Abstract
Objective: This review aimed to systematically synthesize and report the clinical outcomes of poly-ADP ribose polymerase inhibitors (PARPis) for maintenance therapy among ovarian cancer (OC) patients.
Methods: This review was based on the updated PRISMA statement 2020. Eligible studies were identified from PubMed and the Cochrane Library from the database inception to October 7, 2021. Randomized controlled trials reporting the clinical outcomes of PARPis as maintenance therapy for OC were included in this review. The Risk of Bias 2 tool was used for the quality assessment of studies.
Results: Out of 26 studies, 10 were eligible. For patients with newly diagnosed disease, compared with placebo, either olaparib or niraparib considerably prolonged progression-free survival (PFS), with hazard ratios (HRs) of 0.59 (95% confidence interval [CI]: 0.49–0.72) and 0.62 (95% CI: 0.50–0.76), respectively. Among recurrent patients, olaparib, niraparib, and rucaparib also achieved higher PFS than placebo, with HRs of 0.39 (95% CI: 0.27–0.55), 0.32 (95% CI: 0.23–0.45), and 0.35 (95% CI: 0.28–0.45), respectively. Regarding adverse events, patients taking PARPis experienced a higher risk of hematologic events than the placebo group.
Conclusions: PARPis as maintenance therapy were beneficial in PFS improvement for OC patients. However, the considerable risk of hematologic events must be considered when using this treatment class.
Introduction
In 2020, ovarian cancer (OC) was the eighth most common malignancy in women, with an incidence of 314,0001. Given its lack of specific symptoms, OC is often detected at later stages, making it the most lethal gynecological cancer, with a 49% five-year survival rate (2011–2017)2, 3, 4. The currently recommended treatment for advanced OC is neo-adjuvant therapy followed by cytoreductive surgery and subsequent adjuvant chemotherapy with platinum compounds (a combination of carboplatin and paclitaxel or docetaxel)5. Although platinum-based chemotherapy has a good response rate, approximately 80% of patients have advanced-stage OC within 18 months6. Therefore, new therapies are needed to improve responsiveness and prolong survival in advanced OC patients.
Poly-ADP ribose polymerase inhibitors (PARPis) are among the most promising therapeutic maintenance treatments for OC7. PARP is a protein family required to repair single-strand breaks by base excision repair. PARP includes PARP1—the best-known—and PARP2. All PARPis currently being developed are believed to block both PARP1 and PARP28. PARPis block SSB repair and lead to the formation of DNA double-strand breaks that cannot be correctly repaired in homologous recombination-deficient (HRD) tumors, such as those with deleterious mutations in breast cancer genes BRCA1 and BRCA2. These are the genes most at risk of HRD expression, which causes the accumulation of DNA aberrations and leads to the synthetically lethal phenotype in cancer cells9.
The Food and Drug Administration and European Medicine Agency have licensed the use of olaparib, olaparib, and rucaparib for advanced OC10. Few systematic reviews have examined PARPis regarding their efficacy and safety in OC treatment11, 12, 13, 14, 15, 16, 17. Given the latest published study on the efficacy of niraparib, conducted at 30 centers in China by Wu et al.18, this systematic review aimed to update the current evidence on the efficacy and safety of PARPis in OC maintenance treatment.
Methods
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (see Appendix 3 for the PRISMA checklist) 19.
Study selection
For inclusion in this review, studies needed to meet the following criteria: (i) targeted advanced OC; (ii) the intervention arm was PARPi monotherapy or PARPi in combination with chemotherapy; (iii) the comparison arm was either placebo or chemotherapy or chemotherapy plus placebo; (iv) reported survival outcomes, with or without adverse events (AEs) or health-related quality of life (HRQoL); (v) designed as phase II or III randomized control trials (RCTs).
Search method
RCTs were searched in PubMed and the Cochrane Library up to October 7, 2021. Additionally, the website https://clinicaltrials.gov/ was considered for unpublished relevant trials that presented outcomes of interest. Data were searched using key terms including “ovarian neoplasms,” “PARP inhibitors,” “olaparib,” “niraparib,” “rucaparib,” “randomized controlled trial”, and “controlled clinical trial”. The full search strategies are presented in Appendix 1.
Article screening process
Articles identified from the databases were initially imported into EndNote to remove duplicates. Two independent reviewers (PNNQ, HTN) conducted title and abstract screening using Rayyan20. The two reviewers then retrieved and reviewed potential full-text papers to determine the articles eligible for the review. The third reviewer (KD) was consulted to address any conflicts.
Data synthesis
Data from all eligible articles were extracted. In trials with more than one publication, data from the most updated publication were extracted. The extracted information covered the study design, the characteristics of the intervention or comparison, and the overall treatment outcomes and associated factors. This process was carried out by two independent reviewers (PNNQ, HTN), and any conflicts were addressed via discussions or reassessed by the third reviewer (KD).
Methodological quality assessment
The Cochrane Risk of Bias tool version 2 (RoB 2) was employed21. The tool consists of five aspects: (i) the randomization process; (ii) any deviations from the intended interventions; (iii) missing outcome data; (iv) the measurement of the outcome; (v) the selection of the reported result. The assessment result was assigned as “low risk”, “high risk”, or “some concerns.” Two reviewers (PNNQ, HTN) performed the assessment independently, and any discrepancies were resolved by consensus.
Results
Study selection
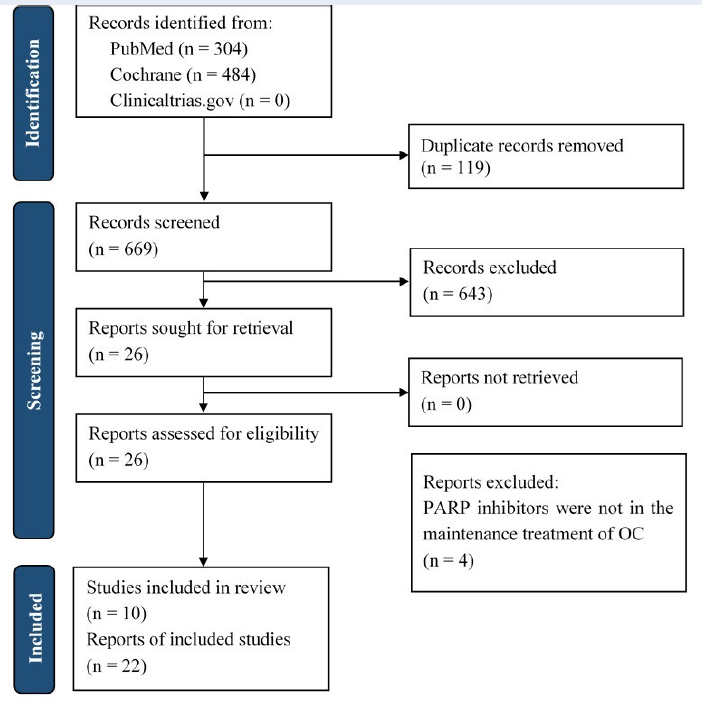
The search located 788 records. Of these, 119 were removed due to duplication, and 669 were screened based on their titles and abstracts. The full texts of 26 articles were retrieved and screened. Finally, 22 articles from 10 RCTs were included in the analysis (Figure 1).
Trial characteristics
Two of the 10 RCTs were phase II trials22, 23, 24, 25, and the others were phase III18, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40. One trial had an open-label design22, whereas the others were double-blind studies18, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40. The SOLO126, 27 and SOLO231, 32, 33 studies included only OC patients with a BRCA mutation, whereas the other eight included all OC patients regardless of their BRCA status18, 22, 23, 24, 25, 28, 29, 30, 34, 35, 36, 37, 38, 39, 40. In those eight studies, 25.3% to 51.3% of patients had the BRCA mutation. Four studies evaluated PARPis in newly diagnosed OC patients, of which two used olaparib26, 28, one used niraparib29, and one used veliparib30. The SOLO126, 27 and PRIMA29 studies compared PARPi monotherapy to placebo, and the PAOLA128 and VELIA30 studies compared a PARPi in combination with bevacizumab or platinum-based chemotherapy. Six studies evaluated PARPis in recurrent OC patients. Of these, Oza et al.22 compared olaparib plus chemotherapy followed by maintenance with olaparib versus chemotherapy alone, whereas the remaining studies, comprising STUDY1923, 24, 25, SOLO231, 32, 33, NOVA34, 35, 36, 37, NORA18, and ARIEL338, 39, 40, compared PARPi monotherapy versus placebo. NORA18 was a dose-adjustment study of niraparib in each patient population based on the weight index and platelet count per all.Table 1 presents the specific characteristics of the selected trials.
| Study, Year of Publication | Setting | Study design | Intervention arm (no. patient) | Control arm (no. patient) | No. patient mBRCA (%) | HR of PFS (95% CI, p value) | HR of OS (95% CI, p value) |
|---|---|---|---|---|---|---|---|
| First-line maintenance treatment | |||||||
| SOLO1, 2018 31 , 33 | International | Phase III, double-blind | olaparib 300 mg twice daily (tablets) (260) | placebo (131) | 391 (100) | 0.30 (0.23 – 0.41, p < 0.001) | 0.95 (0.6 – 1.53) |
| PAOLA1, 2019 28 | International | Phase III, double-blind | olaparib 300 mg twice daily (tablets) plus bevacizumab (537) | bevacizumab (269) | 241 (29,9) | 0.59 (0.49 – 0.72, p < 0.0001) | - |
| PRIMA, 2019 29 | International | Phase III, double-blind | niraparib 300 mg once daily (487) | placebo (246) | 223 (30,4) | 0.62 (0.50 – 0.76, p < 0.001) | 0.70 (0.44 – 1.11) |
| VELIA, 2019 30 | International | Phase III, double-blind | veliparib 150 mg twice daily plus pc and carboplatin followed by veliparib 300/400 mg twice daily maintenance (the veliparib-throughout group) (382); | placebo plus pc followed by placebo maintenance (375) | 298 (26,1) | 0.44 (0.28 – 0.68, p < 0.001) | - |
| Second-line maintenance treatment | |||||||
| STUDY19, 2012 23 , 24 , 25 | International | Phase II, double-blind | olaparib 400 mg twice a day (capsules) (136) | placebo (129) | 136 (51,3) | 0.35 (0.25 – 0.49, p < 0.0001) | 0.73 (0.55 – 0.95, p = 0.02138) |
| Oza et al ., 2014 22 | International | Phase II, open-label | olaparib 200 mg twice daily plus paclitaxel and carboplatin followed by olaparib 400 mg twice daily maintenance (capsules) (81) | paclitaxel and carboplatin alone without further treatment (81) | 41 (25,3) | 0.51 (0.34 – 0.77, p = 0.0015) | 1.17 (0.79 – 1.73, p = 0.44) |
| SOLO2, 2017 31 , 32 , 33 | International | Phase III, double-blind | olaparib 300 mg twice daily (tablets) (196) | placebo (99) | 295 (100) | 0.30 (0.22 – 0.41, p < 0.0001) | 0.74 (0.54 – 1.00, p = 0.054) |
| NOVA, 2016 34 , 35 , 36 , 37 | International | Phase III, double-blind | niraparib 300 mg once daily (203) | placebo (350) | 250 (45,2) | 0.27 (0.17 – 0.41, p < 0.0001) | - |
| NORA, 2020 18 | Multi-centre in China | Phase III, double-blind | niraparib 300 mg once daily niraparib 200 mg once daily (< 77 kg or a platelet count < 150 x10 3 /ul) (177) | placebo (88) | 100 (37,7) | 0.32 (0.23 – 0.45, p < 0.0001) | - |
| ARIEL3, 2017 38 , 39 , 40 | International | Phase III, double-blind | rucaparib 600mg twice daily (375) | placebo (189) | 196 (34,8) | 0.36 (0.3 – 0.45, p < 0.0001) | - |
| Abbreaviatons : -: not available; BRCAm : breast cancer susceptibility gene mutation; HR : hazard ratio; PFS : progression-free survival; OS : overall survival, CI : confidence interval, no .: number | |||||||
| Outcome | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Median PFS, months | HR (95%, CI) | Two-side P | Data maturity | Median OS, months | HR (95%, CI) | Two-side P | ||
| SOLO1 26 | Olaparib | NR | 0.30 (0.23 – 0.41) | < 0.001 | 21% | - | 0.95 (0.6 – 1.53) | - |
| Placebo | 13.8 | - | ||||||
| PRIMA 29 | Niraparib | 13.8 | 0.62 (0.50 – 0.76) | < 0.001 | Interim analysis | - | 0.70 (0.44 – 1.11) | - |
| Placebo | 8.2 | - | ||||||
| PAOLA1 28 | Olaparib + bev | 22.1 | 0.59 (0.49 – 0.72) | < 0.001 | _ | _ | _ | _ |
| Placebo | 16.6 | _ | _ | _ | ||||
| PAOLA1 28 (HRD including BRCAm subset) | Olaparib + bev | 34.7 | 0.44 (0.28-0.68) | < 0.001 | _ | _ | _ | _ |
| Placebo | 22.0 | _ | _ | _ | ||||
| VELIA 30 | Veliparib | 23.5 | 0.68 (0.56 – 0.83) | < 0.001 | _ | _ | _ | _ |
| Placebo | 17.3 | _ | _ | _ | ||||
| VELIA 30 (BRCAm subset) | Veliparib | NR | 0.44 (0.28 – 0.68) | < 0.001 | _ | _ | _ | _ |
| Placebo | 5.5 | _ | _ | _ | ||||
| Abbreviations : _: not available; bev : bevacizumab; HRD : homologous-recombination deficiency; BRCAm : breast cancer susceptibility gene mutation; HR : hazard ratio; PFS : progression-free survival; OS : overall survival | ||||||||
| Outcome Trial | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Median PFS, months | HR (95%, CI) | Two-side P | Data maturity | Median PFS, months | HR (95%, CI) | Two-side P | ||
| STUDY 19 , 23 , 25 | Olaparib | 8.4 | 0.35 (0.25 – 0.49) | < 0.0001 | 79% | 29.8 | 0.73 (0.55 – 0.95) | 0.02138 |
| Placebo | 4.8 | 27.8 | ||||||
| STUDY 19 , 23 , 25 (BRCAm subset) | Olaparib | 11.2 | 0.18 (0.1 – 0.31) | < 0.0001 | 79% | 34.9 | 0.62 (0.42 - 0.93) | 0.02140 |
| Placebo | 4.3 | 30.2 | ||||||
| SOLO2 31 , 33 | Olaparib | 19.1 | 0.30 (0.22 – 0.41) | < 0.0001 | 61% | 51.7 | 0.74 (0.54 – 1.00) | 0.054 |
| Placebo | 5.5 | 38.8 | ||||||
| NORA 18 | Niraparib | 18.3 | 0.32 (0.23 – 0.45) | < 0.0001 | NR | NR | 0.64 (0.29 – 1.42) | 0.267 |
| Placebo | 5.4 | NR | ||||||
| NORA 18 (BRCAm subset) | Niraparib | NR | 0.22 (0.12 – 0.39) | < 0.0001 | _ | _ | _ | _ |
| Placebo | 5.5 | _ | _ | _ | ||||
| ARIEL3 38 | Rucaparib | 10.8 | 0.36 (0.3 – 0.45) | < 0.0001 | _ | _ | _ | _ |
| Placebo | 5.4 | _ | _ | _ | ||||
| ARIEL3 38 (BRCAm subset) | Rucaparib | 16.6 | 0.23 (0.16 – 0.34) | < 0.001 | _ | _ | _ | _ |
| Placebo | 5.4 | _ | _ | _ | ||||
| NOVA 34 (BRCAm subset) | Niraparib | 21.0 | 0.27 (0.17 – 0.41) | < 0.0001 | _ | _ | _ | _ |
| Placebo | 5.5 | _ | _ | _ | ||||
| Oza et al . 22 | Olaparib plus chemotherapy | 12.2 | 0.51 (0.34 – 0.77) | 0.0012 | 60% | 33.8 | 1.17 (0.79 – 1.73) | 0.44 |
| Chemotherapy | 9.6 | 37.6 | ||||||
| Oza et al . 22 (BRCAm subset) | Olaparib plus chemotherapy | NR | 0.21 (0.08 – 0.55) | 0.0015 | 60% | NR | 1.28 (0.39 – 4.18) | 0.69 |
| Chemotherapy | 9.7 | 39.2 | ||||||
| Abbreviations : _ : not available; NR : Not reported; BRCAm : breast cancer susceptibility gene mutation; HR : hazard ratio; PFS : progression-free survival; OS : overall survival | ||||||||
| Trial | Intervention | No. of patients | All grade AEs (%) | Grade 3/4 AEs (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nausea | Vomiting | Fatigue | Anemia | Anemia | Neutropenia | Thrombocytopenia | Increased AST/ALT | |||
| SOLO1 26 | Olaparib | 260 | 77 | 40 | 63 | 39 | 22 | 9 | 1 | NR |
| Placebo | 130 | 38 | 15 | 42 | 10 | 2 | 5 | 2 | NR | |
| SOLO2 33 | Olaparib | 195 | 76 | 37 | 67 | 46 | 21 | 8 | 3 | NR |
| Placebo | 99 | 35 | 19 | 40 | 10 | 2 | 4 | 1 | NR | |
| STUDY 19 , 23 | Olaparib | 136 | 71 | 34 | 52 | 21 | 5 | 4 | NR | NR |
| Placebo | 129 | 36 | 14 | 39 | 5 | <1 | <1 | NR | NR | |
| PAOLA1 28 | Olaparib + bev | 535 | 53 | 22 | 53 | 41 | 17 | 6 | 2 | NR |
| Placebo + bev | 267 | 22 | 11 | 32 | 10 | <1 | 3 | <1 | NR | |
| Oza et al . 22 | Olaparib + chemotherapy + | 81 | 50 | 29 | 20 | 12 | 8 | 5 | 0 | NR |
| Chemotherapy + | 81 | 6 | 7 | 9 | 9 | 2 | 0 | 0 | NR | |
| PRIMA 29 | Niraparib | 484 | 57.4 | 22.3 | 34.7 | 63.4 | 31 | 12.8 | 28.7 | NR |
| Placebo | 244 | 27.5 | 11.9 | 29.5 | 17.6 | 1.6 | 1.2 | 0.4 | NR | |
| NOVA 34 | Niraparib | 367 | 73.6 | 34.3 | 59.4 | 50.1 | 25.3 | 19.6 | 33.8 | NR |
| Placebo | 179 | 35.2 | 16.2 | 41.3 | 6.7 | 0 | 1.7 | 0.6 | NR | |
| ARIEL3 38 | Rucaparib | 372 | 76 | 37 | 71 | 39 | 22 | 8 | 5 | 10 |
| Placebo | 189 | 37 | 15 | 45 | 6 | 1 | 2 | 0 | 0 | |
| VELIA 30 | Veliparib throughout | 377 | 80 | 49 | 69 | 64 | 38 | 58 | 28 | NR |
| Veliparib combination | 376 | 72 | 35 | 62 | 65 | 41 | 62 | 31 | NR | |
| Control | 371 | 68 | 36 | 60 | 53 | 26 | 49 | 8 | NR | |
| Abbreviations : AEs : Adverse events; bev : bevacizumab; + : AEs in maintenance phase; AST : aspartate aminotransferase; ALT : alanine aminotransferase; NR : not report | ||||||||||
Risk of bias assessment
The risk of bias was assessed using RoB 2, evaluating the PFS and OS outcomes of each study21. All 10 studies evaluated via PFS and five studies evaluated via OS were classified as having a low risk of bias for both outcomes. Appendix 2 refers to the risk of bias table.
Efficacy of PARPis as first-line maintenance
Four trials investigated PARPis in first-line maintenance treatment26, 28, 29, 30. The results of the PFS and OS outcomes are synthesized in Table 2.
According to the findings from the two trials SOLO126 and PRIMA29 comparing olaparib and niraparib monotherapy to placebo, PFS improved significantly. Among BRCA-mutation patients in the SOLO1 study26, olaparib lowered the risk of progression or death by 70%, with a hazard ratio (HR) of 0.30 and a 95% confidence interval (CI) of 0.23–0.41. Similarly, among all patients regardless of HRD status in the PRIMA study, the PFS favored niraparib over placebo (HR: 0.62; 95% CI: 0.50–0.76). In the HRD tumor subgroup, the PFS also improved significantly (HR: 0.43; 95% CI: 0.31–0.59). Concerning OS, both SOLO1 and PRIMA found no remarkable difference between olaparib or niraparib and the placebo group, with HRs of 0.95 (95% CI: 0.6–1.53) and 0.70 (95% CI: 0.44–1.11), respectively.
In PAOLA1, advanced OC patients were treated with either bevacizumab or olaparib plus bevacizumab28. According to the findings, this combination with olaparib vs. bevacizumab alone extended investigator-assessed PFS by 5.5 months (HR: 0.59; 95% CI: 0.49–0.72) and blinded-independent-central-reviewed PFS by 7.8 months (HR: 0.63, 95% CI: 0.51–0.77). A subgroup analysis indicated that the HRs for PFS in somatic-BRCA-mutation patients or HRD-positive patients were significantly lower, with HRs of 0.31 (95% CI: 0.20–0.47) and 0.33 (95% CI: 0.25–0.45), respectively.
The VELIA study assessed an intervention consisting of veliparib plus first-line platinum chemotherapy, followed by maintenance veliparib30. After monitoring for 28 months, in the overall population, the median PFS of the veliparib-throughout and placebo groups were 23.5 months and 17.3 months, respectively (HR: 0.68; 95% CI: 0.56–0.83). In the HRD-positive patients, these figures were 34.7 months and 22.0 months, respectively (HR: 0.44, 95% CI: 0.28–0.6).
Efficacy of PARPis as second-line or beyond maintenance
A total of six trials were performed in the recurrent setting: STUDY1923, 25, SOLO231, 33, Oza et al.22, NOVA34, NORA18, and ARIEL38. The PFS and OS outcomes of these studies are presented in Table 3.
The STUDY1923, 25 and SOLO231, 33 trials revealed a considerably better PFS for olaparib over placebo. In the STUDY19 trial23, 25, patients receiving olaparib 400 mg (capsule) had a 3.6-month longer PFS than those without (HR: 0.35; 95% CI: 0.25–0.49). Patients with germline had a longer median PFS of 6.9 months. Olaparib demonstrated a small increase in OS over placebo (29.8 vs. 27.8 months; HR: 0.73; 95% CI: 0.55–0.95) in the overall population. In the germline BRCA mutation (BRCAm) population, OS in the olaparib and placebo groups was 34.9 months versus 30.2 months (HR: 0.62; 95% CI: 0.42–0.93). In the SOLO2 trial, the PFS in olaparib-treated patients decreased by 70% versus placebo (HR: 0.30; 95% CI: 0.22–0.41). An updated analysis of OS with data maturity of 61% showed a longer time to death in olaparib versus placebo (HR: 0.74; 95% CI: 0.54–1.00).
The NOVA34 and NORA18 studies evaluated the efficacy of niraparib compared with placebo. In the NOVA trial, the results demonstrated a remarkable benefit of PFS for niraparib versus placebo in the germline BRCA cohort (HR: 0.27; 95% CI: 0.17–0.41). In the NORA trial, the PFS of the dose-adjusted niraparib group versus the placebo group showed a prolongation of 12.9 months (HR: 0.32; 95% CI: 0.23–0.45). In the germline BRCAm group, the PFS in the niraparib group decreased by 78% versus placebo (HR: 0.22; 95% CI: 0.12–0.39). The OS analysis showed a slight increase in survival for niraparib over placebo (HR: 0.64; 95% CI: 0.29–1.42).
The ARIEL338 trial evaluated the efficacy of rucaparib versus placebo. The PFS of the rucaparib group improved significantly compared with placebo in all three populations: the overall population, germline or somatic BRCAm, and positive HRD patients. In the overall cohort, the PFS of rucaparib versus placebo was 10.8 and 5.4 months, respectively (HR: 0.36; 95% CI: 0.3–0.45). Rucaparib showed the greatest PFS benefit over placebo in the germline or somatic BRCAm subset (HR: 0.23; 95% CI: 0.16–0.34).
Using olaparib plus platinum-based chemotherapy and then maintenance with olaparib, the trial of Oza et al.22 demonstrated a more favorable PFS in the olaparib combination group compared with only treating with chemotherapy. The PFS was considerably prolonged in the olaparib combination group compared to placebo (HR: 0.51; 95% CI: 0.34–0.77). The greatest clinical benefit was in BRCAm patients; the PFS of those in the olaparib combination group decreased by approximately 80% compared to the control group (HR: 0.21; 95% CI: 0.08–0.55). The updated OS showed no appreciable differences between the two groups (HR: 1.17; 95% CI: 0.79–1.73); similarly, the OS result in the BRCAm population was not statistically significant (HR: 1.28; 95% CI: 0.39–4.18; P=0.69).
Safety of PARPis
The proportion of patients in PARPi groups experiencing any adverse events (AEs) was higher than that in control groups (Table 4). The two most frequent AEs reported were nausea and fatigue. Regarding grade 3 and 4 AEs, hematologic AEs in PARPi groups were much more common than in comparison groups, in which anemia was the most prevalent AE. In particular, this AE ranged from 5% to 22% with olaparib22, 23, 26, 33, 25% to 31% with niraparib29, 34, 19% with rucaparib38, 38% to 41% with veliparib combination30, and 17% with olaparib plus bevacizumab28. Notably, the most grade 3 or 4 hematologic-related AEs were documented with niraparib and veliparib. Grade 3 or higher neutropenia with niraparib and veliparib was recorded in up to 19.6% and 62% of patients, respectively30, 34. Similarly, grade 3 or 4 thrombocytopenia occurred up to 33.8% and 31% in those treated with niraparib and veliparib, respectively. However, these grade 3 or higher AEs were manageable for the majority of cases through dose reduction or interruption.
Quality of life of patients treated with PARPis
Eight out of the 10 RCTs reported HRQoL outcomes. Four were indicated for first-line maintenance therapy27, 28, 29, 30, and four were indicated for the maintenance treatment of recurrent OC24, 32, 35, 40. A total of six scales appeared in eight studies: EORTC QLQ-C30, FACT-O, FOSI, TOI, NFOSI-18, and EQ-5D-3L/5L. All study results showed that the HRQoL scores were almost in favor of PARPis compared with the comparison group. However, the results did not achieve statistical significance between the two groups. Additionally, the scores after PARPi treatment were almost unchanged compared with the baseline scores, meaning that PARPis did not appear to add to the burden or have a detrimental effect on HRQoL.
Discussion
All 10 studies and 20 trial-related articles were systematically reviewed for PFS, OS, AEs, and HRQoL outcomes of PARPis in OC maintenance treatment. Most of the studies were phase III, multi-center, and double-blinded. All the included RCTs were high-quality and well-designed. The results of our systematic review highlight that PARPi agents, either as single-agent maintenance therapy or in combination with chemotherapy or other targeted therapies, significantly improved PFS in both recurrent and primary settings. The results also indicated no statistically significant difference in OS outcomes between the PARPi and comparison groups for either first-line or recurrent OC maintenance treatment. Concerning AEs, comparing the PARPi groups to their respective control groups (placebo, chemotherapy alone, anti-angiogenic alone), the use of PARPis increased the likelihood of severe anemia. A review of all HRQoL results in all eight of 10 studies showed no appreciable difference in the quality-of-life scores between the PARPi and comparison groups24, 27, 28, 29, 30, 32, 35, 40.
Olaparib has the strongest evidence for OC maintenance treatment when compared to rucaparib or veliparib. More than half of the included studies evaluated olaparib for outcomes such as PFS, OS, AEs, and HRQoL, in either primary or platinum-sensitive recurrent OC maintenance treatment. All the olaparib studies found olaparib to be more effective as a maintenance regimen alone or in combination with chemotherapy or bevacizumab than in a comparison group. The greatest clinical benefit of olaparib was found in BRCAm patients. However, AEs of all grades and grade 3 or 4 appeared more frequent in the olaparib groups than in the control groups. At the dose of 300 mg or 400 mg twice per day, the most common AEs were nausea, fatigue, vomiting, and anemia. Only two studies evaluated the efficacy and safety of rucaparib and veliparib, and the outcome of OS was incompletely reported for both drugs, leading to difficulties in synthesis and analysis. With rucaparib, the maturity of OS at the reported time (April 15, 2017) was only 22%; this outcome will be updated when data maturity reaches 80%, so no report currently exists on the OS with rucaparib. With veliparib, the OS data is also not mature enough to report. Although PFS can only evaluate the treatment effect and represent the direct clinical benefit, OS is considered the most reliable cancer endpoint41. Therefore, to offer the most comprehensive evidence of the efficacy of rucaparib and veliparib in OS prolongation compared with placebo in the maintenance treatment of OC, more follow-up time is needed to produce final results.
BRCA1 and BRCA2 serve as crucial factors in the DNA repair mechanism of healthy cells. According to previous data, as many as 50% of high-grade serous OCs are HRD-positive42. The risk of mutations is 39–44% for BRCA1 and 11–17% for BRCA2 43. The DNA repair genes most at risk of HRD expression are BRCA1 and BRCA244; a BRCA mutation can result in HRD and cause tumor recurrence. The genetic interaction between PARP and BRCA is referred to as synthetic lethal, so PARPi can act against BRCA1 or BRCA2 mutations. This theory of synthetic lethality has now been demonstrated through the results of RCTs assessing the performance of PARPis in OC patients who are HRD-positive or have BRCA mutations. PARPis have more clinical benefits in BRCAm for first-line maintenance of OC28, 30. This suggests prognostic significance for HRD expression testing before treatment with PARPis. Moreover, in studies by Marchetti et al.45 and Rivera et al.46, the application of next-generation sequencing–based BRCA tumor tissue detection in formalin-fixed paraffin-embedded OC specimens proved that the BRCA gene might predict patient prognosis. Therefore, three PARPis (olaparib, niraparib, rucaparib) have now been licensed for the treatment of BRCAm OC patients, and preclinical tests for HRD expression or BRCAm have been recommended in the current OC guidelines47, 48.
All RCTs investigated AEs during the trials. Patients in the PARPi groups experienced AEs more frequently than those in the control groups. The most common grade 3 or higher AEs of PARPi in both first-line maintenance therapy and maintenance of recurrent OC was anemia. The most frequent AEs at the highest levels with PARPis were nausea and fatigue or asthenia. Notably, hematologic AEs, including anemia, thrombocytopenia, and neutropenia, were the most prevalent with niraparib and veliparib. Additionally, common grade 3 or higher AEs with rucaparib were AST/ALT increases. However, all these grade 3 and 4 AEs were managed successfully through dose adjustment. Eight out of the 10 studies assessed HRQoL, and they all agreed that the PARPi and control groups did not differ clinically24, 27, 28, 29, 30, 32, 35, 40. The conclusions of Yizi Wang49 and Aoki50 also align with our study results. This indicates that AEs involved in the maintenance therapy did not adversely impact HRQoL and were offset by the favored benefit of PFS.
A meta-analysis by Yifan Jiang12 found that PARPis improved PFS more than placebo or chemotherapy alone, and OS improvement was not shown significantly in the PARPi groups compared with the comparison groups. The AE results of this meta-analysis also revealed that the PARPi group encountered a higher rate of AEs than the comparison group. Other meta-analyses gave similar results11, 13, 14, 15, 16, 17. The results from the meta-analyses are consistent with the results of our systematic review. However, when compared with previous meta-analyses11, 12, 13, 14, 15, 16, 17, our study sought and aggregated results from more studies with larger sample sizes. We also aggregated HRQoL results that no meta-analyses since 2018 have reported; our systematic review thus provides comprehensive evidence regarding PARPis in the maintenance treatment of OC. Our systematic review can be combined with economic evaluation studies to better inform coverage, nationally and internationally, and decisions for PARPis in the treatment of OC.
This systematic review has several strengths. First, the systematic review method gives the highest level of evidence for health policymaking. Additionally, the research team used a comprehensive search strategy based on clear and specific criteria and then selected the most up-to-date articles for each outcome of each study. All phases, from searching to screening to data extraction, were performed independently by the two investigators. Therefore, little chance existed of missing related articles. Second, all the included trials were multicenter so the results are highly representative and generalizable for most OC patients. Third, the evidence was of a high standard due to the low risk of bias in all included studies.
Our research also has some limitations. First, this systematic review only aggregated evidence from the PubMed and Cochrane Libraries, thus omitting studies from other databases. However, these databases are the two main libraries for publishing RCT studies. Furthermore, we looked for unpublished relevant studies on the website https://clinicaltrials.gov, so the probability of missing a study was considerably low. The inclusion of only English-language research in this systematic review was a second drawback; studies in other languages may have been excluded during the search and screening phases. However, the majority of RCTs are published in English, so the probability of missing a study was low. Third, in terms of OS outcomes, the data of four studies— PAOLA1, VELIA, NOVA, and ARIEL3—have not matured enough to report so the most comprehensive results are not available yet. Finally, the review reported a single efficacy for each drug, the overall efficacy of which has not been estimated. The overall efficacy of the PARPis can be estimated by meta-analysis or network analysis; however, this is beyond the focus of this review.
Conclusions
PARPis considerably improved PFS irrespective of BRCA mutations in OC patients. However, no remarkable difference was witnessed in OS between the PARPi and comparison groups; hence, a longer follow-up time was needed. The considerable risk of hematology-related events with PARPis must be considered in clinical use.
Abbreviations
OC: Ovarian cancer; FIGO: International Federation of Genecology and Obstetrics; PARPi: poly-ADP ribose polymerase inhibitors; SSB: single-strand breaks; DNA: deoxyribonucleic acid; DSBs: double-strand breaks; HRD: homologous recombination-deficient; BRCA: breast cancer gene; FDA: US Food and Drug Administration; EMA: European Medicine Agency; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs: randomized control trials; PFS: progression-free survival; OS: overall survival; AE: adverse event; HRQoL: health-related quality of life; RoB 2: the Cochrane risk-of-bias tool version 2; HR: hazard ratio; CI: confidence interval; BRCAm: BRCA mutation; EORTC QLQ-C30: European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire; FACT-O: Functional Assessment of Cancer Therapy-Ovarian; FOSI: Functional Assessment of Cancer Therapy Ovarian Cancer Symptom Index - 8 Item Version; TOI: Trial Outcome Index; NFOSI-18: National Comprehensive Cancer Network/Functional Assessment of Cancer Therapy Ovarian Cancer Symptom Index - 18 Item Version; EQ-5D-3L/5L: European Quality of Life 5 Dimensions 3 or 5 Level Version
Acknowledgments
None.
Author’s contributions
PQ, PN, and KD conceptualized and designed the study. PQ, KD, HN, and PN collected and summarized data. TP and DN were involved in the data interpretation. PQ and PN drafted the first manuscript. All authors made a major contribution to the revision. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Sung
H.,
Ferlay
J.,
Siegel
R.L.,
Laversanne
M.,
Soerjomataram
I.,
Jemal
A.,
Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer Journal for Clinicians.
2021;
71
(3)
:
209-49
.
View Article PubMed Google Scholar -
Goff
B.A.,
Mandel
L.S.,
Melancon
C.H.,
Muntz
H.G.,
Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. Journal of the American Medical Association.
2004;
291
(22)
:
2705-12
.
View Article PubMed Google Scholar -
CDC-Centers for Disease Control and Prevention. Stage Distribution (%) of New Cancer Cases, All Ages, All Races and Ethnicities, Female 2018 [Available from: https://gis.cdc.gov/Cancer/USCS/#/StageatDiagnosis/.
.
-
American cancer society. Treating ovarian cancer 2018 [Available from: https://www.cancer.org/content/dam/CRC/PDF/Public/8776.00.pdf.
.
-
Cancer Statistic Center. 5-year relative survival, 2011-2017 of gynecological cancers 2021 [Available from: https://cancerstatisticscenter.cancer.org/#!/data-analysis/SurvivalByStage.
.
-
Luvero
D.,
Milani
A.,
Ledermann
J.A.,
Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Therapeutic Advances in Medical Oncology.
2014;
6
(5)
:
229-39
.
View Article PubMed Google Scholar -
Franzese
E.,
Centonze
S.,
Diana
A.,
Carlino
F.,
Guerrera
L.P.,
Di Napoli
M.,
PARP inhibitors in ovarian cancer. Cancer Treatment Reviews.
2019;
73
:
1-9
.
View Article PubMed Google Scholar -
Rouleau
M.,
Patel
A.,
Hendzel
M.J.,
Kaufmann
S.H.,
Poirier
G.G.,
PARP inhibition: PARP1 and beyond. Nature Reviews. Cancer.
2010;
10
(4)
:
293-301
.
View Article PubMed Google Scholar -
Pearre
D.C.,
Tewari
K.S.,
Targeted treatment of advanced ovarian cancer: spotlight on rucaparib. Therapeutics and Clinical Risk Management.
2018;
14
:
2189-201
.
View Article PubMed Google Scholar -
Lheureux
S.,
Gourley
C.,
Vergote
I.,
Oza
A.M.,
Epithelial ovarian cancer. Lancet.
2019;
393
(10177)
:
1240-53
.
View Article PubMed Google Scholar -
Hao
J.,
Liu
Y.,
Zhang
T.,
He
J.,
Zhao
H.,
An
R.,
Efficacy and safety of PARP inhibitors in the treatment of advanced ovarian cancer: an updated systematic review and meta-analysis of randomized controlled trials. Critical Reviews in Oncology/Hematology.
2021;
157
:
103145
.
View Article PubMed Google Scholar -
Jiang
Y.,
Zhao
J.,
Zhang
L.,
Tian
S.,
Yang
T.,
Wang
L.,
Evaluation of the Efficacy and Safety of PARP Inhibitors in Advanced-Stage Epithelial Ovarian Cancer. Frontiers in Oncology.
2020;
10
:
954
.
View Article PubMed Google Scholar -
Yang
Y.,
Du
N.,
Xie
L.,
Jiang
J.,
Mo
J.,
Hong
J.,
The efficacy and safety of the addition of poly ADP-ribose polymerase (PARP) inhibitors to therapy for ovarian cancer: a systematic review and meta-analysis. World Journal of Surgical Oncology.
2020;
18
(1)
:
151
.
View Article PubMed Google Scholar -
Ruscito
I.,
Bellati
F.,
Ray-Coquard
I.,
Mirza
M.R.,
du Bois
A.,
Gasparri
M.L.,
Incorporating Parp-inhibitors in Primary and Recurrent Ovarian Cancer: A Meta-analysis of 12 phase II/III randomized controlled trials. Cancer Treatment Reviews.
2020;
87
:
102040
.
View Article PubMed Google Scholar -
Shao
F.,
Liu
J.,
Duan
Y.,
Li
L.,
Liu
L.,
Zhang
C.,
Efficacy and safety of PARP inhibitors as the maintenance therapy in ovarian cancer: a meta-analysis of nine randomized controlled trials. Bioscience Reports.
2020;
40
(3)
:
BSR20192226
.
View Article PubMed Google Scholar -
Staropoli
N.,
Ciliberto
D.,
Giudice
T. Del,
Iuliano
E.,
Cucè
M.,
Grillone
F.,
The Era of PARP inhibitors in ovarian cancer: Class Action or not? A systematic review and meta-analysis. Critical Reviews in Oncology/Hematology.
2018;
131
:
83-9
.
View Article PubMed Google Scholar -
Wiggans
A.J.,
Cass
G.K.,
Bryant
A.,
Lawrie
T.A.,
Morrison
J.,
Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer. Cochrane Database of Systematic Reviews.
2015;
2015
(5)
:
CD007929
.
View Article PubMed Google Scholar -
Wu
X.H.,
Zhu
J.Q.,
Yin
R.T.,
Yang
J.X.,
Liu
J.H.,
Wang
J.,
Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial. Annals of Oncology : Official Journal of the European Society for Medical Oncology.
2021;
32
(4)
:
512-21
.
View Article PubMed Google Scholar -
Page
M.J.,
McKenzie
J.E.,
Bossuyt
P.M.,
Boutron
I.,
Hoffmann
T.C.,
Mulrow
C.D.,
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed.).
2021;
372
(71)
:
n71
.
View Article PubMed Google Scholar -
Ouzzani
M.,
Hammady
H.,
Fedorowicz
Z.,
Elmagarmid
A.,
Rayyan-a web and mobile app for systematic reviews. Systematic Reviews.
2016;
5
(1)
:
210
.
View Article PubMed Google Scholar -
Sterne
J.A.,
Savović
J.,
Page
M.J.,
Elbers
R.G.,
Blencowe
N.S.,
Boutron
I.,
RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.).
2019;
366
:
l4898
.
View Article PubMed Google Scholar -
Oza
A.M.,
Cibula
D.,
Benzaquen
A.O.,
Poole
C.,
Mathijssen
R.H.,
Sonke
G.S.,
Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. The Lancet. Oncology.
2015;
16
(1)
:
87-97
.
View Article PubMed Google Scholar -
Ledermann
J.,
Harter
P.,
Gourley
C.,
Friedlander
M.,
Vergote
I.,
Rustin
G.,
Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. The Lancet. Oncology.
2014;
15
(8)
:
852-61
.
View Article PubMed Google Scholar -
Ledermann
J.A.,
Harter
P.,
Gourley
C.,
Friedlander
M.,
Vergote
I.,
Rustin
G.,
Quality of life during olaparib maintenance therapy in platinum-sensitive relapsed serous ovarian cancer. British Journal of Cancer.
2016;
115
(11)
:
1313-20
.
View Article PubMed Google Scholar -
Friedlander
M.,
Matulonis
U.,
Gourley
C.,
du Bois
A.,
Vergote
I.,
Rustin
G.,
Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. British Journal of Cancer.
2018;
119
(9)
:
1075-85
.
View Article PubMed Google Scholar -
Moore
K.,
Colombo
N.,
Scambia
G.,
Kim
B.G.,
Oaknin
A.,
Friedlander
M.,
Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. The New England Journal of Medicine.
2018;
379
(26)
:
2495-505
.
View Article PubMed Google Scholar -
Friedlander
M.,
Moore
K.N.,
Colombo
N.,
Scambia
G.,
Kim
B.G.,
Oaknin
A.,
Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): a randomised, phase 3 trial. The Lancet. Oncology.
2021;
22
(5)
:
632-42
.
View Article PubMed Google Scholar -
Ray-Coquard
I.,
Pautier
P.,
Pignata
S.,
Pérol
D.,
González-Martín
A.,
Berger
R.,
Investigators
PAOLA-1,
Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. The New England Journal of Medicine.
2019;
381
(25)
:
2416-28
.
View Article PubMed Google Scholar -
González-Martín
A.,
Pothuri
B.,
Vergote
I.,
DePont Christensen
R.,
Graybill
W.,
Mirza
M.R.,
Investigators
PRIMA/ENGOT-OV26/GOG-3012,
Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. The New England Journal of Medicine.
2019;
381
(25)
:
2391-402
.
View Article PubMed Google Scholar -
Coleman
R.L.,
Fleming
G.F.,
Brady
M.F.,
Swisher
E.M.,
Steffensen
K.D.,
Friedlander
M.,
Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. The New England Journal of Medicine.
2019;
381
(25)
:
2403-15
.
View Article PubMed Google Scholar -
Pujade-Lauraine
E.,
Ledermann
J.A.,
Selle
F.,
Gebski
V.,
Penson
R.T.,
Oza
A.M.,
SOLO2/ENGOT-Ov21 investigators
Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet. Oncology.
2017;
18
(9)
:
1274-84
.
View Article PubMed Google Scholar -
Friedlander
M.,
Gebski
V.,
Gibbs
E.,
Davies
L.,
Bloomfield
R.,
Hilpert
F.,
Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. The Lancet. Oncology.
2018;
19
(8)
:
1126-34
.
View Article PubMed Google Scholar -
Poveda
A.,
Floquet
A.,
Ledermann
J.A.,
Asher
R.,
Penson
R.T.,
Oza
A.M.,
SOLO2/ENGOT-Ov21 investigators
Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet. Oncology.
2021;
22
(5)
:
620-31
.
View Article PubMed Google Scholar -
Mirza
M.R.,
Monk
B.J.,
Herrstedt
J.,
Oza
A.M.,
Mahner
S.,
Redondo
A.,
Investigators
ENGOT-OV16/NOVA,
Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. The New England Journal of Medicine.
2016;
375
(22)
:
2154-64
.
View Article PubMed Google Scholar -
Oza
A.M.,
Matulonis
U.A.,
Malander
S.,
Hudgens
S.,
Sehouli
J.,
Del Campo
J.M.,
Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): results from a double-blind, phase 3, randomised controlled trial. The Lancet. Oncology.
2018;
19
(8)
:
1117-25
.
View Article PubMed Google Scholar -
Del Campo
J.M.,
Matulonis
U.A.,
Malander
S.,
Provencher
D.,
Mahner
S.,
Follana
P.,
Niraparib Maintenance Therapy in Patients With Recurrent Ovarian Cancer After a Partial Response to the Last Platinum-Based Chemotherapy in the ENGOT-OV16/NOVA Trial. Journal of Clinical Oncology.
2019;
37
(32)
:
2968-73
.
View Article PubMed Google Scholar -
Mirza
M.R.,
Benigno
B.,
D∅rum
A.,
Mahner
S.,
Bessette
P.,
Barceló
I.B.,
Long-term safety in patients with recurrent ovarian cancer treated with niraparib versus placebo: results from the phase III ENGOT-OV16/NOVA trial. Gynecologic Oncology.
2020;
159
(2)
:
442-8
.
View Article PubMed Google Scholar -
Coleman
R.L.,
Oza
A.M.,
Lorusso
D.,
Aghajanian
C.,
Oaknin
A.,
Dean
A.,
investigators
ARIEL3,
Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet.
2017;
390
(10106)
:
1949-61
.
View Article PubMed Google Scholar -
Ledermann
J.A.,
Oza
A.M.,
Lorusso
D.,
Aghajanian
C.,
Oaknin
A.,
Dean
A.,
Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. The Lancet. Oncology.
2020;
21
(5)
:
710-22
.
View Article PubMed Google Scholar -
Oza
A.M.,
Lorusso
D.,
Aghajanian
C.,
Oaknin
A.,
Dean
A.,
Colombo
N.,
Patient-Centered Outcomes in ARIEL3, a Phase III, Randomized, Placebo-Controlled Trial of Rucaparib Maintenance Treatment in Patients With Recurrent Ovarian Carcinoma. Journal of Clinical Oncology.
2020;
38
(30)
:
3494-505
.
View Article PubMed Google Scholar -
FDA. Guidance for Industry. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics 2018 [Available from: https://www.fda.gov/media/71195/download.
.
-
Bell
D.,
Berchuck
A.,
Birrer
M.,
Chien
J.,
Cramer
D.W.,
Dao
F.,
Cancer Genome Atlas Research Network
Integrated genomic analyses of ovarian carcinoma. Nature.
2011;
474
(7353)
:
609-15
.
View Article PubMed Google Scholar -
Petrucelli
N.,
Daly
M.B.,
Pal
T.,
BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. 1998 Sep 4 [Updated 2023 Sep 21]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1247/.
.
-
Zhang
H.,
Liu
T.,
Zhang
Z.,
Payne
S.H.,
Zhang
B.,
McDermott
J.E.,
Investigators
CPTAC,
Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell.
2016;
166
(3)
:
755-65
.
View Article PubMed Google Scholar -
Marchetti
C.,
De Leo
R.,
Musella
A.,
D'Indinosante
M.,
Capoluongo
E.,
Minucci
A.,
BRCA Mutation Status to Personalize Management of Recurrent Ovarian Cancer: A Multicenter Study. Annals of Surgical Oncology.
2018;
25
(12)
:
3701-8
.
View Article PubMed Google Scholar -
Rivera
D.,
Paudice
M.,
Gismondi
V.,
Anselmi
G.,
Vellone
V.G.,
Varesco
L.,
Ligurian BRCA Working Group
Implementing NGS-based BRCA tumour tissue testing in FFPE ovarian carcinoma specimens: hints from a real-life experience within the framework of expert recommendations. Journal of Clinical Pathology.
2021;
74
(9)
:
596-603
.
View Article PubMed Google Scholar -
American Cancer Society. Treating Ovarian Cancer 2018 [Available from: https://www.cancer.org/content/dam/CRC/PDF/Public/8776.00.pdf.
.
-
Redondo
A.,
Guerra
E.,
Manso
L.,
Martin-Lorente
C.,
Martinez-Garcia
J.,
Perez-Fidalgo
J.A.,
SEOM clinical guideline in ovarian cancer. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico.
2020;
23
(5)
:
961-8
.
View Article Google Scholar -
Wang
Y.,
Ren
F.,
Song
Z.,
Wang
X.,
Zhang
C.,
Ouyang
L.,
PARP Inhibitors in Patients With Newly Diagnosed Advanced Ovarian Cancer: A Meta-Analysis of Randomized Clinical Trials. Frontiers in Oncology.
2020;
10
:
530354
.
View Article Google Scholar -
Aoki
D.,
Chiyoda
T.,
PARP inhibitors and quality of life in ovarian cancer. The Lancet. Oncology.
2018;
19
(8)
:
1012-4
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 12 (2023)
Page No.: 6090-6102
Published on: 2023-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3545 times
- PDF downloaded - 1199 times
- XML downloaded - 105 times
- Appendix downloaded - 957 times
 Biomedpress
Biomedpress



