Abstract
Background: Thalassemia is a common inherited hemoglobin disorder in Malaysia. Regular transfusions of packed red cells are required to treat transfusion-dependent thalassemia. These transfusions, although lifesaving, may lead to complications such as red blood cell (RBC) alloantibody formation. This study aimed to determine the incidence of RBC alloantibodies in thalassemia patients who regularly received blood transfusions at our center.
Methods: This study included 33 thalassemia patients who received regular transfusions at Dungun Hospital, Terengganu, Malaysia, from 2017 to 2023. Laboratory data and clinical presentation information were obtained. Antibody screening was conducted to identify potential alloantibodies that could react with donor blood. All samples that tested positive for alloantibodies were investigated further to determine antibody specificity.
Results: The rate of RBC alloimmunization in the studied thalassemia patients was 36.4%. Six alloantibodies were detected in the patient cohort, with some individuals developing antibodies of single or multiple specificities. The most frequently present alloantibodies were against the Rh blood group system.
Conclusion: The development of alloantibodies related to blood transfusions can create challenges in managing transfusion-dependent thalassemia patients. It is advisable to incorporate extended-matched phenotyping into care protocols to mitigate the risk of alloimmunization and minimize the chances of these patients developing blood transfusion-related alloantibodies.
Introduction
Thalassemia is a most common autosomal recessive disorder that affects a significant proportion of the population in tropical countries like Malaysia. As per current estimates, around 6.8% of Malaysians are thalassemia carriers, which puts them at risk of developing anemia to varying degrees1. Hb E thalassemia and beta thalassemia are the predominant inherited hematological disorders of beta-globin in Malaysia2, 3.
Patients with severe thalassemia rely on blood transfusions every 3–4 weeks to treat anemia4. According to the current guidelines of the Thalassemia International Federation, blood transfusions are recommended for pre-transfusion Hb levels of 9–10 g/dL in patients with transfusion-dependent thalassemia5. Recent studies have shown that the incidence of red cell alloantibodies is higher among thalassemia patients than in the general population, which could be attributed to the frequent blood transfusions required by thalassemia patients6. Such alloantibodies arise when the immune system recognizes surface antigens on donor RBCs as foreign, thereby triggering the production of antibodies targeting these antigens4.
The existence of alloantibodies can add complexity by delaying the process of matching blood for transfusion and potentially increasing the risk of hemolytic transfusion reactions. Managing these risks during transfusions becomes even more challenging when autoantibodies coexist with alloantibodies7.
Thalassemia patients need to be closely monitored for the development of alloantibodies, as this can increase the risk of transfusion reactions and make it more difficult to find compatible blood for future transfusions. Regular blood tests and screening for alloantibodies should be performed to ensure the safety and effectiveness of transfusions for thalassemia patients.
Hence, this study aimed to (1) determine the incidence of RBC alloantibodies and (2) identify the types of alloantibodies among thalassemia patients who received regular blood transfusions at our center, Dungun Hospital, Terengganu, Malaysia. Knowing the incidence of alloantibodies among thalassemia patients will assist clinicians and transfusions in predicting the level of difficulty in searching for compatible blood.
| Demographic data | Frequency | Percentage |
| Gender | ||
| Male | 14 | 42.4 |
| Female | 19 | 57.6 |
| Age | ||
| < 12 years | 12 | 36.4 |
| 12 – 18 years | 7 | 21.2 |
| >18 years | 42.4 | |
| Diagnosis | ||
| Beta thalassemia major | 11 | 33.3 |
| Alpha thalassemia | 3 | 9.1 |
| Hb E Beta thalassemia | 15 | 45.5 |
| Hb Adana Pakse | 2 | 6.1 |
| Beta thalassemia trait with ESRF | 1 | 3.0 |
| Hb H Constant Spring | 1 | 3.0 |
| Numbers of transfusions | ||
| < 50 | 20 | 60.6 |
| 51 - 100 | 11 | 33.3 |
| 101 - 150 | 1 | 3.0 |
| >150 | 1 | 3.0 |
| Blood group | ||
| A | 8 | 24.2 |
| B | 8 | 24.2 |
| AB | 4 | 12.1 |
| O | 13 | 39.4 |
| Alloantibody specificities | Number (Percentage %) |
| Anti-E | 4 (33.3) |
| Anti-Jka | 1 (8.3) |
| Anti-Mia | 1 (8.3) |
| Anti-E, anti-c | 4 (33.3) |
| Anti-E, anti-Jka | 1 (8.3) |
| Anti-E, anti-c, anti-Leb | 1 (8.3) |
| Parameters | Antibody | P - value | |
| Positive n (%) | Negative n (%) | ||
| Gender | |||
| Male | 6 (42.9) | 8 (57.1) | 0.506 |
| Female | 6 (31.6) | 13 (64.8) | |
| Age | |||
| <12 years | 4 (33.3) | 8 (66.7) | 0.915 |
| 12 – 18 years | 3 (42.29) | 4 (57.1) | |
| >18 years | 5 (35.7) | 9 (64.3) | |
| Number of transfusions | |||
| <50 | 6 (30.0) | 14 (70.0) | 0.382 |
| 51-100 | 5 (45.5) | 6 (54.5) | |
| 101-150 | 0 (0.0) | 1 (100.0) | |
| >150 | 1 (100.0) | 0 (0.0) | |
| Blood group | |||
| A | 4 (50.0) | 4 (50.0) | 0.581 |
| B | 3 (37.5) | 5 (62.5) | |
| AB | 2 (50.0) | 2 (50.0) | |
| O | 3 (23.1) | 10 (76.9) | |
Materials and Methods
Study design
This retrospective study was conducted in the Pathology Unit of Dungun Hospital from 2017 to 2023. Secondary data from thalassemia patients who received regular transfusions and were screened for alloantibody formation were collected. A total of 33 patients were enrolled, and their demographic data, including gender, age, blood group, total number of transfusion units, frequency of transfusion, and clinical diagnosis, were extracted from the e-Delphyn Blood Bank System. The inclusion criteria for this study were patients with thalassemia who had previously received at least one blood transfusion. Patients with thalassemia who had already passed away were excluded. Approval for this study was obtained from the Medical Research and Ethics Committee (MREC) of the Ministry of Health Malaysia (ID: NMRR ID-22-02377-NMR). The study was conducted following the principles outlined in the Declaration of Helsinki.
Laboratory procedures
Blood samples were collected from patients in EDTA tubes for ABO/RhD blood grouping and antibody screening/identification following standard protocols. ABO and RhD blood groupings were done using the ABO-D/reverse grouping system (Bio-Rad Laboratories, DiaMed GmbH, Cressier, Switzerland). Antibody screening and identification were performed to detect alloantibodies that may react with donor blood. The screening was done using a three-cell panel (Diacell, Bio-Rad, Switzerland) in three phases: immediate spin, 37 °C, and anti‑human globulin. All samples that tested positive for alloantibodies underwent further investigation to ascertain antibody specificity using a commercial 11-cell identification panel (Diapanel, Bio-Rad, Cressier sur Morat, Switzerland). Regarding the transfusion policy, all patients received ABO and Rh(D) crossmatch-compatible blood transfusions. If alloantibodies were detected, antigen-negative crossmatch-compatible blood was given.
Statistical analysis
All data were analyzed using the Statistical Package for the Social Sciences (SPSS) software version 26. The prevalence of RBC alloimmunization and the alloantibody specificities were described using descriptive statistics. A chi-squared analysis was performed to evaluate the correlation of gender, age, number of blood transfusions, and blood group with the development of alloantibodies.
Results
As seen in Table 1, our study involved 33 transfusion-dependent thalassemia patients comprising 14 (42.4%) men and 19 (57.6%) women aged between 4 and 62 years. The majority of patients were 18 years or older. Of the 33 patients dependent on transfusion, 15 (45.5%) had Hb E beta thalassemia and 11 (33.3%) had beta thalassemia major. The quantity of packed cells transfused to patients in this study ranged from 10 to 646 units. Most of the patients (20 [60.6%]) had received <50 units of packed cells. The frequencies of the ABO blood groups were as follows: 13 (39.4%) group O, eight (24.2%) group A, eight (24.2%) group B, and four (12.1%) group AB. All patients were RhD antigen positive.
Of the 33 patients screened for alloantibodies, 12 (36.4%) tested positive (Figure 1). The prevalence of alloimmunization was found to be 36.4% (95% CI: 2.80–5.75%).
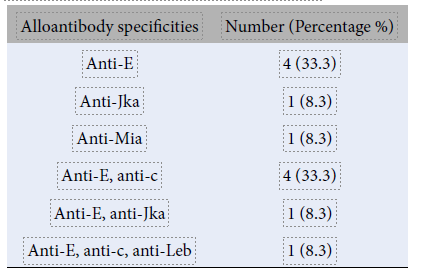
Six alloantibodies were detected in this study, with some individuals developing antibodies of single or multiple specificities. Among the individuals with single red-cell antibodies, the most common alloantibody was anti-E (33.3%), followed by anti-Jka (8.3%) and anti-Mia (8.3%). Among those with multiple antibodies, anti-E and anti-c were the most common combinations (33.3%; Table 2).
There was no significant association between alloantibody formation and gender (p=0.506), age at transfusion (p=0.915), number of blood transfusions (p=0.382), and blood group (p=0.581); see Table 3.
Discussion
RBC alloimmunization is an immune response against foreign RBC antigens that can occur after sensitization through multiple blood transfusions or pregnancies. It is one of the complications that patients dependent on RBC transfusions may encounter8. The process of alloimmunization is complex and influenced by various factors. The main contributing elements include differences in RBC antigens between the blood donor and recipient, the recipient’s immune status, and the immunomodulatory effect of allogeneic blood transfusions on the recipient’s immune system9.
RBC alloimmunization rates have been examined in multi-transfused thalassemia patients in several studies. In a study conducted in Saudi Arabia, the alloimmunization rate in thalassemia patients was 11.97%10. Another study in Iran reported an alloimmunization rate of 16.4% in thalassemia patients11. A study in Iraq found that 5.8% of transfusion-dependent thalassemia patients experienced alloimmunization12. Additionally, a study in Malaysia reported an alloimmunization rate of 8.6% in thalassemia patients13. In our study, the rate of alloimmunization was higher at 36.4%. Among the included patients, 45.5% had HbE beta thalassemia, while 33% had beta thalassemia major. These findings highlight the risk of alloimmunization in multi-transfused thalassemia patients and the need for extended phenotyping to prevent complications. This could be attributed to our inability to provide phenotypically matched blood to transfusion-dependent thalassemia patients who test negative in antibody screening. In contrast, in some other developed countries, the practice is to provide phenotypically matched blood as soon as a patient is diagnosed with thalassemia, regardless of their antibody screening status.
In addition, the increased alloimmunization rate can be attributed to several factors: (1) diversity of thalassemia patients: our study included a diverse population of thalassemia patients with varying genetic backgrounds, treatment histories, and blood transfusion regimens. This diversity may have contributed to the observed differences in alloimmunization rates; (2) genetic factors: thalassemia patients often exhibit genetic variations that may predispose them to higher rates of alloimmunization; and (3) duration of observation and sample size: the duration of our study might have influenced the observed alloimmunization rates. A more extended follow-up period and a larger sample size could provide a more comprehensive understanding of the temporal dynamics of alloimmunization.
RBC alloimmunization is a significant complication that can occur following a blood transfusion. It can lead to delays in transfusions, shortened in vivo survival of donated blood, and difficulties finding compatible blood for future transfusions. In addition, RBC alloimmunization can lead to the development of autoantibodies and cause hemolytic transfusion reactions. In some cases, these reactions can be fatal8.
Over 300 RBC antigens have been identified and categorized into 36 systems, the better known of which include ABO, Rh, Kell, Duffy, Kidd, and MNS. This extensive array of antigens raises the potential for RBC alloimmunization with various antibody specificities14. Different studies have reported variable antibody specificities in multi-transfused patients. This variability can be attributed to differences in antigen frequencies between populations and the effects of genetics on the response to this antigenic stimulation.
In several studies, the K and Rh system antigens were predominantly involved in stimulating the production of alloantibodies. This is expected, given the immunogenicity of these antigens15. In our study, anti-E and anti-c were the most frequently detected alloantibodies in the Rh blood group system. These findings align with most other studies, which also identified anti-E as the most frequent antibody in these patients15. Most of these antibodies are in the form of immunoglobulin (Ig) G. The order of immunogenicity for different Rh antigens is as follows: D>c>E>C>e2. Interestingly, no anti-Kell antibodies were detected in this study, in accordance with a previous study by Dhawan et al8. This may be because, in Western populations, Kell antibodies are frequently detected due to the presence of the K antigen in approximately 10% of Caucasians. In contrast, in Malaysia, over 99% of individuals are Kell-negative; thus, the likelihood of Kell antibody formation is extremely low16, 17.
Another alloantibody detected in our study was anti-Jka, which belongs to the Kidd blood group system. Anti-Jka antibodies pose a significant risk as they can be difficult to detect in routine blood crossmatches because of their amnestic nature. They frequently cause delayed hemolytic transfusion reactions (DHTRs) because they tend to become undetectable between transfusions following sensitization18. However, a strong anamnestic response is observed upon subsequent re-exposure through blood transfusion or pregnancy. Studies showed that approximately 52% of Jka antibodies disappear within months. Therefore, it is recommended that individuals who have developed alloimmunization carry a special immunohematology antibody card to ensure they receive antigen-negative blood lifelong18.
In our study, we observed the presence of anti-Mia antibodies in one patient. According to Prathiba et al., anti-Mia antibodies are the third most commonly occurring antibodies in general and antenatal patients in Malaysia19. Anti-Mia is most frequently reported in Chinese patients due to its high prevalence in this population (15%)20. The significance of the anti-Mia antibody should not be underestimated as there have been reports of clinically significant hemolytic transfusion reactions (HTRs) and hemolytic disease of the fetus and newborn19.
One patient in this study was found to have anti-Leb, which belongs to the Lewis blood system. These antibodies are categorized as naturally occurring non-red-cell IgM antibodies with a propensity to react at lower temperatures. They are typically generated by individuals possessing the Le (a−b−) phenotype and are occasionally observed in pregnant women during specific temporary periods21. Anti-Lewis antibodies typically exhibit non-reactivity at a temperature of 37 °C and are commonly considered clinically insignificant. Nonetheless, it is important to note that clinically significant anti-Lewis antibodies capable of inducing HTRs do exist, and such occurrences have been documented22.
In this study, six out of 12 patients were identified as having multiple antibodies; five had a mixture of two antibodies, and one had three antibodies. The combination of anti-E and anti-c was the most common among patients with multiple alloantibodies23. This is consistent with the findings of other studies, which indicate that the probability of developing additional antibodies is three times higher in alloimmunized patients19, 24. Therefore, patients who have been transfused multiple times with a single alloantibody are at risk of developing multiple alloantibodies in the future. It is worth noting that anti-E is the alloantibody observed with the highest frequency19.
Although this study found no correlation between alloantibodies and gender, age, number of blood transfusions, or blood group, which is consistent with the findings of Kajiyazdi et al., addressing red cell alloimmunization in multi-transfused thalassemia patients remains a significant medical concern11. By continually advancing our understanding and clinical practices in this area, we can enhance the quality of life and improve the long-term outcomes for these patients, allowing them to lead healthier and more fulfilling lives.
The present study had a small sample size of only 33 patients, which may limit the generalizability of the findings to a broader population of thalassemia patients. It is important to note that the specific characteristics and diversity of the patient group included in the study may only partially represent the thalassemia population. Therefore, caution should be exercised when extrapolating the results to different settings or demographics. For future studies, it is recommended that the sample be collected in larger centers, such as central hospitals, where more thalassemia patients are available.
Conclusion
In conclusion, our investigation of alloantibody incidence among thalassemia patients sheds light on important aspects of the immune response and transfusion outcomes within this population. Despite the limitations of the small sample size, our study provides valuable insights into the dynamics of alloimmunization in thalassemia patients. Our study also highlights the importance of tailored transfusion strategies and ongoing vigilance in managing thalassemia patients to minimize the risks associated with alloimmunization. Future research with larger and more diverse cohorts is crucial to validate and extend our findings, providing a more comprehensive understanding of alloantibody incidence in thalassemia.
Abbreviations
None.
Acknowledgments
None.
Author’s contributions
SA and NR are responsible for the writing of the article. MM, MHR and RHZ helped in data collection. RAAR contributed to statistical analysis and data interpretation. AD and NANA participated in sequence alignment. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Approval for this study was obtained from the Medical Research and Ethics Committee (MREC) of the Ministry of Health Malaysia, with the study identified as NMRR ID-22-02377-NMR. The study was conducted following the principles outlined in the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Mohd Ibrahim
H.,
Muda
Z.,
Othman
I.S.,
Mohamed Unni
M.N.,
Teh
K.H.,
Thevarajah
A.,
Observational study on the current status of thalassaemia in Malaysia: a report from the Malaysian Thalassaemia Registry. BMJ Open.
2020;
10
(6)
:
e037974
.
View Article PubMed Google Scholar -
Ismail
W. Ismahanisa,
Al-Hassan
F.M.,
Knight
A.,
Prevalence of red cell alloantibodies among multi transfused dependent thalassemia patient in the Malaysian state of Penang. Journal of Scientific Research and Development.
2016;
2016
(3)
:
62-6
.
-
Zulkeflee
R.H.,
Bahar
R.,
Abdullah
M.,
Application of Targeted Next-Generation Sequencing for the Investigation of Thalassemia in a Developing Country: A Single Center Experience. Diagnostics.
2023;
13
(8)
:
1379
.
View Article Google Scholar -
Davoudi-Kiakalayeh
A.,
Mohammadi
R.,
Pourfathollah
A.A.,
Siery
Z.,
Davoudi-Kiakalayeh
S.,
Alloimmunization in thalassemia patients: new insight for healthcare. International Journal of Preventive Medicine.
2017;
8
(1)
:
101
.
View Article PubMed Google Scholar -
Bain
B.,
2021 Guidelines For The Management of Transfusion Dependent Thalassaemia (TDT), 4th EditionCapellini MD, Farmakis D, Porter J and Taher A (Eds). Thalassaemia International Federation, 2021, ISBN-13 978-9963-717-18-7. Br J Haematol. 2023;200(4):532. doi:https://doi.org/10.1111/bjh.18567. 2023
.
View Article Google Scholar -
Thedsawad
A.,
Taka
O.,
Wanachiwanawin
W.,
Prevalence and clinical significances of red cell alloimmunization and red cell bound immunoglobulin G in polytransfused patients with thalassemias. Hematology (Amsterdam, Netherlands).
2019;
24
(1)
:
208-14
.
View Article PubMed Google Scholar -
El-Beshlawy
A.,
Salama
A.A.,
El-Masry
M.R.,
El Husseiny
N.M.,
Abdelhameed
A.M.,
A study of red blood cell alloimmunization and autoimmunization among 200 multitransfused Egyptian β thalassemia patients. Scientific Reports.
2020;
10
(1)
:
21079
.
View Article PubMed Google Scholar -
Dhawan
H.K.,
Kumawat
V.,
Marwaha
N.,
Sharma
R.R.,
Sachdev
S.,
Bansal
D.,
Alloimmunization and autoimmunization in transfusion dependent thalassemia major patients: study on 319 patients. Asian Journal of Transfusion Science.
2014;
8
(2)
:
84-8
.
View Article PubMed Google Scholar -
Sahu
A.,
Parida
P.,
Mahapatra
S.,
Sahoo
B.B.,
Detection of red cell alloantibodies in thalassaemia patients. International Journal of Contemporary Pediatrics.
2020;
7
(2)
:
419
.
View Article Google Scholar -
Kuriri
F.A.,
Ahmed
A.,
Alanazi
F.,
Alhumud
F.,
Ageeli Hakami
M.,
Atiatalla Babiker Ahmed
O.,
Red Blood Cell Alloimmunization and Autoimmunization in Blood Transfusion-Dependent Sickle Cell Disease and β-Thalassemia Patients in Al-Ahsa Region, Saudi Arabia. Anemia.
2023;
2023
:
3239960
.
View Article PubMed Google Scholar -
Kajiyazdi
M.,
Koochakzadeh
L.,
Kajiyazdi
M.,
Khoshhal
F.,
Hashemi
A.,
Khabazkhoob
M.,
Prevalence of Alloantibodies in Thalassemia Patients and Its Relationship With AgeGender and Blood Group 2023.
Google Scholar -
Abdulqader
A.M.,
Mohammed
A.I.,
Mohammed
N.I.,
Red Cell Alloimmunization and Autoimmunization in Multi-Transfused Thalassemia Patients in Sulaymaniyah Province-Iraq. The Korean Journal of Clinical Laboratory Science.
2020;
52
(2)
:
98-104
.
View Article Google Scholar -
Nasir
A.,
Hassan
R.,
Haslina
N.,
Noor
M.,
Red cell immunization in multiply transfused Malay thalassemic patients. Southeast Asian journal of tropical medicine and public health.
2006;
37
(5)
:
1015
.
-
Valle Neto
O.G.,
Alves
V.M.,
Pereira
G.A.,
Moraes-Souza
H.,
Martins
P.R.,
Clinical and epidemiological profile of alloimmunized and autoimmunized multi-transfused patients against red blood cell antigens in a blood center of Minas Gerais. Hematology, Transfusion and Cell Therapy.
2018;
40
(2)
:
107-11
.
View Article PubMed Google Scholar -
El-Beshlawy
A.,
Salama
A.A.,
El-Masry
M.R.,
El Husseiny
N.M.,
Abdelhameed
A.M.,
A study of red blood cell alloimmunization and autoimmunization among 200 multitransfused Egyptian β thalassemia patients. Scientific Reports.
2020;
10
(1)
:
21079
.
View Article PubMed Google Scholar -
Hajar
C.G.N.,
Zefarina
Z.,
Extended blood group profiles for Malays, Chinese, and Indians in Peninsular Malaysia. The Egyptian Journal of Medical Human Genetics.
2020;
21
(1)
:
51
.
View Article Google Scholar -
Patel
J.,
Shukla
R.,
Gupte
S.,
Red cell alloimmunization in multitransfused patients and multiparous women. Indian Journal of Hematology & Blood Transfusion : An Official Journal of Indian Society of Hematology and Blood Transfusion.
2009;
25
(2)
:
49-52
.
View Article PubMed Google Scholar -
Mittal
K.,
Sood
T.,
Bansal
N.,
Bedi
R.K.,
Kaur
P.,
Kaur
G.,
Clinical Significance of Rare Maternal Anti Jka Antibody. Indian Journal of Hematology & Blood Transfusion : An Official Journal of Indian Society of Hematology and Blood Transfusion.
2016;
32
(4)
:
497-9
.
View Article PubMed Google Scholar -
Yousuf
R.,
Abdul Aziz
S.,
Yusof
N.,
Leong
C.F.,
Incidence of red cell alloantibody among the transfusion recipients of Universiti Kebangsaan Malaysia medical centre. Indian Journal of Hematology {&}amp; Blood Transfusion : An Official Journal of Indian Society of Hematology and Blood Transfusion.
2013;
29
(2)
:
65-70
.
View Article PubMed Google Scholar -
Chao
Y.H.,
Wu
K.H.,
Lu
J.J.,
Shih
M.C.,
Peng
C.T.,
Chang
C.W.,
Red blood cell alloimmunisation among Chinese patients with β-thalassaemia major in Taiwan. Blood Transfusion.
2013;
11
(1)
:
71-4
.
View Article PubMed Google Scholar -
Reyhaneh
K.,
Frequency & specificity of RBC alloantibodies in patients due for surgery in Iran. The Indian journal of medical research.
;
138
(2)
:
252
.
-
Gayathri
A.M.,
Gupta
D.,
Case series investigation on the Lewis system antibodies encountered during a routine screening in a tertiary care hospital-based blood center. Asian Journal of Transfusion Science.
2020;
14
(1)
:
54-6
.
View Article PubMed Google Scholar -
Chaudhari
C.N.,
Red cell alloantibodies in multiple transfused thalassaemia patients. Medical Journal, Armed Forces India.
2011;
67
(1)
:
34-7
.
View Article PubMed Google Scholar -
S Abdullah
S.Z.,
Hassan
M.N.,
Ramli
M.,
Abdullah
M.,
Mohd Noor
N.H.,
Red Blood Cell Alloimmunization and Its Associated Factors among Chronic Liver Disease Patients in a Teaching Hospital in Northeastern Malaysia. Diagnostics (Basel).
2023;
13
(5)
:
886
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 1 (2024)
Page No.: 6135-6141
Published on: 2024-01-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 3277 times
- PDF downloaded - 1230 times
- XML downloaded - 126 times
 Biomedpress
Biomedpress



