Abstract
Research has suggested that people with polycystic ovary syndrome (PCOS) have an increased risk of developing type 2 diabetes or prediabetes before the age of 40 years. Erythema multiforme major refers to a severe generalized rash with limited desquamation and mucous membrane involvement with blister formation. This case report will help all health care professionals be aware and vigilant regarding the adverse drug reaction caused by the combination of glimepiride, voglibose, metformin, and clomiphene citrate. A female patient in her early 30s visited the outpatient department for follow-up with a history of PCOS and type 2 diabetes. Her main complaints were itching, redness, and a pink and brown skin allergy on the face, neck, shoulder, breast, abdomen, and back, along with breast tenderness or discomfort. She had been prescribed oral clomiphene citrate 100 mg, vitamin D 10,000 IU twice weekly, and calcium 1,250 mg twice daily for PCOS and a combination of glimepiride 2 mg, voglibose 0.2 mg, and metformin 500 mg SR daily for type 2 diabetes. An adverse drug reaction (ADR) was found probable based on assessment with the Naranjo scale. ADRs such as maculopapular rash following erythema multiforme major are fatal. They can cause stress and anxiety and reduce the patient's quality of life. This case will help physicians and clinicians in the early detection and management of ADRs.
Introduction
Research has suggested that people with polycystic ovary syndrome (PCOS) have an increased risk of developing type 2 diabetes or prediabetes before the age of 40 years1, 2. Approximately one in 10 women of childbearing age have PCOS, and around 9.4% of people in the United States have diabetes2. PCOS affects the ovaries, leading to the formation of small cysts on their surface. These disturb the menstruation cycle and impair normal functioning mainly because ovulation and condition often result in an irregular release of eggs, causing infertility2. PCOS is also associated with diabetes mellitus and causes an imbalance of endocrine hormones, mainly insulin3, 4. People who are overweight or obese (BMI of 30 kg/m2 or greater) are at higher risk of developing both PCOS and type 2 diabetes mellitus (DM)5. Common PCOS symptoms include acne, fertility problems, hair loss, hirsutism, and irregular menstruation or missed periods6.
Erythema multiforme major (EMM) refers to a severe generalized rash with limited desquamation and mucous membrane involvement with blister formation7.
Case Presentation
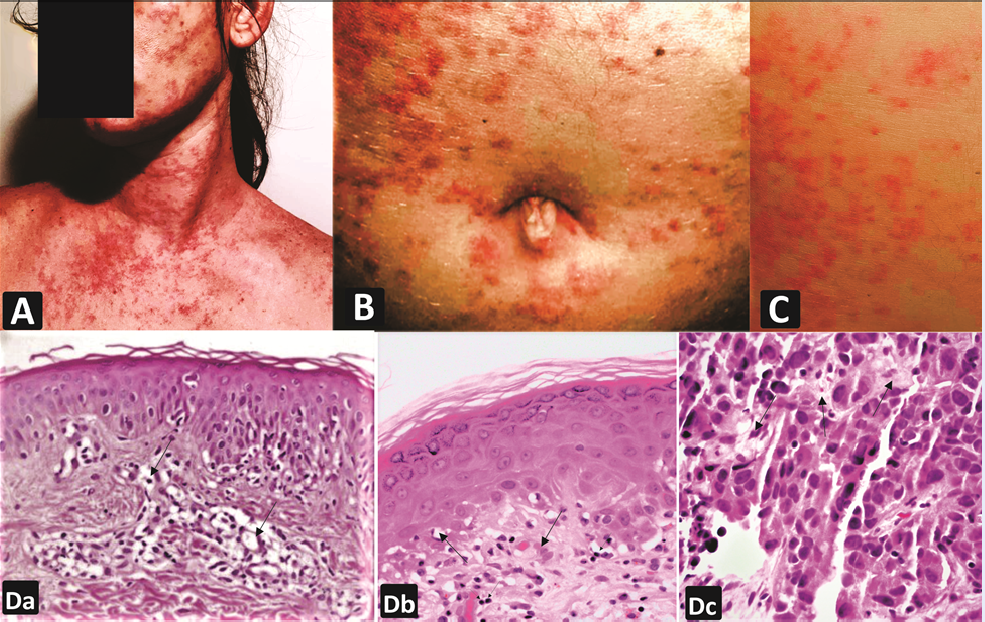
A female patient in her early 30s visited the outpatient department for follow-up. She was referred to the dermatology department with a primary complaint of itching, redness, and skin allergy on the face, neck, shoulder, breast, stomach, and back. She also complained of breast tenderness, discomfort, and loss of taste. She had a history of recently diagnosed PCOS and type 2 DM for the last seven years. The eruption had begun over the breast and abdomen, with redness that progressed to her neck, face, and back region. The development of widespread, rash-like lesions measuring 5–6 cm in diameter and varying in size, even spreading to the trunk, is shown in Figure 1.
On interviewing the patient, the medical and family history revealed that her mother had experienced the same reaction in adulthood. The patient had a past medical history of uncontrolled DM over the last seven years, as well as migraine, and she was allergic to dust mites, pollen, mold, and ground nuts. She was on oral gliclazide 3 mg daily and a tablet containing a combination of caffeine 100 mg, ergotamine 1 mg, paracetamol 250 mg, and prochlorperazine 2.5 mg (SOS). The patient had been taking this combination of drugs for the last 6–7 years. For the last 4 days, she had been on glimepiride 2 mg, voglibose 0.2 mg, and metformin 500 mg SR daily for DM. Her physician had changed antidiabetic medications because she did not respond well to gliclazide 3 mg daily.
The EMM reaction with various-shaped lesions had appeared in the first weeks of treatment when she began the alternative medications of glimepiride 2 mg, voglibose 0.2 mg, and metformin 500 mg (SR) daily with oral clomiphene citrate 100 mg. Vitamin D 10,000 IU twice weekly and calcium 1,250 mg twice daily had also been prescribed for PCOS.
Investigations
The patient was physically diagnosed with maculopapular rash following EMM, but no other abnormality or deformity was found. On the recommendation of a gynecologist, dermatologist, and endocrinologist, a differential diagnosis and the following tests were performed: pregnancy test, TSH test for hypothyroidism, prolactin for hyperprolactinemia, total testosterone for evaluation of ovarian tumor, total testosterone for hyperthecosis, DHEA-S for adrenal tumor, 24-hour urine free cortisol for Cushing’s syndrome, fasting blood glucose, HbA1c for diabetes, and 75 g oral glucose tolerance test for insulin resistance or glucose intolerance. The patient’s blood pressure was normal at 120/70 mmHg.
After 2 hours, the glucose level was 285 mg/dL, indicating diabetes with insulin resistance. The testosterone value of 147 ng/dL was normal. The DHEA-S value of 18.7 µmol/L showed no adrenal tumor. An elevated level of prolactin was found, confirming mild hyperprolactinemia. The 17-hydroxyprogesterone test was normal at <6 nmol/L, and the 24-hour urine free cortisol was also normal. Other investigations, such as the urine examination and chest x-ray, were as expected. On evaluation, no clinical sign of systemic lupus erythematosus was found. The anti-nuclear antibody test was negative. An x-ray of the abdomen showed no evidence of gallstones or pancreatic calcification. No evidence of pancreatic necrosis or dilatation of the intrahepatic ducts was seen on an ultrasound of the abdomen. Three cutaneous punch biopsies were also performed. The skin biopsy of the target lesion revealed intraepithelial edema associated with exocytosis. The biopsy of the breast sample showed severe edema and polymorphonuclear infiltrate in the superficial dermis. The presence of high CD1+ immune-labeled cells was also observed (Figure 1).
After 20 days of discontinuation of the combination of glimepiride, voglibose, metformin, and clomiphene citrate, a re-challenge test was performed after obtaining patient consent. Six hours after the first dose was administered, mild eruptions developed with itching and redness of the neck and face, loss of taste, and chest tiredness and discomfort. The patient declined to give other samples for biopsy due to a lack of money and time. One day after drug administration, the reaction became more severe and spread over the neck and breast. Treatment was initiated, and the patient recovered.
Treatment
The patient’s vital signs, electrolyte levels, and FBC were monitored daily. The progression of skin rashes was also observed daily. The treatment included the oral antihistamine levocetirizine 5 mg, β-methasone dipropionate 0.05% ointment, methylprednisolone 32 mg/ day for five days, and topical calamine lotion at night. The dose of methylprednisolone was reduced to 16 mg after 5 days then to 8 mg after another 5 days. On assessing this adverse drug reaction (ADR), oral glimepiride, voglibose, and metformin, when prescribed with clomiphene citrate, were the suspected drugs. However, the breast tenderness or discomfort was caused only by oral clomiphene citrate 100 mg. The combination of glimepiride 2 mg, voglibose 0.2 mg, and metformin 500 mg SR daily was stopped immediately when it was confirmed as the cause of the ADR. A causality assessment of the ADR was conducted using the Naranjo scale. On examination, an ADR was found probable and caused by the combination of glimepiride, voglibose, and metformin. Vitamin D and calcium were assessed as having no role in this ADR.
Outcome and Follow-Up
The patient had been taking glimepiride 2 mg, voglibose 0.2 mg, and metformin 500 mg SR daily, along with clomiphene citrate 100 mg. On follow-up, the signs subsided when this combination was withdrawn and treatment was initiated; the patient began to recover.
Discussion
PCOS is a common hormonal disorder among girls and women during their reproductive years6. EMM refers to hypersensitivity disorders characterized by symmetric red, patchy lesions8. In PCOS, androgen level increases disturb the normal functioning of the endocrine system. Global experts believe that PCOS and diabetes are related to each other in women. In PCOS, the resistance of insulin receptors leads the pancreas to produce a high level of insulin. The release of more insulin than normal (hyperinsulinemia) causes the ovaries to produce too much testosterone, which can prevent normal ovulation9. The pathogenic hypersensitivity mechanisms involved in the development of EMM are under research. Drug-like acarbose can induce generalized erythema multiforme of the same class as voglibose10. Treatment with a triple combination of glimepiride, metformin, and voglibose aids in reaching the target HbA1c in cases when the initial HbA1c is high. Additionally, it can increase beta-cell volume or activity and lower the risk of macrovascular and microvascular problems.
In summary, evidence supports that oral clomiphene citrate was responsible for worsening the reaction and causing breast tenderness or discomfort and loss of taste. The ADR of breast tenderness or discomfort caused by clomiphene citrate is well-documented11.
Conclusion
The regimen of 2 mg of glimepiride, 0.2 mg of voglibose, and 500 mg of metformin was used once a day as SR for type II diabetes. After applying the Naranjo scale, an ADR was determined likely. ADRs, including maculopapular dermatitis secondary to multifaceted erythema major, may be deadly, induce tension and worry, and lower the quality of life. This example will assist medical professionals in identifying and managing ADRs early.
Learning Points/Take-Home Messages
Abbreviations
ADR: Adverse drug reaction, BMI: Body Mass Index, DHEA-S: Dehydroepiandrosterone sulfate, DM: Diabetes Mellitus, EMM: Erythema multiforme major, FBC: Full Blood Count, HbA1c: Glycated haemoglobin, Kg: Kilogram, PCOS: Polycystic ovary syndrome
Acknowledgments
We thank the patient for allowing us to share her details, Guru Gobind Singh Medical College and Hospital, Faridkot, and thank ISF College of Pharmacy, Moga, Punjab.
Author’s contributions
All equally contributed to this work, from data collection, and write the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The present case is collected in ongoing study. Informed consent was obtained from the patient. All procedures performed in studies involving human participants followed the institutional and national research committee's ethical standards and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The present study is approved by IEC of ISF College of Pharmacy, Moga, Punjab (Ref. No. ECR/296/Indt/PB/2023/ISFCP/139) and Guru Gobind Singh Medical College, Faridkot, Punjab (Ref. No. EC/NEW/INST/2023/PB/0219).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Forslund
M.,
Landin-Wilhelmsen
K.,
Trimpou
P.,
Schmidt
J.,
Brännström
M.,
Dahlgren
E.,
Type 2 diabetes mellitus in women with polycystic ovary syndrome during a 24-year period: importance of obesity and abdominal fat distribution. Human Reproduction Open.
2020;
2020
(1)
.
View Article PubMed Google Scholar -
Wang
E.T.,
Calderon-Margalit
R.,
Cedars
M.I.,
Daviglus
M.L.,
Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstetrics and Gynecology.
2005;
23
(1)
:
1-7
.
View Article PubMed Google Scholar -
De Leo
V.,
Musacchio
M.C.,
Cappelli
V.,
Massaro
M.G.,
Morgante
G.,
Petraglia
F.,
Genetic, hormonal and metabolic aspects of PCOS: an update. Reproductive Biology and Endocrinology.
2016;
14
(1)
:
38
.
View Article PubMed Google Scholar -
Ghigo
E.,
Porta
M.,
Diabetes secondary to endocrine and pancreatic disorders. Diabetes Second to Endocr Pancreat Disord..
2014;
22
:
1-183
.
View Article Google Scholar -
Jindal
A.,
Gupta
M.,
Sharma
G.,
Mohan
G.,
Tikoo
D.,
Comparative evaluation of voglibose versus pioglitazone on glycaemic control and lipid profile in patients of type 2 diabetes mellitus on glimepiride and metformin in punjabi population. International Journal of Basic and Clinical Pharmacology.
2012;
1
(3)
:
160
.
View Article Google Scholar -
Hoeger
K.M.,
Legro
R.S.,
Welt
C.K.,
Polycystic ovary syndrome (PCOS) patient guide. In: Polycystic Ovary Syndrome (PCOS), The Endocrine Society. ; 2014:1-2..
.
-
Hidajat
C.,
Loi
D.,
Drug-mediated rash: erythema multiforme versus Stevens-Johnson syndrome. BMJ Case Reports.
2014;
2014
:
1-4
.
View Article PubMed Google Scholar -
Ozuguz
P.,
Kacar
S.D.,
Ozuguz
U.,
Karaca
S.,
Tokyol
C.,
Erythroderma secondary to gliclazide: a case report. Cutaneous and Ocular Toxicology.
2014;
33
(4)
:
342-4
.
View Article PubMed Google Scholar -
Baptiste
C.G.,
Battista
M.C.,
Trottier
A.,
Baillargeon
J.P.,
Insulin and hyperandrogenism in women with polycystic ovary syndrome. The Journal of Steroid Biochemistry and Molecular Biology.
2010;
122
(1-3)
:
42-52
.
View Article PubMed Google Scholar -
Kono
T.,
Hayami
M.,
Kobayashi
H.,
Ishii
M.,
Taniguchi
S.,
Acarbose-induced generalised erythema multiforme. Lancet.
1999;
354
(9176)
:
396-7
.
View Article PubMed Google Scholar -
Sovino
H.,
Sir-Petermann
T.,
Devoto
L.,
Clomiphene citrate and ovulation induction. Reproductive Biomedicine Online.
2002;
4
(3)
:
303-10
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 12 (2023)
Page No.: 6086-6089
Published on: 2023-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4346 times
- PDF downloaded - 1419 times
- XML downloaded - 106 times
 Biomedpress
Biomedpress



