrome. American Journal of Obstetrics and Gynecology.
2013;
208
(1)
:
3-18
.
View Article PubMed Google Scholar -
Huber
A.,
Diehl
W.,
Zikulnig
L.,
Bregenzer
T.,
Hackelöer
B.J.,
Hecher
K.,
Perinatal outcome in monochorionic twin pregnancies complicated by amniotic fluid discordance without severe twin-twin transfusion syndrome. Ultrasound in Obstetrics & Gynecology.
2006;
27
(1)
:
48-52
.
View Article PubMed Google Scholar -
Casella
A.,
Moccia
S.,
Frontoni
E.,
Paladini
D.,
De Momi
E.,
Mattos
L.S.,
Inter-foetus membrane segmentation for TTTS using adversarial networks. Annals of Biomedical Engineering.
2020;
48
(2)
:
848-59
.
View Article PubMed Google Scholar -
Banek
C.S.,
Hecher
K.,
Hackeloer
B.J.,
Bartmann
P.,
Long-term neurodevelopmental outcome after intrauterine laser treatment for severe twin-twin transfusion syndrome. American Journal of Obstetrics and Gynecology.
2003;
188
(4)
:
876-80
.
View Article PubMed Google Scholar -
Senat
M.V.,
Deprest
J.,
Boulvain
M.,
Paupe
A.,
Winer
N.,
Ville
Y.,
Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. The New England Journal of Medicine.
2004;
351
(2)
:
136-44
.
View Article PubMed Google Scholar -
Quintero
R.A.,
Dickinson
J.E.,
Morales
W.J.,
Bornick
P.W.,
Bermúdez
C.,
Cincotta
R.,
Stage-based treatment of twin-twin transfusion syndrome. American Journal of Obstetrics and Gynecology.
2003;
188
(5)
:
1333-40
.
View Article PubMed Google Scholar -
Quintero
R.A.,
Morales
W.J.,
Allen
M.H.,
Bornick
P.W.,
Johnson
P.K.,
Kruger
M.,
Staging of twin-twin transfusion syndrome. Journal of Perinatology.
1999;
19
(8 Pt 1)
:
550-5
.
View Article PubMed Google Scholar -
Saunders
N.J.,
Snijders
R.J.,
Nicolaides
K.H.,
Twin-twin transfusion syndrome during the 2nd trimester is associated with small intertwin hemoglobin differences. Fetal Diagnosis and Therapy.
1991;
6
(1-2)
:
34-6
.
View Article PubMed Google Scholar -
Simpson
L.L.,
Marx
G.R.,
Elkadry
E.A.,
D'Alton
M.E.,
Cardiac dysfunction in twin-twin transfusion syndrome: a prospective, longitudinal study. Obstetrics and Gynecology.
1998;
92
(4 Pt 1)
:
557-62
.
View Article PubMed Google Scholar -
Rychik
J.,
Zeng
S.,
Bebbington
M.,
Szwast
A.,
Quartermain
M.,
Natarajan
S.,
Speckle tracking-derived myocardial tissue deformation imaging in twin-twin transfusion syndrome: differences in strain and strain rate between donor and recipient twins. Fetal Diagnosis and Therapy.
2012;
32
(1-2)
:
131-7
.
View Article PubMed Google Scholar -
Rychik
J.,
Tian
Z.,
Bebbington
M.,
Xu
F.,
McCann
M.,
Mann
S.,
The twin-twin transfusion syndrome: spectrum of cardiovascular abnormality and development of a cardiovascular score to assess severity of disease. American Journal of Obstetrics and Gynecology.
2007;
197
(4)
:
392. e1-. e8
.
View Article Google Scholar -
Stirnemann
J.J.,
Mougeot
M.,
Proulx
F.,
Nasr
B.,
Essaoui
M.,
Fouron
J.C.,
Profiling fetal cardiac function in twin-twin transfusion syndrome. Ultrasound in Obstetrics & Gynecology.
2010;
35
(1)
:
19-27
.
View Article PubMed Google Scholar -
Mieghem
T. Van,
Doné
E.,
Gucciardo
L.,
Klaritsch
P.,
Allegaert
K.,
Bree
R. Van,
Amniotic fluid markers of fetal cardiac dysfunction in twin-to-twin transfusion syndrome. American Journal of Obstetrics and Gynecology.
2010;
202
(1)
:
48. e1-. e7
.
View Article Google Scholar -
Khalil
A.,
Liu
B.,
Controversies in the management of twin pregnancy. Ultrasound in Obstetrics & Gynecology.
2021;
57
(6)
:
888-902
.
View Article PubMed Google Scholar -
Roberts
D.,
Neilson
J.P.,
Kilby
M.D.,
Gates
S.,
Interventions for the treatment of twin‐twin transfusion syndrome. Cochrane Database of Systematic Reviews..
2014;
2014
(1)
:
CD002073
.
View Article Google Scholar -
Haverkamp
F.,
Lex
C.,
Hanisch
C.,
Fahnenstich
H.,
Zerres
K.,
Neurodevelopmental risks in twin-to-twin transfusion syndrome: preliminary findings. European Journal of Paediatric Neurology.
2001;
5
(1)
:
21-7
.
View Article PubMed Google Scholar -
Lewi
L.,
Schoubroeck
D. Van,
Gratacós
E.,
Witters
I.,
Timmerman
D.,
Deprest
J.,
Monochorionic diamniotic twins: complications and management options. Current Opinion in Obstetrics & Gynecology.
2003;
15
(2)
:
177-94
.
View Article PubMed Google Scholar -
Middeldorp
J.M.,
Sueters
M.,
Lopriore
E.,
Klumper
F.J.,
Oepkes
D.,
Devlieger
R.,
Fetoscopic laser surgery in 100 pregnancies with severe twin-to-twin transfusion syndrome in the Netherlands. Fetal Diagnosis and Therapy.
2007;
22
(3)
:
190-4
.
View Article PubMed Google Scholar -
Gemert
M.J. van,
Umur
A.,
Tijssen
J.G.,
Ross
M.G.,
Twin-twin transfusion syndrome: etiology, severity and rational management. Current Opinion in Obstetrics & Gynecology.
2001;
13
(2)
:
193-206
.
View Article PubMed Google Scholar -
D'Antonio
F.,
Benlioglu
C.,
Sileo
F.G.,
Thilaganathan
B.,
Papageorghiou
A.,
Bhide
A.,
Perinatal outcomes of twin pregnancies affected by early twin-twin transfusion syndrome: A systematic review and meta-analysis. Acta Obstetricia et Gynecologica Scandinavica.
2020;
99
(9)
:
1121-34
.
View Article PubMed Google Scholar -
Peeters
S.H.,
Van Zwet
E.W.,
Oepkes
D.,
Lopriore
E.,
Klumper
F.J.,
Middeldorp
J.M.,
Learning curve for fetoscopic laser surgery using cumulative sum analysis. Acta Obstetricia et Gynecologica Scandinavica.
2014;
93
(7)
:
705-11
.
View Article PubMed Google Scholar -
Duy Anh
N.,
Duy An
N.,
Huyen Thuong
P.T.,
Thu Ha
N.T.,
Sim
N.T.,
Sy Hung
H.,
The Efficacy of Fetoscopic Laser Surgery in Twin-Twin Transfusion Syndrome: A Preliminary Vietnamese Study. La Clinica Terapeutica.
2022;
173
(3)
:
265-73
.
PubMed Google Scholar -
Sago
H.,
Hayashi
S.,
Saito
M.,
Hasegawa
H.,
Kawamoto
H.,
Kato
N.,
The outcome and prognostic factors of twin-twin transfusion syndrome following fetoscopic laser surgery. Prenatal Diagnosis.
2010;
30
(12-13)
:
1185-91
.
View Article PubMed Google Scholar -
Rossi
A.C.,
D'Addario
V.,
Laser therapy and serial amnioreduction as treatment for twin-twin transfusion syndrome: a metaanalysis and review of literature. American Journal of Obstetrics and Gynecology.
2008;
198
(2)
:
147-52
.
View Article PubMed Google Scholar -
Akkermans
J.,
Peeters
S.H.,
Klumper
F.J.,
Lopriore
E.,
Middeldorp
J.M.,
Oepkes
D.,
Twenty-five years of fetoscopic laser coagulation in twin-twin transfusion syndrome: a systematic review. Fetal Diagnosis and Therapy.
2015;
38
(4)
:
241-53
.
View Article PubMed Google Scholar -
Glennon
C.L.,
Shemer
S.A.,
Palma-Dias
R.,
Umstad
M.P.,
The history of treatment of twin-to-twin transfusion syndrome. Twin Research and Human Genetics.
2016;
19
(3)
:
168-74
.
View Article PubMed Google Scholar -
D'Antonio
F.,
Khalil
A.,
Dias
T.,
Thilaganathan
B.,
Bahamie
A.,
Bhide
A.,
(STORK)
Southwest Thames Obstetric Research Collaborative,
Weight discordance and perinatal mortality in twins: analysis of the Southwest Thames Obstetric Research Collaborative (STORK) multiple pregnancy cohort. Ultrasound in Obstetrics & Gynecology.
2013;
41
(6)
:
643-8
.
View Article PubMed Google Scholar -
Chang
Y.L.,
Chmait
R.H.,
Bornick
P.W.,
Allen
M.H.,
Quintero
R.A.,
The role of laser surgery in dissecting the etiology of absent or reverse end-diastolic velocity in the umbilical artery of the donor twin in twin-twin transfusion syndrome. American Journal of Obstetrics and Gynecology.
2006;
195
(2)
:
478-83
.
View Article PubMed Google Scholar -
Ishii
K.,
Hayashi
S.,
Nakata
M.,
Murakoshi
T.,
Sago
H.,
Tanaka
K.,
Ultrasound assessment prior to laser photocoagulation for twin-twin transfusion syndrome for predicting intrauterine fetal demise after surgery in Japanese patients. Fetal Diagnosis and Therapy.
2007;
22
(2)
:
149-54
.
View Article PubMed Google Scholar -
Kontopoulos
E.V.,
Quintero
R.A.,
Chmait
R.H.,
Bornick
P.W.,
Russell
Z.,
Allen
M.H.,
Percent absent end-diastolic velocity in the umbilical artery waveform as a predictor of intrauterine fetal demise of the donor twin after selective laser photocoagulation of communicating vessels in twin-twin transfusion syndrome. Ultrasound in Obstetrics & Gynecology.
2007;
30
(1)
:
35-9
.
View Article PubMed Google Scholar -
Martínez
J.M.,
Bermúdez
C.,
Becerra
C.,
López
J.,
Morales
W.J.,
Quintero
R.A.,
The role of Doppler studies in predicting individual intrauterine fetal demise after laser therapy for twin-twin transfusion syndrome. Ultrasound in Obstetrics {&}amp; Gynecology.
2003;
22
(3)
:
246-51
.
View Article PubMed Google Scholar -
Habli
M.,
Michelfelder
E.,
Cnota
J.,
Wall
D.,
Polzin
W.,
Lewis
D.,
Prevalence and progression of recipient-twin cardiomyopathy in early-stage twin-twin transfusion syndrome. Ultrasound in Obstetrics & Gynecology.
2012;
39
(1)
:
63-8
.
View Article PubMed Google Scholar -
Mieghem
T. Van,
Klaritsch
P.,
Doné
E.,
Gucciardo
L.,
Lewi
P.,
Verhaeghe
J.,
Assessment of fetal cardiac function before and after therapy for twin-to-twin transfusion syndrome. American journal of obstetrics and gynecology.
2009;
200
(4)
:
400. e1-. e7
.
View Article Google Scholar -
Gapp-Born
E.,
Sananes
N.,
Weingertner
A.S.,
Guerra
F.,
Kohler
M.,
Fritz
G.,
Predictive value of cardiovascular parameters in twin-to-twin transfusion syndrome. Ultrasound in Obstetrics {&}amp; Gynecology.
2014;
44
(4)
:
427-33
.
View Article PubMed Google Scholar -
Van Mieghem
T.,
Martin
A.M.,
Weber
R.,
Barréa
C.,
Windrim
R.,
Hornberger
L.K.,
Fetal cardiac function in recipient twins undergoing fetoscopic laser ablation of placental anastomoses for Stage IV twin-twin transfusion syndrome. Ultrasound in Obstetrics {&}amp; Gynecology.
2013;
42
(1)
:
64-9
.
View Article PubMed Google Scholar -
Morris
R.K.,
Selman
T.J.,
Harbidge
A.,
Martin
W.I.,
Kilby
M.D.,
Fetoscopic laser coagulation for severe twin-to-twin transfusion syndrome: factors influencing perinatal outcome, learning curve of the procedure and lessons for new centres. BJOG.
2010;
117
(11)
:
1350-7
.
View Article PubMed Google Scholar -
Choudhry
N.K.,
Fletcher
R.H.,
Soumerai
S.B.,
Systematic review: the relationship between clinical experience and quality of health care. Annals of Internal Medicine.
2005;
142
(4)
:
260-73
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 2 (2024)
Page No.: 6224-6232
Published on: 2024-02-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 3411 times
- PDF downloaded - 1122 times
- XML downloaded - 107 times
 Biomedpress
Biomedpress

