Abstract
Introduction: The para-Bombay blood phenotype is a rare blood group variant, with a limited number of cases reported worldwide.
Case Summary: Here, we present the case of a pregnant woman with clinically significant anti-IH antibodies and incompatible cross-matching with group O packed cells detected during pre-transfusion testing. Three years later, samples were collected again from the subject and showed that her anti-IH antibody levels had reduced. Her para-Bombay blood type was then confirmed using a polymerase chain reaction with sequence-specific primers of the ABO gene and Sanger sequencing of the FUT1 and FUT2 genes. This patient can only undergo transfusion therapy with Bombay or para-Bombay matched blood group RBCs due to the pre-formed anti-IH in her serum.
Conclusion: The systematic identification of blood groups and antibodies helped prevent transfusion-related adverse events and contributed to safe transfusion practice.
Introduction
Individuals with the para-Bombay blood phenotype lack clinically relevant H, A, and B antigens on their red blood cell (RBC) membranes. This is due to the inactivation of the FUT1 gene, which codes for H antigen, a precursor necessary to synthesize A and B antigens present on RBCs. However, the FUT2 gene that codes for H antigen in body secretions remains active in para-Bombay individuals1, 2. Therefore, a weak H-like antibody, termed anti-IH, which is reactive at low temperatures, is usually present in the serum of para-Bombay individuals.
Patient Information
A 29-year-old Malay primigravid woman at 38 weeks of gestation presented with false labor. A blood sample was collected and subjected to group screen and hold (GSH).
Forward and reverse grouping at room temperature was performed via column agglutination and revealed the sample to be group O and RhD positive. The blood sample was then subjected to standard immunohematological methods for RBC antibody screening and identification using the DiaMed ID gel microtyping system (Diamed ID-Diacell, Diamed, Switzerland). Antibody screening revealed 3+ reactions with all three screening cells. Antibody identification tests showed pan-agglutination with the 11 panel cells but a negative result for the auto-control. A polyspecific direct Coombs test (DCT) was also negative. Cross-matching at the anti-human globulin (AHG) phase showed incompatibility with five units of group O RhD-positive packed cells. Further tests were performed using anti-H lectin and O cells, where only 3+ reactions with O cells were observed. An adsorption-elution test returned negative results for A and B antigens. Further testing with O Bombay and O cord blood was not performed due to the unavailability of these types of red cells. A secretor saliva test was also not performed because the patient had been discharged home and had given birth at another hospital. She did not need any transfusion throughout this admission. At this point, her blood sample was diagnosed as para-Bombay with anti-H/IH antibody.
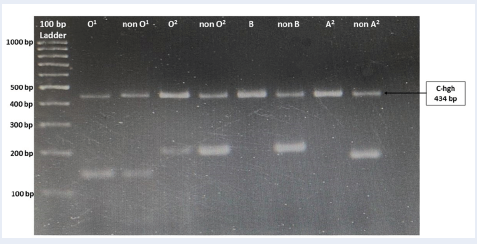
The patient returned 3 years later to enquire further about her rare blood group because she planned to conceive. The immunohematological workup was repeated. Extended ABO grouping revealed a similar pattern for forward and reverse grouping, except that interaction with O cells was not detected. However, after the serum-to-cell ratio was increased, a reaction of 2+ with O cell was seen at 4 °C, room temperature, and 37 °C. The saliva secretor test showed positivity for H substance; thus, the patient was tested as a secretor (i.e., Leb antigen-positive). A repeat adsorption-elution also produced similar results. A test with O cord blood (with an increased serum-to-cell ratio) showed negative results at room temperature, 4 °C, and the AHG phase. The results showed the presence of H antigen with the Le (a-b+) phenotype (Table 1). Serologically, this case was concluded with the patient’s diagnosis as para-Bombay with the presence of anti-IH. However, the latter anti-IH antibody reaction was weaker than the previous reaction. Genomic DNA from the patient blood sample was extracted using a commercially available kit (Bio Basic, Canada). The genomic DNA was then subjected to ABO, FUT1, and FUT2 genotyping using polymerase chain reaction with sequence-specific primers (PCR-SSP) and Sanger sequencing techniques. She was observed to have the O1/O2 genotype (i.e., O blood group, and refer to Figure 1).
However, she was homozygous for the 328G>A (Ala110Thr) missense mutation (i.e., the FUT*01W.02 allele) and the 390C>T (Asn130) synonymous mutation in the FUT1 and FUT2 genes, respectively (Figure 2, Figure 3). Thus, genotyping analyses revealed that the patient had the para-Bombay blood type.
| 2018 | 2021 | |
|---|---|---|
| ABO and Rh grouping (gel card method) | ||
| Anti-A | 0 | 0 |
| Anti-B | 0 | 0 |
| Anti-H lectin | 0 | 0 |
| Anti-D | 4+ | 4+ |
| A1 cell | 4+ | 4+ |
| A2 cell | 4+ | 4+ |
| B cell | 4+ | 4+ |
| O cell (4 °C, room RT and 37 °C) | 3+ | 0 2+ (increase serum to cell ratio) |
| Testing plasma with red cell of O cord blood (4 °C, RT and 37 °C) | ND | 0 (increase serum to cell ratio) |
| Antibody screening (3 cell panels) | 3+ for all panel cells | Negative for all panel cells |
| Antibody identification (11 cell panels) | 3+ for all panel cells | Negative for all panel cells |
| Autocontrol | Negative | Negative |
| Adsorption-Elution test | Negative. | Negative |
| Saliva secretor test | ND | Presence of H substance |
| RBC phenotyping | ||
| Lea | Negative | ND |
| Leb | Positive | ND |
Discussion
In this report, we identified a FUT1 missense mutation (328G>A) in a patient with the para-Bombay O phenotype, which results from H antigen deficiency (Figure 2). Serologically, only H antigen could be detected in the saliva test, and genotyping showed a synonymous mutation (Se390Se390) in the FUT2 gene (Figure 3)3, 4. ABO, FUT1, and FUT2 genotyping was performed using PCR-SSP [1] and Sanger sequencing2.
The FUT1 and FUT2 genes (both located on chromosome 19) code for enzymes responsible for the synthesis of H antigen in hemopoietic tissues and body secretions (e.g., saliva and sweat), respectively. H antigen is then converted to A or B antigen encoded by the A and B genes on chromosome 9. The A and B genes are active in both hemopoietic and endodermal tissues5. Therefore, mutational events in FUT1 and FUT2 genes may produce the Bombay and para-Bombay phenotypes and carriers that will produce isoantibodies against A, B, and H antigens following transfusion or gestation6, 7. The Bombay and para-Bombay phenotypes are caused by inactivating mutations of both the FUT1 and FUT2 genes and the FUT1 gene only, respectively8.
Individuals with para-Bombay secretors (hh/Sese or hh/SeSe) lack H antigen on their RBCs but not in body secretions. Para-Bombay individuals generally preserve some H antigen on RBCs, with weak anti-H activity, which is frequently observed only at 4 °C or by using absorption and elution methods9. However, in this case, anti-IH had wide thermal amplitude, reacting at 37 °C in the AHG phase, which made it clinically significant, as previously reported in several hemolytic transfusion reaction cases10, 11, 12.
Conclusion
Benign cold agglutinins, if left undetected, may occasionally become clinically significant antibodies causing hemolytic transfusion reactions. Women with red cell antibodies, especially those with a risk of fetal anemia or cases where a compatible red cell donor for transfusion may be difficult to find, should undergo pre-pregnancy counseling with an expert clinician. The role of transfusion medicine specialists in the categorization of antibodies and the selection of suitable blood is vital to uphold safe transfusion practices. The efficient analysis of all blood group discrepancies, together with comprehensive immunohematological testing, ensures a reputable and vibrant role in an arrangement for transfusion therapy in individuals with rare phenotypes.
Abbreviations
DCT: direct Coombs test; RT: room temperature; ND: not done; RBC: red blood cell; GSH: group screen and hold; AHG: anti-human globulin; PCR-SSP: polymerase chain reaction with sequence-specific primers
Acknowledgments
We would like to thank all the staff at the Universiti Sains Malaysia, Kubang Kerian, Kelantan.
Author’s contributions
Conceptualization: Nur Nasuha Ibrahim and Noor Haslina Mohd Noor Methodology: Che Ghazali Norul Hajar, Siti Nor Assyuhada Mat-Ghani, Xiao Hong Cai and Hisham Atan Edinur. Formal analysis: Salfarina Iberahim, Zefarina Zulkafli and Shafini Mohamed Yusoff. Writing—original draft preparation: Nur Nasuha Ibrahim and Noor Haslina Mohd Noor. Writing—review and editing: Rosnah Bahar, Marini Ramli, Wan Suriana Wan Ab Rahman, and Marne Abdullah. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Gassner
C.,
Schmarda
A.,
Nussbaumer
W.,
Schönitzer
D.,
ABO glycosyltransferase genotyping by polymerase chain reaction using sequence-specific primers. Blood.
1996;
88
(5)
:
1852-6
.
View Article PubMed Google Scholar -
Yip
S.P.,
Chee
K.Y.,
Chan
P.Y.,
Chow
E.Y.,
Wong
H.F.,
Molecular genetic analysis of para-Bombay phenotypes in Chinese: a novel non-functional FUT1 allele is identified. Vox Sanguinis.
2002;
83
(3)
:
258-62
.
View Article PubMed Google Scholar -
Guo
Z.H.,
Xiang
D.,
Zhu
Z.Y.,
Wang
J.L.,
Zhang
J.M.,
Liu
X.,
[Analysis on FUT1 and FUT2 gene of 10 para-Bombay individuals in China]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi.
2004;
21
(5)
:
417-21
.
PubMed Google Scholar -
Kim
M.S.,
Kim
J.S.,
Park
H.,
Chung
Y.,
Kim
H.,
Ko
D.H.,
The First Case of Para-Bombay Blood Type Encountered in a Korean Tertiary Hospital. Journal of Korean Medical Science.
2019;
34
(39)
:
e258
.
View Article PubMed Google Scholar -
Saleh
R.M.,
Zefarina
Z.,
Che Mat
N.F.,
Chambers
G.K.,
Edinur
H.A.,
Transfusion Medicine and Molecular Genetic Methods. International Journal of Preventive Medicine.
2018;
9
(1)
:
45
.
View Article PubMed Google Scholar -
Lei
H.,
Shen
Y.,
Wang
Y.,
Su
N.,
Wang
X.,
Cai
X.,
A Para-Bombay Blood Group Case Associated with a Novel FUT1 Mutation c.361G>A. Transfusion Medicine and Hemotherapy ; Offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie.
2021;
48
(4)
:
254-8
.
View Article PubMed Google Scholar -
Cai
X.H.,
Fang
C.J.,
Jin
S.,
Liu
X.,
Fan
L.F.,
Yang
X.P.,
H blood-group deficiency has a high frequency in Lahu Chinese. Transfusion Medicine (Oxford, England).
2011;
21
(3)
:
209-10
.
View Article PubMed Google Scholar -
Cai
X.H.,
Jin
S.,
Liu
X.,
Fan
L.F.,
Lu
Q.,
Xiang
D.,
Molecular genetic analysis for the para-Bombay blood group revealing two novel alleles in the FUT1 gene. Blood Transfusion.
2011;
9
(4)
:
466-8
.
View Article PubMed Google Scholar -
Chacko
M.P.,
Mathan
A.,
Daniel
D.,
Para-Bombay: A blind spot in blood grouping?. Asian Journal of Transfusion Science.
2011;
5
(2)
:
182-3
.
View Article PubMed Google Scholar -
Darabi
K.,
Makar
R.S.,
Acute hemolysis of transfused A2 red cells by an auto-HI antibody. Transfusion.
2008;
48
(5)
:
964-8
.
View Article PubMed Google Scholar -
Chenna
D.,
Shastry
S.,
Kandasamy
D.,
Guduri
P.R.,
Anti-IH: An Innocuous Antibody Complicating Pre-Transfusion Testing. Malaysian J Med Health Sci.
2020;
14
:
391-3
.
-
Ibanez
C.,
Habibi
A.,
Mekontso-Dessap
A.,
Chadebech
P.,
Chami
B.,
Bierling
P.,
Anti-HI can cause a severe delayed hemolytic transfusion reaction with hyperhemolysis in sickle cell disease patients. Transfusion.
2016;
56
(7)
:
1828-33
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 1 (2024)
Page No.: 6142-6145
Published on: 2024-01-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3479 times
- PDF downloaded - 1295 times
- XML downloaded - 112 times
 Biomedpress
Biomedpress




