Abstract
Regenerative medicine-based therapies are considered promising for some chronic diseases, such as osteoarthritis (OA). Because OA is the most common disease in many countries, significant efforts have long been made to develop effective treatments. Current therapies for OA include hyaluronic acid, platelet-rich plasma, bone marrow aspirate concentrates, the stromal vascular fraction from adipose tissue, bone marrow-derived mesenchymal stem cells, and adipose tissue-derived mesenchymal stem cells. Clinical trials testing these agents for OA treatment have been performed for over 10 years. In this review, we summarize and compare the effects of these agents for treating knee OA based on recent meta-analyses.
Introduction
Osteoarthritis (OA), especially knee OA, is the most common form of arthritis. As of 2019, it affected more than 32.5 million people in the U.S. and more than 528 million people worldwide1. It is a significant contributor to years lived with disability and is more prevalent in people older than 55 years. Moreover, 60% of people living with OA are women. OA is characterized by symptoms including pain, swelling, stiffness, and difficulty moving. Among the types of OA, knee OA is most common among older people. It can cause a significant decrease in quality of life, as due to pain and difficulty in moving, patients cannot participate in home, work, or social activities. This can negatively impact mental health, sleep, and relationships.
A recent study showed an association between joint inflammation signs, especially inflammation in the synovial membrane, and pain in patients with OA2. Therefore, almost all OA cases are treated with anti-inflammatory agents.
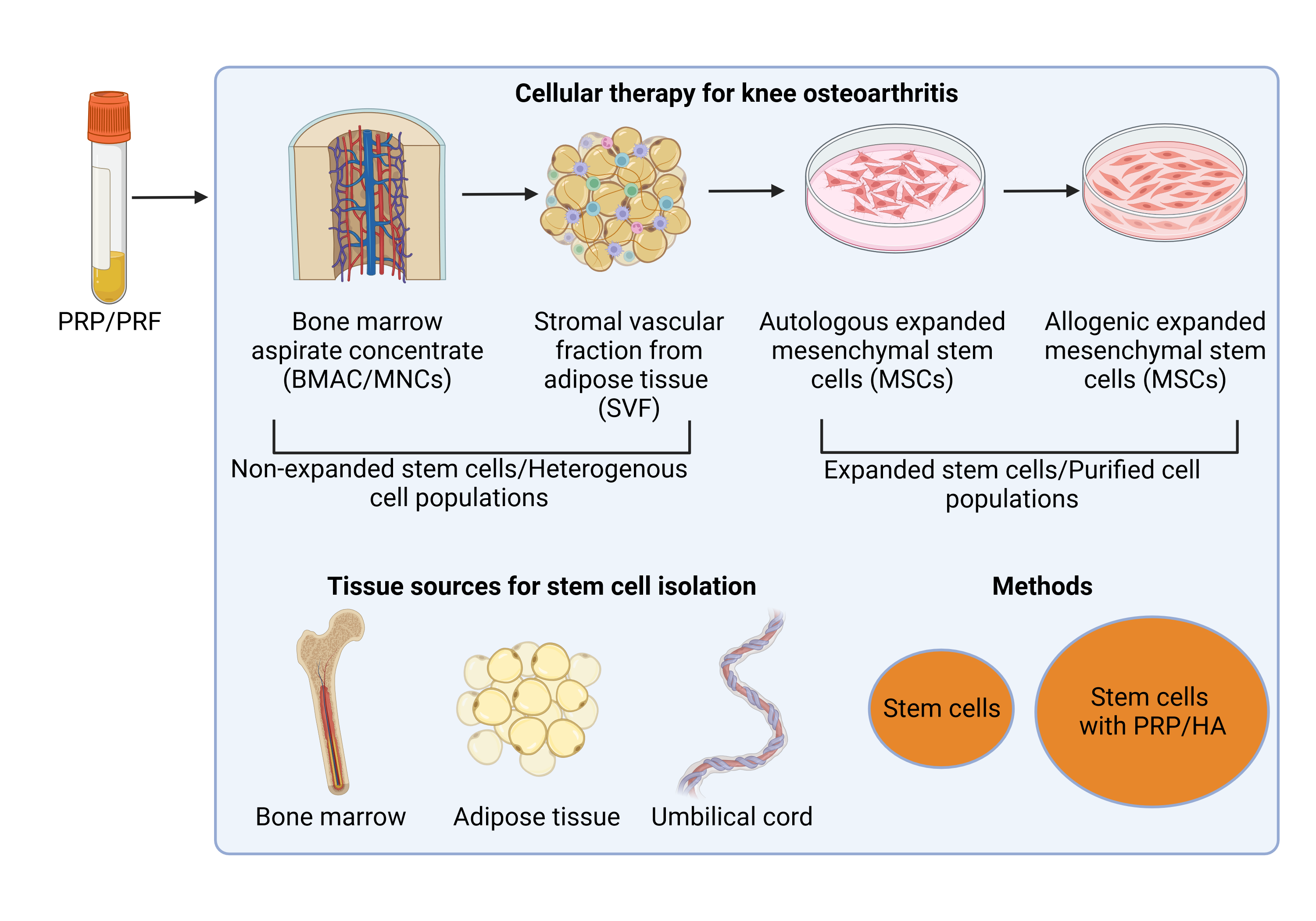
Current treatments for OA include noncellular agents, such as hyaluronic acid (HA) and platelet-rich plasma (PRP), and cellular agents, such as stem cells from bone marrow and adipose tissue (Figure 1). To summarize and compare their treatment efficacies, this review summarizes several meta-analyses on OA treatment using noncellular and cellular therapies.
Current approaches for treating knee osteoarthritis
Hyaluronic acid
HA is used to treat OA in the form of 6000 to 7000 kDa protein at concentrations of 2–4 mg/mL3. HA positively affects the knee by working as a lubricant at low shear rates and providing shock absorption during movement. It also has anti-inflammatory effects and contributes to proteoglycan synthesis4.
To investigate the therapeutic effects of HA in knee OA, Vincent et al. (2020) performed a meta-analysis of the effects of HA on knee OA in 1177 patients. The authors reported significant improvements in the Western Ontario and McMaster University index function subscores5. However, recently, Mao et al. (2023) systematically analyzed 15 studies involving 951 knees injected with HA after arthroscopic knee surgery. Although the meta-analysis showed that HA injection was safe, this therapy did not support pain relief or functional recovery6.
In 2020, Zheng et al. performed a meta-analysis of 10 randomized controlled trials involving 998 patients to compare the effects of HA injection alone with those of combination therapy comprising acupuncture and HA injection. The analysis revealed that the combination of acupuncture and HA injection significantly reduced pain, as measured by the visual analog scale (VAS), and improved knee function, as measured by the Lysholm knee scoring scale (LKSS)7.
Platelet-rich plasma
The use of PRP is a new strategy for improving knee OA. PRP is plasma enriched with platelets. Currently, there are three types of PRP, including pure PRP, which lacks leukocytes; leukocyte-poor PRP, which contains a few leukocytes; and leukocyte-rich plasma, which contains leukocytes. Because of its high concentration of platelets, PRP is considered to contain a pool of growth factors and anti-inflammatory agents. Therefore, it has been widely used in treating knee OA for many years. However, to date, the use of PRP as a therapy for knee OA has not been recommended by societies or committees. The first consensus regarding the use of PRP for treating knee OA came from French-speaking experts in 20208. Their publications included 25 recommendations related to the use of PRP for knee OA and agreed that PRP treatment was appropriate for treating knee OA. These authors also suggested that leukocyte-poor PRP is preferred for patients with knee OA8.
However, in a study published in JAMA 2021, Bennell et al. (2021) showed that compared with a placebo, PRP injection did not significantly alter symptoms or joint structures at 12 months. These findings did not support PRP use for knee OA management9. We think that several factors caused the failure of PRP injection in this study. The most important factor may be the dose of platelets used. The authors reported a PRP dose of 5 mL, 325.103/mm3. In another study by Bansal et al. (2021), 10 billion platelets (a dose more than fivefold higher than that used by Bennell et al.) were confirmed to have long-term effects on moderate knee OA10.
Several different publications about the efficacy of treatment for knee OA show that efficacy depends on the dose of platelets used. Therefore, identifying the best techniques to prepare PRP is essential to ensure good treatment efficacy. Patients’ platelet counts are checked before their blood is collected to prepare PRP. To our knowledge, there are currently four platelet dose types, namely, low (< 1 billion), average (1–3 billion), high (3–5 billion), and very high (> 5 billion). Depending on the clinical response, a suitable dose should be recommended by medical doctors.
Bone marrow aspirate concentrates
Bone marrow aspirate concentrates (BMACs) are mononuclear cells (MNCs) derived from bone marrow aspirates. Bone marrow is known as a source of stem cells. It contains various types of stem cells, including hematopoietic stem cells, mesenchymal stem cells, and endothelial progenitor cells11. BMACs are prepared by centrifuging bone marrow in gel or Ficoll to eliminate red blood cells and some mature leukocytes. Traditionally, BMACs are prepared by centrifuging 60–90 mL of bone marrow at 2400 rpm for 10 minutes to obtain platelet-poor plasma and a buffy coat layer for a second centrifugation at a higher speed (approximately 3400 rpm for 6 minutes). The resulting cell pellet is collected and resuspended in platelet-poor plasma12. Therefore, compared with whole bone marrow, bone marrow concentrates contain MNCs with a greater percentage of stem cells. The quality of BMACs depends on the harvest site and the patient’s age13. Cavallo et al. (2022) showed that younger patients had three times more MNCs than older patients did, and the number of MNCs was four times greater in BMACs from the iliac crest than in those from the tibia13. Muthu et al. (2023) also confirmed this finding. Thus, age affects the number of MNCs. The MNC count is also significantly reduced in patient populations with comorbidities14.
Keeling et al. (2022) analyzed eight studies with 299 knees treated with BMACs. The results showed that BMAC injection effectively improved pain in knee OA patients with short- to mid-term follow-up15. A recent study by Rascovic et al. (2023) involving 111 patients also showed that BMAC therapy is effective, especially for younger patients with milder OA16. The combination of intra-articular and subchondral BMAC injection can provide clinical and imaging benefits for up to 24 months in patients with knee OA17.
Stromal vascular fraction from adipose tissue
The stromal vascular fraction (SVF) from adipose tissue is a new candidate for treating knee OA. Like MNCs or BMACs, SVFs are freshly isolated from adipose tissue; therefore, they contain a variety of cells enriched with stromal stem cells. Although several types of stem cells are found in SVFs, mesenchymal stem cells (MSCs) are the most important types of stem cells that benefit knee OA patients. SVFs can be easily prepared by digesting adipose tissue with collagenase to disrupt fat cells and release nucleated cells. The MSCs inside SVFs are considered the main component that can help to reduce pain and stimulate cartilage regeneration. The biological functions of MSCs have been described in many publications18, 19, 20.
In 2019, Hong et al. performed a double-blinded clinical trial in which SVF was used to treat knee OA. The study included 60 patients with K-L grades II to III divided into two groups. Group 1 included mice with SVF injected into one side of the knee joint, and group 2 included mice with HA injected on the other side. The results revealed that SVF-treated knees exhibited improved mean VAS and WOMAC scores, while the scores in the control group worsened21. A recent publication by Goncharov et al. (2023) analyzed 22 clinical studies using SVFs for knee OA; most of those studies showed the therapeutic benefits of SVF injection for knee OA22.
The effects of SVFs on knee OA depend on the dose of SVF cells. A high dose of SVF cells (50.106 SVF cells) showed better results than did low-dose SVF cells (25.106 SVF cells)23; however, at a dose of 25.106 SVF cells, the WOMAC score and VAS and KOOS improved24. In a recent publication, Kim et al. (2023) confirmed that the cartilage lesion size and number of SVF cells strongly influence the clinical outcome of knee OA treatment25. The therapeutic effects of SVF can persist for the first 2 years in patients with knee OA grades 2–3, and the positive effects of the injection disappear in the third year26.
Expanded mesenchymal stem cells
Expanded MSCs constitute the next generation of cellular therapy for knee OA. In contrast to SVFs or BMACs, in which the percentage of MSCs inside the space is low, expanded MSCs comprise pure MSCs expanded from the SVF, BMAC, or umbilical cord blood (UCB)/tissue. MSCs can be isolated and expanded for several applications. MSCs used to treat knee OA have various sources, such as bone marrow, adipose tissue, umbilical cord tissue, and UCB. In several early studies, autologous MSCs were injected into joints, and most current applications use allogeneic MSCs for this purpose. In fact, MSCs exhibit low immunogenicity and act as immune modulators and anti-inflammatory agents. For this reason, MSCs have rapidly become excellent candidates for treating knee OA18, 19, 20.
To provide an overview of this therapy, Long et al. (2022) summarized and analyzed the results of 28 RCTs using MSCs for knee OA (27) with 1494 participants. They concluded that MSC injection is effective for treating knee OA, and the curative effect should be maintained for no less than 12 months, during which the WOMAC pain score, VAS score, WOMAC stiffness score, and WOMAC physical function improve27. In another review, 12 articles comprising 539 patients and 576 knees treated with a single intra-articular injection of MSCs for knee OA were analyzed by Kyriakidis et al. (2023)28, who reported that MSC injection is a safe and effective treatment for K-L grade I-III knee OA. Interestingly, a triple-blinded, placebo-controlled, randomized trial compared the treatment efficacy of allogeneic MSCs (from adipose tissue) to that of normal saline (control group) and confirmed that allogeneic MSC injection is safe and significantly improves treatment efficacy29.
The efficacy of this therapy depends on various factors, including the dose of cells, the kind of MSCs, and the grade of knee OA. The efficacy of MSC injection also depends on the cell dose. Huang et al. (2023) reported three popular doses of adipose-derived stem cells used to treat knee OA, including a low dose (0–25.106 cells), an elevated dose (25–50.106 cells), and a high dose (> 50.106 cells), based on 16 studies. The authors suggested that a high dose had the best treatment effects; however, adverse effects also increased with increasing doses30.
Wei et al. (2021), based on eight studies with 203 knee OA patients, evaluated the differences in the efficacy of several types of MSCs used to treat this disease. The authors concluded that MSCs from adipose tissue are the most effective at improving function31.
Some allogeneic MSCs have been successfully developed into “off-the-shelf” MSC products for knee OA and have been approved in some countries as stem cell drugs. A system that contains allogeneic UCB-derived MSCs was first approved by the Korean FDA for knee OA treatment. Multiple RCTs investigating the use of Cartistem (a combination of UCB-MSCs with HA) for large, full-thickness cartilage defects in older patients showed that Cartistem improved the cartilage defect grade, as well as pain and function, for up to 5 years compared to microfracture32. Another product, an off-the-shelf MSC product for knee OA named StemOne, has been approved in India33. This approval is based on the results of a multicenter, double-blinded, randomized, placebo-controlled study published by Gupta et al. (2023)34. StemOne comprises vials containing 25.106 pooled allogeneic bone marrow-derived MSCs in 1 mL of CryoStor CS5 plus 1 mL of PlasmaLyte-A. In the abovementioned phase 3 RCT, a total of 65 patients received one dose of StemOne (treatment group) and 2 mL of 20 mg HA (placebo group). The results showed that StemOne injection is safe and effective for the treatment of Grades II and III OA. This therapy relieves pain and stiffness, improves physical function, and prevents the worsening of cartilage quality for more than 12 months34. Another allogeneic MSC-based treatment from adipose tissue that is being developed as an off-the-shelf therapy for knee OA is Cartilatist. This is a product consisting of adipose tissue-derived MSCs resuspended in MSCCryosave OTS (Regenmedlab, HCMC, Vietnam) developed by the Stem Cell Institute (University of Science, Vietnam National University, Ho Chi Minh City, Vietnam). This product is currently undergoing clinical trials. Like Cartilatist, ElixCyte, which was developed by UnicoCell (Taiwan), also contains allogeneic AT-MSCs35. This product is also undergoing clinical trials35.
Comparisons of the therapeutic effects of knee osteoarthritis therapies
A comparison between hyaluronic acid and platelet-rich-plasma
Although both HA and PRP are shown to be beneficial for treating knee OA, which treatment is the best is a common question for most clinical doctors. Due to differences in their mechanisms of action, PRP exhibited better clinical effects than HA in most clinical studies.
In 2016, Duymus et al. compared the effects of PRP, HA, and ozone for knee OA in a clinical trial. In this study, 102 patients with mild-to-moderate or moderate knee OA and a history of at least 1 year were included. In the PRP group, patients were intra-articularly injected with two doses of PRP; in the HA group, patients were injected with a single dose of HA; and in the ozone group, patients were injected with four doses of ozone. All patients in the three groups showed significant improvement after the first month of follow-up. However, at the 3-month follow-up visit, the WOMAC and VAS scores in the PRP and HA groups were significantly greater than those in the ozone group. In the sixth month, the therapeutic effects were maintained and similar in both the PRP and HA groups but disappeared in patients treated with ozone injections. In the twelfth month, the effects of PRP injections were clearly greater than those of HA36. These observations were checked and confirmed by Raeissadat et al. (2021). Raeissadat et al. (2021) compared the effects of the intra-articular injection of PRP, plasma rich in growth factor (PRGF), HA, and ozone therapy for knee OA. In this study, 238 patients were randomly divided into four groups: HA (three doses weekly), PRP (two doses at a 3-week interval), PRGF (two doses at a 3-week interval), and ozone (three doses weekly). The results showed that ozone injection had rapid effects but improved short-term results after 2 months. Until the 6-month point, the effects of PRP, PRGF, and HA were superior to those of ozone. However, up to 12 months of follow-up, symptoms improved in only patients who received PRP or PRGF37.
In a systematic review and meta-analysis published in 2022, Costa et al. analyzed 40 studies with 3034 participants to compare the effects of PRP therapy with those of HA, corticosteroids, and saline38. At the 6-month follow-up, PRP therapy was as effective as other therapies, and in some studies, it was even more effective than other therapies38.
In a recent publication, Chen et al. (2023) analyzed the effects of PRP versus HA for knee OA based on 30 articles with 2733 patients. The results also confirmed that PRP injection was better than HA injection; in particular, the WOMAC and IKDC scores were better in the PRP group than in the HA group at the last follow-up timepoint39. Additionally, in this analysis, Chen et al. showed that LP-PRP appeared to be superior to LR-PRP in terms of functional recovery but not in terms of pain relief. They also showed that there were no differences between single PRP injections and triple PRP injections39. However, in another analysis, Peng et al. (2022) compared the effects of LR-PRP and HA and showed that although LR-PRP had no significant pain relief effect, LR-PRP injection demonstrated better overall outcomes than HA injection40. Kim et al. (2022) compared the effects of LP-PRP to those of HA and showed that LP-PRP injection improved pain and function in patients with knee OA for 12 months and was superior to HA regardless of the leukocyte concentration or number of injections41. In addition, in a previous publication, Kim et al. (2021) showed that LR-PRP can cause more adverse effects than LP-PRP; patients treated with LR-PRP experienced significantly greater pain than those treated with LP-PRP, and LR-PRP was associated with a significantly greater rate of swelling than was LP-PRP42. However, according to Abbas et al. (2022), LP-PRP is preferred to LR-PRP according to the SUCRA rankings; nevertheless, this preference is not important in clinical practice43.
An RCT with 7 years of follow-up showed that PRP was also more effective than HA in terms of survival, reintervention rates, VAS score, and WOMAC score, with higher satisfaction than was observed in patients with HA, although there were no significant differences in the imaging evaluation between the PRP and HA groups44.
Which kinds of stem cells are the best for treating knee osteoarthritis?
In general, MSCs from adipose tissue, bone marrow, SVFs from adipose tissue, and bone marrow adenocarcinoma (BMAC) are beneficial for treating knee OA. Almost all the studies reviewed here showed that these treatments improved the VAS score, KOOS, WOMAC score, and MRI results without significant adverse effects45.
A meta-analysis of 1042 patients from 27 studies was performed to compare the therapeutic effects of PRP, HA, and BMAC. Belk et al. (2023) reported no difference in outcome scores between PRP and BMAC, but both the PRP and BMAC outcome scores were better than those for patients receiving HA injection46. A recent study showed that autologously expanded AT-MSCs are better than PRP, especially at 12- and 24-month follow-ups47.
Bolia et al. (2021) compared the clinical efficacy of BMAC and SVF for knee OA48 based on 10 studies with 472 patients (233 patients with BMAC, 239 patients with SVF). The analysis showed that SVF injection had a greater effect on pain than BMAC injection. However, more complications were associated with SVF injection than with BMAC injection (67% of patients with SVF injection vs. 50% of patients with BMAC injection)48.
Allogenic UCB-MSCs were better than BMAC for cartilage regeneration in knee OA49. In a study of 176 patients with knee OA, Yang et al. (2022) compared the therapeutic efficacy of BMAC and UCB-MSCs and reported similar clinical outcomes between the two groups; however, UCB-MSC implantation was more effective at promoting cartilage regeneration than was BMAC implantation50. UCB-MSC implantation is comparable to SVF implantation in knee OA51.
To compare treatment efficacy between BM-MSCs and AT-MSCs, Han et al. (2020) analyzed nine RCTs with 377 patients52. According to the VAS and WOMAC scores, AT-MSCs are better than BM-MSCs for treating knee OA52. Wei et al. (2021) analyzed 203 knee OA patients in eight studies using three types of MSCs: BM-MSCs, AD-MSCs, and UC-MSCs. The analysis suggested that AD-MSCs are the most effective at relieving pain, while UC-MSCs are the most effective at improving function31. Jeyaraman et al. (2021) also confirmed that MSCs from adipose tissue were better than BM-MSCs for treating knee OA53. Indeed, after 24 months of implantation, AD-MSCs had significantly better Lysholm scores than BM-MSCs did53.
To compare the therapeutic effects of PRP, MSCs from adipose tissue, and MSCs from bone marrow with HA and normal saline in treating knee OA, Zhao et al. (2021) analyzed 43 studies involving 6 months of follow-up and concluded that MSCs from adipose tissue are the best kind of treatment for relieving pain, while leukocyte-poor PRP is the most effective for functional improvement. At the 12-month follow-up, both MSCs from adipose tissue and LP-PRP had clinical pain relief effects54.
Which dose of stem cells is best for treating knee osteoarthritis?
Matas et al. (2019) reported that two doses of UC-MSCs (on day 0 and after 6 months, 20.106 cells per dose) were better than a single dose of 20.106 UC-MSCs55. In a recent publication, Sadri et al. (2023) used a high dose of 100.106 allogeneic AT-MSCs to treat knee OA patients. At this high dose, the authors found that intra-articular injection is safe, as indicated by significant improvements in laboratory data, MRI findings, and clinical examination at the 12-month follow-up29. Based on 14 studies with 564 patients, Muthu et al. (2021) categorized the treatments into four doses, namely < 10.106 MSCs, 10–50.106 MSCs, 50–100.106 MSCs, and > 100.106 MSCs, and recommended that a dose of 50–100.106 MSCs confers superior benefits56.
Based on 16 studies, Huang et al. (2023) grouped the doses of stem cells used for treating knee OA into three types: low dose (0–25.106 cells), high dose (25–50.106 cells), and high dose (> 50.106 cells)30. In general, a high dose of stem cells results in a better response; however, the adverse effects also increase with increasing doses30.
Non-expanded stem cells or expanded stem cells: which are better?
Muthu et al. (2021) investigated the following question: is culture expansion necessary in autologous MSC therapy to obtain superior results in treating knee OA? A meta-analysis was performed, with 17 studies including 767 patients. Based on the improvements in the VAS, WOMAC, Lysholm, and KOOS scores, the authors concluded that in vitro culture of autologous MSCs is unnecessary for obtaining superior results57. In a meta-analysis, Kim et al. (2023) also showed that autologous AD-MSCs and SVF injections had similar efficacy in treating knee OA58.
In a rabbit model of knee OA, Anjiki et al. (2023) compared the therapeutic effects of SVF (a type of nonexpanded stem cells) and AT-MSCs. The authors found that SVF had better effects on chondrocytes. The SVF group showed greater expression of collagen II and SOX9 in the cartilage, greater expression of TGF-beta and IL-10 in the synovium, lower expression of MMP-13, and a lower M1/M2 macrophage ratio than the ADSC group. These findings revealed that SVF cells were superior to ADSCs59. However, in terms of clinical findings, Yokota et al. (2019) showed that AT-MSCs were better than SVFs at treating knee OA after 6 months of follow-up60 or 2 years of follow-up61.
Should treatments for knee osteoarthritis be combined for greater efficacy?
Huang et al. (2022) compared the effects of combinations of PRP with different hyaluronans and PRP alone in 99 patients with K-L grade II OA. Although this combination is safe, the efficacy of the two regimens did not differ significantly62. In contrast to the findings of Huang et al. (2022), Sun et al. (2021) reported that the combination of PRP with Hyajoint Plus helped reduce pain better than PRP alone at 6 months63. However, in a recent analysis by Howlader et al. (2023), based on six studies, five of which were RCTs, the authors suggested that the combination of PRP and HA yields outcomes comparable to those of PRP therapy alone at the 12-month follow-up64; however, at the 24-month follow-up, the combination has the potential to yield superior outcomes compared to PRP alone64.
Zhao et al. (2022) compared the effects of MSC injection alone and MSC in combination with PRP injection for the treatment of knee OA65 in a meta-analysis of six RCTs including 493 patients. Although the results showed that the combination of MSCs and PRP had good clinical efficacy in improving pain and joint functions, there were no significant differences between the MSC plus PRP group and the MSC alone group65.
Which grade of OA is suitable for treatment?
Bakowski et al. (2023) followed 59 knee OA patients treated with intra-articular injection of autologous adipose tissue. They found that patients with stage II disease and a normal BMI are most likely to benefit from this therapy, while patients with stage IV disease are not satisfied with this therapy66. Similarly, Kuwasawa et al. (2023) reported a study in which expanded AT-MSCs were used for knee OA patients with K-L grades 2, 3, and 4. The data demonstrated that intra-articular administration of AT-MSCs to knee OA patients improved KOOS at 6 months; however, the difference was more significant for K-L grade 2 or 3 knees than for K-L grade 4 knees.
Conclusion
Several strategies are currently used to treat knee OA, ranging from the use of accelerated therapies such as HA or PRP injection to the use of heterogeneous cell populations (BMAC or SVF) or pure cell populations (MSCs from adipose tissue, bone marrow, or the umbilical cord). Although almost all therapies show some benefits in almost all patients with knee OA according to short-term follow-up studies, their treatment efficacy differs over long-term follow-up. Based on the results of the meta-analyses presented in this review, we propose that increasing the efficacy from HA to PRP, BMAC, SVF, BM-MSCs, UC-MSCs, and AT-MSCs. Almost all the analyses showed that expanded MSCs are effective at treating knee OA, but the best candidate for reducing pain is AT-MSCs. Because these findings were based on meta-analyses, well-designed clinical trials should be performed to confirm these observations.
Abbreviations
AT: Adipose tissue, AT-MSC: Adipose tissue derived MSCs, BMAC: Bone marrow aspirate concentrate, BM-MSC: Bone marrow derived MSCs, HA: Hyaluronic acid, MNC: Mononuclear cells, MSC: Mesenchymal stem cell, OA: Osteoarthritis, PRP: Platelet rich plasma, SVF: Stromal vascular fraction, UC-MSC: Umbilical cord derived MSCs
Acknowledgments
None
Author’s contributions
LTBP and VBN drafted the manuscript, PVP suggested the ideas, finalized the manuscript, and drew the figure 1. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
https://www.who.int/news-room/fact-sheets/detail/osteoarthritis.
.
-
Dainese
P.,
Wyngaert
K.V.,
De Mits
S.,
Wittoek
R.,
Van Ginckel
A.,
Calders
P.,
Association between knee inflammation and knee pain in patients with knee osteoarthritis: a systematic review. Osteoarthritis and Cartilage.
2022;
30
(4)
:
516-34
.
View Article PubMed Google Scholar -
Martin-Alarcon
L.,
Schmidt
T.A.,
Rheological effects of macromolecular interactions in synovial fluid. Biorheology.
2016;
53
(2)
:
49-67
.
View Article PubMed Google Scholar -
Altman
R.D.,
Manjoo
A.,
Fierlinger
A.,
Niazi
F.,
Nicholls
M.,
The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskeletal Disorders.
2015;
16
(1)
:
321
.
View Article PubMed Google Scholar -
Vincent
P.,
Lucas de Couville
T.,
Thomas
T.,
Intra-Articular Hyaluronic Acid for Knee Osteoarthritis: A Postmarket, Open-Label, Long-Term Historical Control Study with Analysis Detailed per Krellgren-Lawrence Radiologic Osteoarthritis Scale Grade. Current Therapeutic Research, Clinical and Experimental.
2020;
92
:
100575
.
View Article PubMed Google Scholar -
Mao
B.,
Pan
Y.,
Zhang
Z.,
Yu
Z.,
Li
J.,
Fu
W.,
Efficacy and Safety of Hyaluronic Acid Intra-articular Injection after Arthroscopic Knee Surgery: A Systematic Review and Meta-analysis. Orthopaedic Surgery.
2023;
15
(1)
:
16-27
.
View Article PubMed Google Scholar -
Zheng
Y.,
Duan
X.,
Qi
S.,
Hu
H.,
Wang
M.,
Ren
C.,
Acupuncture Therapy plus Hyaluronic Acid Injection for Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Evidence-Based Complementary and Alternative Medicine.
2020;
2020
:
4034105
.
View Article PubMed Google Scholar -
Eymard
F.,
Ornetti
P.,
Maillet
J.,
Noel
É.,
Adam
P.,
Legré-Boyer
V.,
GRIP (Groupe de Recherche sur les Injections de PRP
PRP Injection Research Group),
Intra-articular injections of platelet-rich plasma in symptomatic knee osteoarthritis: a consensus statement from French-speaking experts. Knee Surgery, Sports Traumatology, Arthroscopy : Official Journal of the ESSKA.
2021;
29
(10)
:
3195-210
.
View Article PubMed Google Scholar -
Bennell
K.L.,
Paterson
K.L.,
Metcalf
B.R.,
Duong
V.,
Eyles
J.,
Kasza
J.,
Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. Journal of the American Medical Association.
2021;
326
(20)
:
2021-30
.
View Article PubMed Google Scholar -
Bansal
H.,
Leon
J.,
Pont
J.L.,
Wilson
D.A.,
Bansal
A.,
Agarwal
D.,
Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Scientific Reports.
2021;
11
(1)
:
3971
.
View Article PubMed Google Scholar -
Kassem
M.,
Abdallah
B.M.,
Human bone-marrow-derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell and Tissue Research.
2008;
331
(1)
:
157-63
.
View Article PubMed Google Scholar -
Chahla
J.,
Mannava
S.,
Cinque
M.E.,
Geeslin
A.G.,
Codina
D.,
LaPrade
R.F.,
Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthroscopy Techniques.
2017;
6
(2)
:
e441-5
.
View Article PubMed Google Scholar -
Cavallo
C.,
Boffa
A.,
de Girolamo
L.,
Merli
G.,
Kon
E.,
Cattini
L.,
Bone marrow aspirate concentrate quality is affected by age and harvest site. Knee Surgery, Sports Traumatology, Arthroscopy : Official Journal of the ESSKA.
2023;
31
(6)
:
2140-51
.
View Article PubMed Google Scholar -
Muthu
S.,
Jeyaraman
M.,
Narula
A.,
Ravi
V.R.,
Gandi
A.,
Khanna
M.,
Factors Influencing the Yield of Progenitor Cells in Bone Marrow Aspiration Concentrate-A Retrospective Analysis of 58 Patients. Biomedicines.
2023;
11
(3)
:
738
.
View Article PubMed Google Scholar -
Keeling
L.E.,
Belk
J.W.,
Kraeutler
M.J.,
Kallner
A.C.,
Lindsay
A.,
McCarty
E.C.,
Bone Marrow Aspirate Concentrate for the Treatment of Knee Osteoarthritis: A Systematic Review. The American Journal of Sports Medicine.
2022;
50
(8)
:
2315-23
.
View Article PubMed Google Scholar -
Rasovic
P.,
Dulic
O.,
Lalic
I.,
Matijevic
R.,
Janjic
N.,
Tosic
M.,
The role of osteoarthritis severity, BMI and age on clinical efficacy of bone marrow aspirate concentrate in the treatment of knee osteoarthritis. Regenerative Medicine.
2023;
18
(9)
:
735-47
.
View Article PubMed Google Scholar -
Kon
E.,
Boffa
A.,
Andriolo
L.,
Di Martino
A.,
Di Matteo
B.,
Magarelli
N.,
Combined subchondral and intra-articular injections of bone marrow aspirate concentrate provide stable results up to 24 months. Knee Surgery, Sports Traumatology, Arthroscopy : Official Journal of the ESSKA.
2023;
31
(6)
:
2511-7
.
View Article PubMed Google Scholar -
Wei
P.,
Bao
R.,
Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence. International Journal of Molecular Sciences.
2022;
24
(1)
:
59
.
View Article PubMed Google Scholar -
Lopa
S.,
Colombini
A.,
Moretti
M.,
de Girolamo
L.,
Injective mesenchymal stem cell-based treatments for knee osteoarthritis: from mechanisms of action to current clinical evidences. Knee Surgery, Sports Traumatology, Arthroscopy : Official Journal of the ESSKA.
2019;
27
(6)
:
2003-20
.
View Article PubMed Google Scholar -
Xiang
X.N.,
Zhu
S.Y.,
He
H.C.,
Yu
X.,
Xu
Y.,
He
C.Q.,
Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Research {&}amp; Therapy.
2022;
13
(1)
:
14
.
View Article PubMed Google Scholar -
Hong
Z.,
Chen
J.,
Zhang
S.,
Zhao
C.,
Bi
M.,
Chen
X.,
Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. International Orthopaedics.
2019;
43
(5)
:
1123-34
.
View Article PubMed Google Scholar -
Goncharov
E.N.,
Koval
O.A.,
Nikolaevich Bezuglov
E.,
Encarnacion Ramirez
M.J.,
Engelgard
M.,
Igorevich
E.I.,
Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review. Medicina (Kaunas, Lithuania).
2023;
59
(12)
:
2090
.
View Article PubMed Google Scholar -
Tsubosaka
M.,
Matsumoto
T.,
Sobajima
S.,
Matsushita
T.,
Iwaguro
H.,
Kuroda
R.,
Comparison of Clinical and Imaging Outcomes of Different Doses of Adipose-Derived Stromal Vascular Fraction Cell Treatment for Knee Osteoarthritis. Cell Transplantation.
2021;
30
:
9636897211067454
.
View Article PubMed Google Scholar -
Tsubosaka
M.,
Matsumoto
T.,
Sobajima
S.,
Matsushita
T.,
Iwaguro
H.,
Kuroda
R.,
The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskeletal Disorders.
2020;
21
(1)
:
207
.
View Article PubMed Google Scholar -
Kim
Y.S.,
Oh
S.M.,
Suh
D.S.,
Tak
D.H.,
Kwon
Y.B.,
Koh
Y.G.,
Cartilage lesion size and number of stromal vascular fraction (SVF) cells strongly influenced the SVF implantation outcomes in patients with knee osteoarthritis. Journal of Experimental Orthopaedics.
2023;
10
(1)
:
28
.
View Article PubMed Google Scholar -
Çimen
O.,
Irg\it
K.S.,
Bekmezci
T.,
Büyüktopçu
Ö.,
\cSahbat
Y.,
Korucu
A.,
Midterm results of intra-articular stromal vascular fraction injection for the treatment of knee osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy : Official Journal of the ESSKA.
2023;
31
(11)
:
5012-7
.
View Article PubMed Google Scholar -
Long
Z.,
Zhang
M.,
Zhang
T.,
Zeng
L.,
Yang
K.,
Yang
T.,
The Effectiveness and Safety of Mesenchymal Stem Cells in the Treatment of Osteoarthritis: A Systematic Review and Meta-analysis of 28 Randomized Controlled Trials. Stem Cells International.
2022;
2022
:
6151866
.
View Article PubMed Google Scholar -
Kyriakidis
T.,
Pitsilos
C.,
Iosifidou
M.,
Tzaveas
A.,
Gigis
I.,
Ditsios
K.,
Stem cells for the treatment of early to moderate osteoarthritis of the knee: a systematic review. Journal of Experimental Orthopaedics.
2023;
10
(1)
:
102
.
View Article PubMed Google Scholar -
Sadri
B.,
Hassanzadeh
M.,
Bagherifard
A.,
Mohammadi
J.,
Alikhani
M.,
Moeinabadi-Bidgoli
K.,
Cartilage regeneration and inflammation modulation in knee osteoarthritis following injection of allogeneic adipose-derived mesenchymal stromal cells: a phase II, triple-blinded, placebo controlled, randomized trial. Stem Cell Research & Therapy.
2023;
14
(1)
:
162
.
View Article PubMed Google Scholar -
Huang
Z.,
Zhang
S.,
Cao
M.,
Lin
Z.,
Kong
L.,
Wu
X.,
What is the optimal dose of adipose-derived mesenchymal stem cells treatment for knee osteoarthritis? A conventional and network meta-analysis of randomized controlled trials. Stem Cell Research & Therapy.
2023;
14
(1)
:
245
.
View Article PubMed Google Scholar -
Wei
Z.J.,
Wang
Q.Q.,
Cui
Z.G.,
Inadera
H.,
Jiang
X.,
Wu
C.A.,
Which is the most effective one in knee osteoarthritis treatment from mesenchymal stem cells obtained from different sources?-A systematic review with conventional and network meta-analyses of randomized controlled trials. Annals of Translational Medicine.
2021;
9
(6)
:
452
.
View Article PubMed Google Scholar -
Lim
H.C.,
Park
Y.B.,
Ha
C.W.,
Cole
B.J.,
Lee
B.K.,
Jeong
H.J.,
Cartistem Research Group
Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cell Implantation Versus Microfracture for Large, Full-Thickness Cartilage Defects in Older Patients: A Multicenter Randomized Clinical Trial and Extended 5-Year Clinical Follow-up. Orthopaedic Journal of Sports Medicine.
2021;
9
(1)
:
2325967120973052
.
View Article PubMed Google Scholar -
Gupta
A.,
StemOneTM/Stempeucel\textregistered: CDSCO Approved, Adult Human Bone Marrow-Derived, Cultured, Pooled, Allogenic Mesenchymal Stem Cells for Knee Osteoarthritis. Biomedicines.
2023;
11
(11)
:
2894
.
View Article PubMed Google Scholar -
Gupta
P.K.,
Maheshwari
S.,
Cherian
J.J.,
Goni
V.,
Sharma
A.K.,
Tripathy
S.K.,
Efficacy and Safety of Stempeucel in Osteoarthritis of the Knee: A Phase 3 Randomized, Double-Blind, Multicenter, Placebo-Controlled Study. The American Journal of Sports Medicine.
2023;
51
(9)
:
2254-66
.
View Article PubMed Google Scholar -
Chen
C.F.,
Hu
C.C.,
Wu
C.T.,
Wu
H.H.,
Chang
C.S.,
Hung
Y.P.,
Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE\textregistered: a phase I/II, randomized, active-control, single-blind, multiple-center clinical trial. Stem Cell Research & Therapy.
2021;
12
(1)
:
562
.
View Article PubMed Google Scholar -
Duymus
T.M.,
Mutlu
S.,
Dernek
B.,
Komur
B.,
Aydogmus
S.,
Kesiktas
F.N.,
Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surgery, Sports Traumatology, Arthroscopy : Official Journal of the ESSKA.
2017;
25
(2)
:
485-92
.
View Article PubMed Google Scholar -
Raeissadat
S.A.,
Ghazi Hosseini
P.,
Bahrami
M.H.,
Salman Roghani
R.,
Fathi
M.,
Gharooee Ahangar
A.,
The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskeletal Disorders.
2021;
22
(1)
:
134
.
View Article PubMed Google Scholar -
Costa
L.A.,
Lenza
M.,
Irrgang
J.J.,
Fu
F.H.,
Ferretti
M.,
How Does Platelet-Rich Plasma Compare Clinically to Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review and Meta-analysis. The American Journal of Sports Medicine.
2023;
51
(4)
:
1074-86
.
View Article PubMed Google Scholar -
Chen
L.,
Jin
S.,
Yao
Y.,
He
S.,
He
J.,
Comparison of clinical efficiency between intra-articular injection of platelet-rich plasma and hyaluronic acid for osteoarthritis: a meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis.
2023;
15
:
1759720x231157043
.
View Article Google Scholar -
Peng
Y.N.,
Chen
J.L.,
Hsu
C.C.,
Chen
C.P.,
Suputtitada
A.,
Intra-Articular Leukocyte-Rich Platelet-Rich Plasma versus Intra-Articular Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-Analysis of 14 Randomized Controlled Trials. Pharmaceuticals (Basel, Switzerland).
2022;
15
(8)
:
974
.
View Article PubMed Google Scholar -
Kim
J.H.,
Park
Y.B.,
Ha
C.W.,
Are leukocyte-poor or multiple injections of platelet-rich plasma more effective than hyaluronic acid for knee osteoarthritis? A systematic review and meta-analysis of randomized controlled trials. Archives of Orthopaedic and Trauma Surgery.
2023;
143
(7)
:
3879-97
.
View Article PubMed Google Scholar -
Kim
J.H.,
Park
Y.B.,
Ha
C.W.,
Roh
Y.J.,
Park
J.G.,
Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthopaedic Journal of Sports Medicine.
2021;
9
(6)
:
23259671211011948
.
View Article PubMed Google Scholar -
Abbas
A.,
Du
J.T.,
Dhotar
H.S.,
The Effect of Leukocyte Concentration on Platelet-Rich Plasma Injections for Knee Osteoarthritis: A Network Meta-Analysis. The Journal of Bone and Joint Surgery. American Volume.
2022;
104
(6)
:
559-70
.
View Article PubMed Google Scholar -
Wang
Z.,
Wang
R.,
Xiang
S.,
Gu
Y.,
Xu
T.,
Jin
H.,
Assessment of the effectiveness and satisfaction of platelet-rich plasma compared with hyaluronic acid in knee osteoarthritis at minimum 7-year follow-up: A post hoc analysis of a randomized controlled trial. Frontiers in Bioengineering and Biotechnology.
2022;
10
:
1062371
.
View Article PubMed Google Scholar -
Shoukrie
S.I.,
Venugopal
S.,
Dhanoa
R.K.,
Selvaraj
R.,
Selvamani
T.Y.,
Zahra
A.,
Safety and Efficacy of Injecting Mesenchymal Stem Cells Into a Human Knee Joint To Treat Osteoarthritis: A Systematic Review. Cureus.
2022;
14
(5)
:
e24823
.
View Article PubMed Google Scholar -
Belk
J.W.,
Lim
J.J.,
Keeter
C.,
McCulloch
P.C.,
Houck
D.A.,
McCarty
E.C.,
Patients With Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone Marrow Aspirate Concentrate Injections Have Better Outcomes Than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-analysis. Arthroscopy.
2023;
39
(7)
:
1714-34
.
View Article PubMed Google Scholar -
Khoury
M.A.,
Chamari
K.,
Tabben
M.,
Alkhelaifi
K.,
Papacostas
E.,
Marín Fermín
T.,
Knee Osteoarthritis: Clinical and MRI Outcomes After Multiple Intra-Articular Injections With Expanded Autologous Adipose-Derived Stromal Cells or Platelet-Rich Plasma. Cartilage.
2023;
14
(4)
:
433-44
.
View Article PubMed Google Scholar -
Bolia
I.K.,
Bougioukli
S.,
Hill
W.J.,
Trasolini
N.A.,
Petrigliano
F.A.,
Lieberman
J.R.,
Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. The American Journal of Sports Medicine.
2022;
50
(5)
:
1451-61
.
View Article PubMed Google Scholar -
Lee
N.H.,
Na
S.M.,
Ahn
H.W.,
Kang
J.K.,
Seon
J.K.,
Song
E.K.,
Allogenic Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Are More Effective Than Bone Marrow Aspiration Concentrate for Cartilage Regeneration After High Tibial Osteotomy in Medial Unicompartmental Osteoarthritis of Knee. Arthroscopy.
2021;
37
(8)
:
2521-30
.
View Article PubMed Google Scholar -
Yang
H.Y.,
Song
E.K.,
Kang
S.J.,
Kwak
W.K.,
Kang
J.K.,
Seon
J.K.,
Allogenic umbilical cord blood-derived mesenchymal stromal cell implantation was superior to bone marrow aspirate concentrate augmentation for cartilage regeneration despite similar clinical outcomes. Knee Surgery, Sports Traumatology, Arthroscopy : Official Journal of the ESSKA.
2022;
30
(1)
:
208-18
.
View Article PubMed Google Scholar -
Kim
Y.S.,
Suh
D.S.,
Tak
D.H.,
Kwon
Y.B.,
Koh
Y.G.,
Adipose-Derived Stromal Vascular Fractions Are Comparable With Allogenic Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells as a Supplementary Strategy of High Tibial Osteotomy for Varus Knee Osteoarthritis. Arthroscopy, Sports Medicine, and Rehabilitation.
2023;
5
(3)
:
e751-64
.
View Article PubMed Google Scholar -
Han
X.,
Yang
B.,
Zou
F.,
Sun
J.,
Clinical therapeutic efficacy of mesenchymal stem cells derived from adipose or bone marrow for knee osteoarthritis: a meta-analysis of randomized controlled trials. Journal of Comparative Effectiveness Research.
2020;
9
(5)
:
361-74
.
View Article PubMed Google Scholar -
Jeyaraman
M.,
Muthu
S.,
Ganie
P.A.,
Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Meta-Analysis of Randomized Controlled Trials. Cartilage.
2021;
13
(1{_}suppl)
:
1532-47
.
View Article PubMed Google Scholar -
Zhao
D.,
Pan
J.K.,
Yang
W.Y.,
Han
Y.H.,
Zeng
L.F.,
Liang
G.H.,
Intra-Articular Injections of Platelet-Rich Plasma, Adipose Mesenchymal Stem Cells, and Bone Marrow Mesenchymal Stem Cells Associated With Better Outcomes Than Hyaluronic Acid and Saline in Knee Osteoarthritis: A Systematic Review and Network Meta-analysis. Arthroscopy.
2021;
37
(7)
.
View Article PubMed Google Scholar -
Matas
J.,
Orrego
M.,
Amenabar
D.,
Infante
C.,
Tapia-Limonchi
R.,
Cadiz
M.I.,
Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Translational Medicine.
2019;
8
(3)
:
215-24
.
View Article PubMed Google Scholar -
Muthu
S.,
Mir
A.A.,
Kumar
R.,
Yadav
V.,
Jeyaraman
M.,
Khanna
M.,
What is the clinically significant ideal mesenchymal stromal cell count in the management of osteoarthritis of the knee? - Meta-analysis of randomized controlled trials. Journal of Clinical Orthopaedics and Trauma.
2021;
25
:
101744
.
View Article PubMed Google Scholar -
Muthu
S.,
Kartheek
R.R.,
Jeyaraman
N.,
Rajendran
R.L.,
Khanna
M.,
Jeyaraman
M.,
Is Culture Expansion Necessary in Autologous Mesenchymal Stromal Cell Therapy to Obtain Superior Results in the Management of Knee Osteoarthritis?-Meta-Analysis of Randomized Controlled Trials. Bioengineering (Basel, Switzerland).
2021;
8
(12)
:
220
.
View Article PubMed Google Scholar -
Kim
K.I.,
Kim
M.S.,
Kim
J.H.,
Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials. The American Journal of Sports Medicine.
2023;
51
(3)
:
837-48
.
View Article PubMed Google Scholar -
Anjiki
K.,
Matsumoto
T.,
Kuroda
Y.,
Fujita
M.,
Hayashi
S.,
Nakano
N.,
Heterogeneous Cells as well as Adipose-Derived Stromal Cells in Stromal Vascular Fraction Contribute to Enhance Anabolic and Inhibit Catabolic Factors in Osteoarthritis. Stem Cell Reviews and Reports.
2023;
19
(7)
:
2407-19
.
View Article PubMed Google Scholar -
Yokota
N.,
Hattori
M.,
Ohtsuru
T.,
Otsuji
M.,
Lyman
S.,
Shimomura
K.,
Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. The American Journal of Sports Medicine.
2019;
47
(11)
:
2577-83
.
View Article PubMed Google Scholar -
Yokota
N.,
Lyman
S.,
Hanai
H.,
Shimomura
K.,
Ando
W.,
Nakamura
N.,
Clinical Safety and Effectiveness of Adipose-Derived Stromal Cell vs Stromal Vascular Fraction Injection for Treatment of Knee Osteoarthritis: 2-Year Results of Parallel Single-Arm Trials. The American Journal of Sports Medicine.
2022;
50
(10)
:
2659-68
.
View Article PubMed Google Scholar -
Huang
H.Y.,
Hsu
C.W.,
Lin
G.C.,
Lin
H.S.,
Chou
Y.J.,
Liou
I.H.,
Comparing efficacy of a single intraarticular injection of platelet-rich plasma (PRP) combined with different hyaluronans for knee osteoarthritis: a randomized-controlled clinical trial. BMC Musculoskeletal Disorders.
2022;
23
(1)
:
954
.
View Article PubMed Google Scholar -
Sun
S.F.,
Lin
G.C.,
Hsu
C.W.,
Lin
H.S.,
Liou
I.S.,
Wu
S.Y.,
Comparing efficacy of intraarticular single crosslinked Hyaluronan (HYAJOINT Plus) and platelet-rich plasma (PRP) versus PRP alone for treating knee osteoarthritis. Scientific Reports.
2021;
11
(1)
:
140
.
View Article PubMed Google Scholar -
Howlader
M.A.,
Almigdad
A.,
Urmi
J.F.,
Ibrahim
H.,
Efficacy and Safety of Hyaluronic Acid and Platelet-Rich Plasma Combination Therapy Versus Platelet-Rich Plasma Alone in Treating Knee Osteoarthritis: A Systematic Review. Cureus.
2023;
15
(10)
:
e47256
.
View Article PubMed Google Scholar -
Zhao
J.,
Liang
G.,
Han
Y.,
Yang
W.,
Xu
N.,
Luo
M.,
Combination of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP) in the treatment of knee osteoarthritis: a meta-analysis of randomised controlled trials. BMJ Open.
2022;
12
(11)
:
e061008
.
View Article PubMed Google Scholar -
B\kakowski
P.,
Kaszyński
J.,
Baka
C.,
Kaczmarek
T.,
Ciemniewska-Gorzela
K.,
B\kakowska-Żywicka
K.,
Patients with stage II of the knee osteoarthritis most likely benefit from the intra-articular injections of autologous adipose tissue-from 2 years of follow-up studies. Archives of Orthopaedic and Trauma Surgery.
2023;
143
(1)
:
55-62
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 12 (2023)
Page No.: 6065-6074
Published on: 2023-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4273 times
- PDF downloaded - 1422 times
- XML downloaded - 148 times
 Biomedpress
Biomedpress



