Abstract
Statins are widely used lipid-lowering drugs that are relatively well-tolerated and have an established safety profile. However, statin therapy has been reported to increase the risk of developing new-onset type 2 diabetes mellitus (NOD2). Although this side effect is rare, research on this topic is still ongoing. This bibliometric study was performed to provide an overview of the dynamics of research on statin-associated NOD2 and insulin resistance from the initial report to the year 2022. Original articles related to statin-associated NOD2 and insulin resistance were selected and retrieved from the Web of Science database. A quantitative analysis of publication trends, the contributions of different countries and funding agencies, and the most highly cited articles were then tabulated. The citation networks and the co-occurrence analysis of keywords of the included articles were illustrated with VOSviewer. A total of 271 research articles were included and analysed. The years 2012 to 2016 were prolific in research on statin-associated NOD2 and insulin resistance, followed by a decreasing trend in publications on this topic in many countries, particularly from 2020 to 2021. However, researchers from South Korea and China seem to have had a continued interest in this research area and the trend in publications increased again in 2022. Based on this trend, it is predicted that the number of pertinent articles in the coming years will be maintained or will continue to rise. The co-occurrence analysis of keywords showed that ``atorvastatin'' occurred more often than other statins. Among the thematic areas of research on statin-associated NOD2 and insulin resistance that were identified in this study were ``heterogeneity'', ``peripheral glucose uptake and insulin sensitivity'', ``tissue inflammation and oxidative stress'' and ``targeted tissues''. This is the first bibliometric study to predict the trends and provide an overview of the progress of research on statin-associated NOD2 and insulin resistance.
Introduction
Statins are lipid-lowering drugs widely used in the primary prevention of atherosclerotic cardiovascular disease. Their benefits in reducing cardiovascular mortality and morbidity are remarkable1. Despite their established safety profile, statins have been reported to enhance the risk of developing new-onset type 2 diabetes mellitus (NOD2) and worsen glucose control in patients with diabetes2, 3, 4. Although an earlier meta-analysis of 112 randomized controlled trials and 64 cohort studies concluded that the overall prevalence of the side effects of statins, including impaired glucose tolerance, was not significant, it revealed the high heterogeneity between studies5, making the conclusions uncertain. The risk of developing NOD2 is influenced by co-morbidities and genetic variations1, whereas it was reported that the type of statins used might not significantly alter the risk5. Based on a more recent systematic review and meta-analysis involving 67 randomized controlled trials enrolling a total of 25,481 individuals, statins minimally but significantly increase diabetic indexes in individuals with adequate or altered glycemic control. The diabetogenic effect of statins does not seem to be determined by the type or dosage of statin prescribed6. Studies have suggested that statins can increase insulin resistance while reducing insulin sensitivity and secretion from pancreatic β-cells7, 8. A recent molecular study using skeletal muscle tissues from patients receiving statins demonstrated that statins aggravated the risk of insulin resistance in the muscle tissues9.
Globally, many studies investigating the effects of statin therapy on the development of NOD2 have been conducted. However, bibliometric analysis of the progress of research into statin-associated NOD2 and insulin resistance has not previously been carried out. Bibliometric analysis can provide an overview of overall research trends and may also identify potential future directions for a particular field of study10. The current analysis aimed to portray: 1) the global output of research into statin-associated NOD2 and insulin resistance over a period of 30 years, with a prediction of the trend of future studies; 2) the presence of any bibliographic factors that may influence the research on statin-associated NOD2 and insulin resistance, such as countries and funding agencies; 3) the countries/regions that have contributed the most to this field of study; 4) the most cited articles on statin-associated NOD2 and insulin resistance and the citation networks; and 5) a co-occurrence analysis of keywords related to this research area. It is important to declare that this bibliometric study does not challenge the use of statins in any way, but rather illustrates the research dynamics of statin-associated NOD2 and insulin resistance over recent decades.
Methods
Search strategy
A search was conducted on September 14, 2023 in the Web of Science (WoS) database using the following search string: ((TS=(hydroxymethylglutaryl-coa reductase inhibitors)) OR TS=(statins)) OR TS=(statin)) OR TS=(pitavastatin)) OR TS=(rosuvastatin)) OR TS=(pravastatin)) OR TS=(lovastatin)) OR TS=(simvastatin)) OR TS=(atorvastatin)) OR TS=(fluvastatin)) AND ((TS=(new onset diabetes mellitus)) OR TS=(insulin resistance)) OR TS=(insulin-resistant)) OR TS=(impaired fasting blood glucose)) OR TS=(impaired fasting blood sugar)) OR TS=(pre-diabetic)) OR TS=(non-diabetic)), with a time span ranging from 1991 to 2022.
Data collection
The search retrieved 2757 articles, which were then screened for inclusion criteria by two independent investigators (H.N.S. and H-S.H.): any discrepancies were solved by consensus. Only original articles, written in English and covering both clinical and experimental studies, were included. Those articles that were included reported on the association of statins with new-onset diabetes mellitus, the association of statins with disease progression in pre-existing diabetes mellitus, the association of statins with the prediction of insulin resistance, consisting of changes in fasting insulin levels, fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), and the homeostatic model assessment of insulin resistance (HOMA-IR) or β-cell function (HOMA-B), which is a marker of basal insulin secretion by pancreatic β-cells. Review articles, editorials, letters, meeting abstracts, book chapters, errata, articles unrelated to statin-associated NOD2 or insulin resistance, articles involving a combination of statins with other medications that focused on the effect of the other medications rather than the statins, articles involving compounds without a separated statin group, articles on the effects of statins on lipid profiles and liver enzymes without statin-associated NOD2 or insulin-resistant indicators, and articles on the effects of statins on pre-existing diabetes mellitus without disease progression were excluded.Figure 1 illustrates the flow chart of the article retrieval process. Based on the inclusion and exclusion criteria, only 271 documents were included in the analysis.
Bibliometric analysis
The data analysis procedure was adapted from Wong et al.10 and was divided into two parts. First, the publication trends (global research output), the countries/regions and funding agencies that have contributed the most to this field of study, and the most cited articles were quantitatively analysed. This analysis was meant to illustrate the impacts and productivity of this field of study. Secondly, the full citation records of the selected documents were exported from the WoS into “.txt” files for the construction of a bibliometric network, which is a citation analysis by documents to demonstrate the relationships among articles that received high levels of citation and a co-occurrence analysis of keywords to determine the research focus. VOSviewer software (Centre for Science and Technology Studies, Leiden University, Netherlands) was used to map these bibliometric networks.
| Funding agencies | Record count | Percentage |
|---|---|---|
| National Institutes of Health (NIH) USA | 19 | 7.01% |
| United States Department of Health Human Services | 19 | 7.01% |
| National Natural Science Foundation of China | 10 | 3.69% |
| Canadian Institutes of Health Research | 7 | 2.58% |
| Pfizer Inc. | 7 | 2.58% |
| Fundacao De Amparo A Pesquisa Do Estado De São Paulo Fapesp | 6 | 2.21% |
| AstraZeneca plc | 5 | 1.85% |
| Research Council of Finland | 5 | 1.85% |
| Conselho Nacional De Desenvolvimento Cientifico E Tecnologico | 4 | 1.48% |
| Coordenacao De Aperfeicoamento De Pessoal De Nivel Superior Capes | 4 | 1.48% |
| Countries/ regions | Total publication | H-index | Citations | 1992-1997 | 1998-2002 | 2003-2007 | 2008-2012 | 2013-2017 | 2018-2022 |
|---|---|---|---|---|---|---|---|---|---|
| Number of documents | |||||||||
| USA | 48 | 26 | 1992 | 2 | 0 | 4 | 11 | 24 | 7 |
| South Korea | 37 | 17 | 811 | 0 | 0 | 1 | 4 | 17 | 15 |
| Japan | 29 | 18 | 1057 | 0 | 0 | 7 | 11 | 7 | 4 |
| China | 27 | 12 | 578 | 0 | 0 | 0 | 4 | 12 | 11 |
| England | 14 | 11 | 1183 | 0 | 1 | 0 | 4 | 6 | 3 |
| Canada | 14 | 13 | 782 | 0 | 1 | 3 | 3 | 4 | 3 |
| Greece | 13 | 11 | 332 | 0 | 0 | 1 | 5 | 5 | 2 |
| Taiwan | 13 | 8 | 163 | 1 | 1 | 1 | 3 | 5 | 2 |
| Brazil | 13 | 9 | 327 | 0 | 0 | 2 | 6 | 3 | 2 |
| India | 11 | 7 | 159 | 0 | 0 | 0 | 1 | 4 | 6 |
| Article title | Authors | Source title | Year of publication | Total citations | Average citations per year | Citations in last 5-year (2018-2022) |
|---|---|---|---|---|---|---|
| Pravastatin and the development of diabetes mellitus - Evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study | Freeman et al . 18 | Circulation | 2001 | 671 | 30.5 | 56 |
| Statin use and risk of diabetes mellitus in postmenopausal women in the women's health initiative | Culver et al . 19 | Archives of Internal Medicine | 2012 | 314 | 28.5 | 66 |
| Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control | Nakata et al . 20 | Diabetologia | 2006 | 226 | 13.3 | 60 |
| Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6-year follow-up study of the METSIM cohort | Cederberg et al . 12 | Diabetologia | 2015 | 206 | 25.8 | 112 |
| Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients | Koh et al. 14 | Journal of the American College of Cardiology | 2010 | 169 | 13.0 | 37 |
| Circulating microRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes | Willeit et al . 21 | Diabetes | 2017 | 156 | 26.0 | 149 |
| Effect of torcetrapib on glucose, insulin, and hemoglobin A(1c) in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial | Barter et al . 22 | Circulation | 2011 | 137 | 11.4 | 55 |
| Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non-insulin dependent diabetic patients | Paolisso et al . 23 | Atherosclerosis | 2000 | 136 | 5.9 | 8 |
| Vascular and metabolic effects of combined therapy with ramipril and simvastatin in patients with type 2 diabetes | Koh et al . 13 | Hypertension | 2005 | 125 | 6.9 | 10 |
| The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study | Sathyapalan et al. 16 | Journal of Clinical Endocrinology & Metabolism | 2009 | 102 | 7.3 | 31 |
| Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism | Caparros-Martin et al . 11 | Microbiome | 2017 | 97 | 16.2 | 96 |
| Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients | Koh et al . 15 | Atherosclerosis | 2009 | 92 | 6.6 | 12 |
| Fluvastatin causes NLRP3 inflammasome-mediated adipose insulin resistance | Henriksbo et al . 24 | Diabetes | 2014 | 90 | 10.0 | 50 |
| A 52-week, randomized, open-label, parallel-group comparison of the tolerability and effects of pitavastatin and atorvastatin on high-density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low-density lipoprotein cholesterol and glucose intolerance | Sasaki et al . 25 | Clinical Therapeutics | 2008 | 87 | 5.8 | 14 |
| Effects of statins on adipose tissue inflammation their inhibitory effect on MyD88-independent IRF3/IFN-beta pathway in macrophages | Abe et al . 17 | Arteriosclerosis Thrombosis and Vascular Biology | 2008 | 77 | 5.1 | 29 |
Results
Publication trends
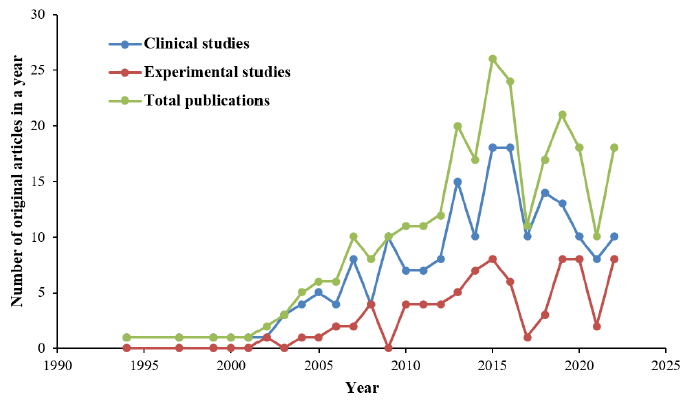
The total number of original research articles related to statin-associated NOD2 and insulin resistance increased from 1 article in 1994 to 10 articles in 2007, and peaked at 26 articles in 2015 (Figure 2). Although there was a decreasing trend of publications until 2017, the number of relevant studies increased again until the year 2019, during which 21 articles were published. From 2020 to 2021, the total number of original articles dramatically decreased to 10 articles in 2021. However, the studies again increased in trend to 18 articles in the year 2022. Clinical studies were more frequent than experimental studies throughout the years. From 1994 until 2022, 192 clinical studies and 79 experimental studies on statin-associated NOD2 and insulin resistance were published. Until the end of year 2022, the 271 articles that were included were cited a total of 7,260 times (including 707 self-citations). Thus, the average number of citations per article was 26.79.
Contributions of funding agencies
Research funding agencies significantly influenced the growth and direction of statin-associated NOD2 and insulin resistance research. Table 1 presents the top 10 funding agencies that were involved in this research area. The National Institutes of Health (NIH) of the United States and the United States Department of Health and Human Services were the top two funders, being acknowledged in 38 documents (14.0%). The National Natural Science Foundation of China was acknowledged in 10 documents (3.7%), followed by the Canadian Institutes of Health Research in 7 documents (2.6%). The two pharmaceutical companies that contributed most to the research were Pfizer Inc. in 7 documents (2.6%) and AstraZeneca plc in 5 documents (1.9%). Other funding agencies in the top 10 list included the Fundação de Amparo à Pesquisa do Estado de São Paulo of Brazil (2.2%), the Research Council of Finland (1.9%), and the Conselho Nacional De Desenvolvimento Cientifico E Tecnologico and Coordenacao De Aperfeicoamento De Pessoal De Nivel Superior Capes (1.5%).
Contributions of countries
The 10 countries that contributed most to research on statin-associated NOD2 and insulin resistance until the end of 2022 are shown in Table 2. ‘Publication by country’ refers to the affiliation country of the authors. The United States was the most productive country and began relevant research in 1994, which then increased steadily. South Korea was the second-most productive country, while the third and fourth were Japan, which boosted its research on this issue between 2008 and 2012, followed by China. Based on the observed trend, the 2013 to 2017 period was the most productive in research related to statin-associated NOD2 and insulin resistance. After that, the majority of the countries produced less research on statin-associated NOD2 and insulin resistance over the final 5 years (2018 to 2022). However, researchers from South Korea and China seemed to maintain their enthusiasm in this research area and their productivity was minimally reduced, by only 1-2 publications, after the prolific period.
The most highly cited articles and citation analysis by documents
Table 3 lists the 15 most cited articles on statin-associated NOD2 and insulin resistance until December 2022. The short-listed articles were published in the period between 2000 and 2017. The most cited article was by Freeman et al. 18, who reported that pravastatin reduced the risk of diabetes mellitus in 5,974 middle-aged men by 30%, while the second most cited article was by Culver et al. 19, who reported that statin therapy augmented the risk of diabetes among 153,840 postmenopausal women. The third most cited article was by Nakata et al. 20. In contrast to the previous two articles, Nakata et al.20 conducted an experimental study showing that atorvastatin mitigated adipocyte maturation and downregulated soluble carrier 2A4 (SLC2A4) expression, leading to insulin resistance and glucose tolerance impairment. Based on the 10 most cited articles, clinical studies were cited most, followed by experimental studies that investigated the molecular mechanisms of statin-associated NOD2. In terms of their continued relevance, the top articles related to research in statin-associated NOD2 and insulin resistance were still being cited within the last 5 years.
Our citation analysis by documents investigated the relationships between articles that received the highest levels of citation (Figure 3). In this figure, each document is represented by a node and labeled as the first author's name, followed by the year. The node size represents the citation number. The paper by Freeman et al.18 had the greatest number of citations, followed by Culver et al. 19 and Nakata et al.20, and this is reflected by their node size. The distance between nodes roughly indicates the relatedness of the documents in terms of their citation links. The further apart two documents are, the weaker their relatedness. The citation link between two documents is represented by a line. However, some of the linked nodes were scarcely visible in their labeling because the image overlapped unless the figure was zoomed in. The isolated nodes indicate no collaboration. Notably, studies by Willeit et al.21, Barter et al.22 and Sasaki et al.25 were considered isolated despite having relatively large node sizes for their high citation numbers.
Co-occurrence analysis of keywords
A total of 1096 keywords (all keywords) were extracted from the 271 original articles using VOSviewer. From these, 121 keywords met the threshold of five occurrences, meaning that a keyword was used at least five times in all the articles. Incomplete or irrelevant keywords or repetition of keywords were excluded from the map, leaving a total of 35 nodes of keywords. As shown in Fig. 4, each node represents a keyword, and its size illustrates the number of occurrences: the bigger the size is, the more the keyword was being used. The connecting line between two nodes indicates that these keywords have co-occurrence link or a potential relationship between them. “Atorvastatin” had the highest frequency of occurrence (107), indicating that it was the most frequently used keyword,followed by “statins” (92 occurrences) and “insulin resistance” (79 occurrences). The nodes “atorvastatin”, “simvastatin”, “pravastatin”, “statins”, “rosuvastatin” and “pitavastatin” were relatively large, were located at the center of the map and were more densely connected to other nodes in comparison with other statin-related keywords, namely “fluvastatin”, “lovastatin” and the class “HMG-CoA reductase inhibitor”, Among the statin-related keywords, “atorvastatin” had the highest keyword occurrence, followed by simvastatin (72), pravastatin (70), rosuvastatin (51), pitavastatin (10), lovastatin (6) and fluvastatin (6). “HMG-CoA reductase inhibitor” had an occurrence frequency of five.
The co-occurrence analysis of keywords is also beneficial in revealing thematic areas in the research on statin-associated NOD2 and insulin resistance. Four themes (clusters) were recognized, and are represented by colors in Figure 4. The 8 nodes in the blue-colored cluster includes “atorvastatin”, “rosuvastatin”, “pitavastatin”, “risk” (71 occurrences), “new-onset diabetes mellitus” (10), “prediabetes” (7), “impaired fasting glucose” (6) and “insulin secretion” (6), which could represent the theme “heterogeneity” in the diabetogenic risk of different statins in different subgroup of patients. The 12 nodes in the red-colored cluster include “simvastatin”, “pravastatin”, “statins”, “lovastatin”, “fluvastatin”, “HMG-CoA reductase inhibitor”, “insulin sensitivity” (17 occurrences), “glucose-uptake” (10), “muscle” (7), “GLUT4” (5), and “mitochondrial dysfunction” (5), which could represent the theme “peripheral glucose uptake and insulin sensitivity” after statin treatment. The 10 nodes in the green-colored cluster includes “metabolic syndrome” (32 occurrences), “inflammation” (28), “C-reactive protein” (19), “oxidative stress” (15), “glucose metabolism” (15), “liver” (9), “beta-cell function” (6), “adiponectin” (23), adipocytokines” (5) and “non-diabetic patients” (6). This could represent the theme “tissue inflammation and oxidative stress” which could be related to a metabolic condition, in particular diabetes. The 5 nodes in the yellow-colored cluster includes “insulin resistance” (79 occurrences), “adipose-tissue” (13), “skeletal-muscle” (13), “PPAR-gamma” (6) and “hepatic steatosis” (5). This could represent the theme “targeted tissues” involving liver, adipocytes and skeletal muscle in statin-associated NOD2 and insulin resistance. These 4 clusters have multiple connections to each other.
Discussion
Research on statin-associated NOD2 and insulin resistance started in the 1990s—a few years after the first statin, lovastatin, was approved by the United States Food and Drug Administration in 1987. This was followed by other semi-synthetic (simvastatin and pravastatin) and synthetic (fluvastatin, atorvastatin, rosuvastatin, and pitavastatin) statins. However, the magnitude of the research on statin-associated NOD2 and insulin resistance over a 30-year analysis period is relatively small when compared to other research areas concerning the benefits of statins26, 27, which is probably because this adverse effect of statin is relatively rare. The studies, which were predominantly clinical (70.8%), were extensively published in influential journals categorized under “cardiovascular system & cardiology” such as the American Journal of Cardiology and Atherosclerosis. The number of articles related to statin-associated NOD2 and insulin resistance increased steadily from 1994 to 2020, with its peak in 2015, indicating continuous research interest in the field. The period 2013 to 2017 was the most prolific period, possibly driven the fact that research in many top countries was boosted in the previous 5 years (2008 to 2012), which was already more than double that of previous years. However, based on the number of articles published in a particular year, the trend in global publications actually decreased in 2017. Therefore, 2012 to 2016 should be taken as the most prolific period of research in this field. These data illustrate the fluctuation and extent of the research on this issue, about 30 years after the introduction of statins to the market. From 2020 onward, there was a dramatic drop in the number of new publications in many countries, especially the United States, which is likely due to fact that the coronavirus disease (COVID-19) pandemic significantly affected scientific research. Starting in early 2020, lockdowns were implemented worldwide to help curb the contagion, and this forced many research institutions and laboratories to cease operation. The pandemic has changed the direction of research and reshaped the allocation of funding, with COVID-19-related research being prioritized. Thus, research not focusing on the COVID-19 issue has suffered enormously28, 29. Moreover, major pharmaceutical companies and funding agencies involved in research on statin-induced NOD2 (e.g., Pfizer Inc. and AstraZeneca plc) are mostly located in the United States and Europe, where the focus on COVID-19 vaccine development was intense. However, as the number of published studies increased in trend again in 2022, it is predicted that the number of related articles in the coming years will be sustained or will continue to rise, further suggesting that research on statin-associated NOD2 and insulin resistance remains relevant.
Over the past three decades, the United States has been the leading country in this field of study in terms of the proportion of publications (18%), followed by South Korea (14%) and Japan (11%). The credibility of the United States as the leader was further supported by its highest H-index and citation number, reflecting highly impactful and quality research by its scholars. Although England was ranked fifth in terms of the number of related articles published, it had the second-highest number of citations after the United States, indicating the high academic impact of the two countries. The citations received by England were mainly attributable to an article by Freeman et al.18, which remains relevant in the field. This is evidenced by its continuous yearly citations, with a good number of citations in the last 5 years, 20 years after publication. Notably, the United States and Taiwan started research in this field relatively early, and the former progressed steadily in studying statin-associated NOD2, with the highest impact observed in 2016. Among the 10 most active countries, it is worth mentioning that five were from Asia, with South Korea taking the lead, followed by Japan, China, Taiwan, and India. The incidence of statin-induced NOD2 may be higher in Asian populations, thus promoting more research in this field within Asia. However, no published data were found to support this idea. In the era of pharmacogenetics and pharmacogenomics, this hypothesis could be tested in future. Developed countries have dominated global contributions to the field of study since sufficient funding, as well as advanced facilities and techniques, are better in these countries. The National Institute of Health, United States Department of Health Human Services, NIH National Heart Lung Blood Institute, and Pfizer Inc. were among the top funding agencies located in the United States.
A good citation number is commonly followed by a good link to other research works. Therefore, most of the 15 most cited articles were connected. However, some research works stand on their own, for instance the papers by Willeit et al. 21, Barter et al.22 and Sasaki et al.25, which had good citation numbers even without a citation network. The article by Willeit et al.21 demonstrated a strong association between miRNA-122 and the risk of new-onset metabolic syndrome and NOD2 in the Anglo-Scandinavian Cardiac Outcomes Trial and Bruneck Study. The article by Barter et al.22, on the other hand, reported the post hoc analysis of a clinical trial on the comparative effects of torcetrapib plus atorvastatin and atorvastatin alone on glycemic control in patients with type 2 diabetes mellitus. Meanwhile, the article by Sasaki et al.25 was a randomized controlled trial evaluating the tolerability and effects of pitavastatin and atorvastatin on glucose metabolism in Japanese patients with glucose intolerance. There are many possible reasons for the solitary nature of these three articles, which could be due to a research focus or findings that are not related to the other articles.
Based on the bibliometric analysis of keywords, atorvastatin has been a significant interest in this area of research, followed by simvastatin, pravastatin, rosuvastatin, and pitavastatin. This indicates that these statins—especially atorvastatin—have received more attention when compared to other statins in the research on statin-associated NOD2 and insulin resistance. The analysis of keywords summarised by this bibliometric study may help other researchers find suitable keywords in their literature searches. In this bibliometric study, we tried to blend the gathered keywords into the postulated mechanisms that had been reported, making the themes or clusters linked together in a more sensible way.
In the 1990s, the effects of statins on lipid profiles in patients with diabetes mellitus were investigated. Although their lipid profiles improved, worsening insulin resistance was observed in these patients30. These findings led to studies on the effect of statins on insulin resistance and its association with glucose metabolism over the next decade. However, the findings of these studies were inconsistent. Some statins showed no effects on plasma adiponectin (a glucose-regulating hormone), leptin, and insulin sensitivity in healthy individuals31, 32, while others showed deterioration33, 34, and a few demonstrated some beneficial effects of statins on these parameters35, 13, both in patients with type 2 diabetes mellitus and in those without diabetes. Furthermore, adiponectin level is positively associated with insulin sensitivity36. Experimental studies were conducted to elucidate the possible mechanism of atorvastatin in inducing NOD2. This drug was demonstrated to increase glucose intolerance and attenuate solute carrier SLC2A4 gene expression, which encodes the glucose transporter 4 (GLUT4) in adipocytes20. This may explain the impairment of peripheral glucose uptake and insulin sensitivity, which is especially observed in diabetic subjects.
While studies on the effects of statins on peripheral glucose uptake and insulin sensitivity continued, the research trend over the following decades also investigated the effects of statins on pancreatic beta cell function in vitro 17, 37 and the progression of pancreatic fibrosis in diabetic rats38, 39, in an attempt to explain the impairment of insulin secretion induced by statins. Some studies showed that pravastatin improved insulin secretion17 and decelerated the progression of pancreatic fibrosis in diabetic rats38, 39. In contrast, a recent study reported that rosuvastatin impaired insulin secretion in a pancreatic cell line37. It was obvious that the different methods and types of cells might partly explain the discrepancies between studies. Other than that, predicted by the keywords “hepatic steatosis” and “liver”, the effect of statins on the liver structure is another noteworthy subarea of this research field, since non-alcoholic fatty liver disease contributes to the development of type 2 diabetes mellitus40.
Skeletal muscle is another targeted tissue involved in the mechanism of insulin resistance and impaired glucose uptake. It was reported that long-term statin treatment induces prolonged activation of 5′-adenosine monophosphate–activated protein kinase (AMPK) α subunit (AMPKα) and reduction in protein kinase B/Akt activation, which consequently leads to metabolic inflexibility and insulin resistance in human skeletal muscle tissue after some time9. Throughout the years, glucose metabolism and insulin signaling pathways have been the focus of research on statin-induced NOD2 to understand the possible mechanisms better. Based on keyword occurrence, the roles of at least two related proteins have been studied regarding the effects of statins in glucose metabolism and insulin signaling: the glucose transporter (GLUT) (especially GLUT4)41, 42, 43 and peroxisome proliferator-activated receptor gamma (PPAR-γ)44, 45. Rosuvastatin was reported to improve peripheral insulin sensitization by increasing the expression of PPAR-γ and GLUT-4 in the white adipose tissue of hyperlipidemic rats44, 45. However, a more recent study using hyperlipidemic mice showed that rosuvastatin did not improve insulin sensitization, while pitavastatin showed minor attenuations in insulin signaling in adipocytes by promoting GLUT4 translocation to the plasma membrane of adipocytes41. Therefore, this collected information could support the link between the cluster “targeted tissues” and the cluster “peripheral glucose uptake and insulin sensitivity” in the co-occurrence analysis of keywords.
Over the last decade, the pleiotropic effects of statins on inflammatory and oxidative stress status have emerged, with inflammation, C-reactive protein and oxidative stress often appearing as keywords. Some studies have reported a reduction in C-reactive protein and TNF-α in statin-treated patients with diabetes46, 47, while other studies reported no significant changes in proinflammatory cytokines in statin-treated prediabetic patients48, 49. A recent study by Henriksbo et al.24 showed that atorvastatin promoted inflammasome-mediated adipose tissue inflammation and interleukin-1β-dependent insulin resistance. The discrepancies in these findings suggest the existence of an independent phenomenon between inflammation and NOD2 in their relationship to statin therapy.
Finally, the statin of choice in prescriptions for different subgroups of patients is predicted to be a future direction of studies in this field aimed at investigating the heterogeneity of the tested statins-simvastatin, atorvastatin, pravastatin, and rosuvastatin- and the heterogeneity in patient subgroups (e.g., non-diabetic, prediabetic, impaired fasting glucose, overt diabetes, and other metabolic syndromes) as supported by the cluster “heterogeneity” proposed in the bibliometric analysis of keywords.
A limitation of this bibliometric study is the use of only a single database. The WoS was selected to ensure that only peer-reviewed and credible journals were included in the sample. However, there is a possibility that a few articles were not included in our sampling. In contrast to other frequently used analytical review methods, such as systematic reviews, which are meant to answer a specific clinical research question, a bibliometric study may not represent a comprehensive clinical understanding of this topic. However, it may give an overview of the research dynamics relevant to this topic and therefore may predict future trends of research.
Conclusions
After the decreasing trend in global publications between 2020 and 2021, research on statin-associated NOD2 and insulin resistance seemed to be gaining momentum again in 2022. It is expected that the research on this issue will persist or even increase in coming years, since Eastern researchers, especially from South Korea and China, seem to have continued enthusiasm. Furthermore, the mechanism of statin-induced diabetes and insulin resistance has not been fully elucidated. Risk stratification represents a way forward for statin-associated NOD2 and insulin resistance that may predict individuals at risk of developing this rare side effect. This does not necessarily discourage adherence to statin therapy in patients but may help give patients more objective reassurance.
Abbreviations
AMPK: 5′-adenosine monophosphate–activated protein kinase, GLUT4: glucose transporter 4, NOD2: new-onset type 2 diabetes mellitus, PPAR-γ: peroxisome proliferator-activated receptor gamma, SLC2A4: soluble carrier 2A4, TNF-α: tumour necrosis factor alpha
Acknowledgments
We thank Universiti Sultan Zainal Abidin for the support in this project under Dana Penyelidikan Universiti 1.0 (UniSZA/2021/DPU1.0/15) project number R0319.
Author’s contributions
Conception, articles acquisition and screening of articles, data analysis and manuscript draft preparation, HNS, YK, H-SH and MN; data retrieval and analysis, AHN and MLN; manuscript revision and editing, NAM, HMSQ, AA, NAMF and NM. All authors have read and agreed to the published version of the manuscript.
Funding
Universiti Sultan Zainal Abidin for the support in this project–grant number UniSZA/2021/DPU1.0/15 (R0319).
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Ward
N.C.,
Watts
G.F.,
Eckel
R.H.,
Statin Toxicity. Circulation Research.
2019;
124
(2)
:
328-50
.
View Article PubMed Google Scholar -
Angelidi
A.M.,
Stambolliu
E.,
Adamopoulou
K.I.,
Kousoulis
A.A.,
Is atorvastatin associated with new onset diabetes or deterioration of glycemic control? Systematic review using data from 1.9 million patients. International journal of endocrinology.
2018;
2018
:
8380192
.
View Article Google Scholar -
Kamran
H.,
Kupferstein
E.,
Sharma
N.,
Karam
J.G.,
Myers
A.K.,
Youssef
I.,
Statins and new-onset diabetes in cardiovascular and kidney disease cohorts: a meta-analysis. Cardiorenal Medicine.
2018;
8
(2)
:
105-12
.
View Article PubMed Google Scholar -
Sunjaya
A.P.,
Sunjaya
A.F.,
Halim
S.,
Ferdinal
F.,
Risk and benefits of statins in glucose control management of type II diabetes. The International Journal of Angiology.
2018;
27
(3)
:
121-31
.
View Article PubMed Google Scholar -
Bytyçi
I.,
Penson
P.E.,
Mikhailidis
D.P.,
Wong
N.D.,
Hernandez
A.V.,
Sahebkar
A.,
Prevalence of statin intolerance: a meta-analysis. European Heart Journal.
2022;
43
(34)
:
3213-23
.
View Article PubMed Google Scholar -
Alvarez-Jimenez
L.,
Morales-Palomo
F.,
Moreno-Cabañas
A.,
Ortega
J.F.,
Mora-Rodríguez
R.,
Effects of statin therapy on glycemic control and insulin resistance: A systematic review and meta-analysis. European Journal of Pharmacology.
2023;
947
:
175672
.
View Article PubMed Google Scholar -
Agarwala
A.,
Kulkarni
S.,
Maddox
T.,
The association of statin therapy with incident diabetes: evidence, mechanisms, and recommendations. Current Cardiology Reports.
2018;
20
(7)
:
50
.
View Article PubMed Google Scholar -
Betteridge
D.J.,
Carmena
R.,
The diabetogenic action of statins - mechanisms and clinical implications. Nature Reviews. Endocrinology.
2016;
12
(2)
:
99-110
.
View Article PubMed Google Scholar -
Grunwald
S.A.,
Haafke
S.,
Grieben
U.,
Kassner
U.,
Steinhagen-Thiessen
E.,
Spuler
S.,
Statins aggravate the risk of insulin resistance in human muscle. International Journal of Molecular Sciences.
2022;
23
(4)
:
2398
.
View Article PubMed Google Scholar -
Wong
S.,
Mah
A.X.,
Nordin
A.H.,
Nyakuma
B.B.,
Ngadi
N.,
Mat
R.,
Emerging trends in municipal solid waste incineration ashes research: a bibliometric analysis from 1994 to 2018. Environmental Science and Pollution Research International.
2020;
27
(8)
:
7757-84
.
View Article PubMed Google Scholar -
Caparrós-Martín
J.,
Lareu
R.,
Ramsay
J.,
Peplies
J.,
Reen
F.,
Headlam
H.,
Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome 9. Microbiome.
2017;
5
(1)
:
95
.
View Article Google Scholar -
Cederberg
H.,
Stancáková
A.,
Yaluri
N.,
Modi
S.,
Kuusisto
J.,
Laakso
M.,
Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Diabetologia.
2015;
58
(5)
:
1109-17
.
View Article PubMed Google Scholar -
Koh
K.K.,
Quon
M.J.,
Han
S.H.,
Ahn
J.Y.,
Jin
D.K.,
Kim
H.S.,
Vascular and metabolic effects of combined therapy with ramipril and simvastatin in patients with type 2 diabetes. Hypertension.
2005;
45
(6)
:
1088-93
.
View Article PubMed Google Scholar -
Koh
K.K.,
Quon
M.J.,
Han
S.H.,
Lee
Y.,
Kim
S.J.,
Shin
E.K.,
Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. Journal of the American College of Cardiology.
2010;
55
(12)
:
1209-16
.
View Article PubMed Google Scholar -
Koh
K.K.,
Quon
M.J.,
Han
S.H.,
Lee
Y.,
Kim
S.J.,
Park
J.B.,
Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis.
2009;
204
(2)
:
483-90
.
View Article PubMed Google Scholar -
Sathyapalan
T.,
Kilpatrick
E.S.,
Coady
A.M.,
Atkin
S.L.,
The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. The Journal of Clinical Endocrinology and Metabolism.
2009;
94
(1)
:
103-8
.
View Article PubMed Google Scholar -
Abe
M.,
Toyohara
T.,
Ishii
A.,
Suzuki
T.,
Noguchi
N.,
Akiyama
Y.,
The HMG-CoA reductase inhibitor pravastatin stimulates insulin secretion through organic anion transporter polypeptides. Drug Metabolism and Pharmacokinetics.
2010;
25
(3)
:
274-82
.
View Article PubMed Google Scholar -
Culver
A.L.,
Ockene
I.S.,
Balasubramanian
R.,
Olendzki
B.C.,
Sepavich
D.M.,
Wactawski-Wende
J.,
Statin use and risk of diabetes mellitus in postmenopausal women in the Women's Health Initiative. Archives of Internal Medicine.
2012;
172
(2)
:
144-52
.
View Article PubMed Google Scholar -
Barter
P.J.,
Rye
K.A.,
Tardif
J.C.,
Waters
D.D.,
Boekholdt
S.M.,
Breazna
A.,
Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation.
2011;
124
(5)
:
555-62
.
View Article PubMed Google Scholar -
Willeit
P.,
Skroblin
P.,
Moschen
A.R.,
Yin
X.,
Kaudewitz
D.,
Zampetaki
A.,
Circulating microRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes.
2017;
66
(2)
:
347-57
.
View Article PubMed Google Scholar -
Nakata
M.,
Nagasaka
S.,
Kusaka
I.,
Matsuoka
H.,
Ishibashi
S.,
Yada
T.,
Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia.
2006;
49
(8)
:
1881-92
.
View Article PubMed Google Scholar -
Sasaki
J.,
Ikeda
Y.,
Kuribayashi
T.,
Kajiwara
K.,
Biro
S.,
Yamamoto
K.,
A 52-week, randomized, open-label, parallel-group comparison of the tolerability and effects of pitavastatin and atorvastatin on high-density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low-density lipoprotein cholesterol and glucose intolerance. Clinical Therapeutics.
2008;
30
(6)
:
1089-101
.
View Article PubMed Google Scholar -
Freeman
D.J.,
Norrie
J.,
Sattar
N.,
Neely
R.D.,
Cobbe
S.M.,
Ford
I.,
Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation.
2001;
103
(3)
:
357-62
.
View Article PubMed Google Scholar -
Henriksbo
B.D.,
Tamrakar
A.K.,
Xu
J.,
Duggan
B.M.,
Cavallari
J.F.,
Phulka
J.,
Statins promote interleukin-1β-dependent adipocyte insulin resistance through lower prenylation, not cholesterol. Diabetes.
2019;
68
(7)
:
1441-8
.
View Article PubMed Google Scholar -
Paolisso
G.,
Barbagallo
M.,
Petrella
G.,
Ragno
E.,
Barbieri
M.,
Giordano
M.,
Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non-insulin dependent diabetic patients. Atherosclerosis.
2000;
150
(1)
:
121-7
.
View Article PubMed Google Scholar -
Devos
P.,
Menard
J.,
Bibliometric analysis of research relating to hypertension reported over the period 1997-2016. Journal of Hypertension.
2019;
37
(11)
:
2116-22
.
View Article PubMed Google Scholar -
Gao
Y.,
Wang
Y.,
Zhai
X.,
He
Y.,
Chen
R.,
Zhou
J.,
Publication trends of research on diabetes mellitus and T cells (1997-2016): A 20-year bibliometric study. PLoS One.
2017;
12
(9)
:
e0184869
.
View Article PubMed Google Scholar -
Becker
G.,
Martin
T.,
Sabo
A.N.,
Bertrand
F.,
Hutt
A.,
Ayme-Dietrich
E.,
Impact of the COVID-19 pandemic on clinical research in hospitals: observational study in the first epicenter of the epidemic during the general lockdown in France. European Review for Medical and Pharmacological Sciences.
2021;
25
(2)
:
1158-62
.
PubMed Google Scholar -
Sohrabi
C.,
Mathew
G.,
Franchi
T.,
Kerwan
A.,
Griffin
M.,
Soleil C Del Mundo
J.,
Impact of the coronavirus (COVID-19) pandemic on scientific research and implications for clinical academic training - A review. International Journal of Surgery.
2021;
86
:
57-63
.
View Article PubMed Google Scholar -
Sheu
W.H.,
Shieh
S.M.,
Shen
D.D.,
Fuh
M.M.,
Jeng
C.Y.,
Chen
Y.D.,
Effect of pravastatin treatment on glucose, insulin, and lipoprotein metabolism in patients with hypercholesterolemia. American Heart Journal.
1994;
127
(2)
:
331-6
.
View Article PubMed Google Scholar -
Bulcão
C.,
Ribeiro-Filho
F.F.,
Sañudo
A.,
Roberta Ferreira
S.G.,
Effects of simvastatin and metformin on inflammation and insulin resistance in individuals with mild metabolic syndrome. American Journal of Cardiovascular Drugs.
2007;
7
(3)
:
219-24
.
View Article PubMed Google Scholar -
Sonmez
A.,
Dogru
T.,
Tasci
I.,
Yilmaz
M.I.,
Pinar
M.,
Naharci
I.,
The effect of fluvastatin on plasma adiponectin levels in dyslipidaemia. Clinical Endocrinology.
2006;
64
(5)
:
567-72
.
View Article PubMed Google Scholar -
Forst
T.,
Pfützner
A.,
Lübben
G.,
Weber
M.,
Marx
N.,
Karagiannis
E.,
Effect of simvastatin and/or pioglitazone on insulin resistance, insulin secretion, adiponectin, and proinsulin levels in nondiabetic patients at cardiovascular risk\textemdashthe PIOSTAT Study. Metabolism: Clinical and Experimental.
2007;
56
(4)
:
491-6
.
View Article PubMed Google Scholar -
Gannagé-Yared
M.H.,
Azar
R.R.,
Amm-Azar
M.,
Khalifé
S.,
Germanos-Haddad
M.,
Neemtallah
R.,
Pravastatin does not affect insulin sensitivity and adipocytokines levels in healthy nondiabetic patients. Metabolism: Clinical and Experimental.
2005;
54
(7)
:
947-51
.
View Article PubMed Google Scholar -
Huptas
S.,
Geiss
H.C.,
Otto
C.,
Parhofer
K.G.,
Effect of atorvastatin (10 mg/day) on glucose metabolism in patients with the metabolic syndrome. The American Journal of Cardiology.
2006;
98
(1)
:
66-9
.
View Article PubMed Google Scholar -
Achari
A.E.,
Jain
S.K.,
Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. International Journal of Molecular Sciences.
2017;
18
(6)
:
1321
.
View Article PubMed Google Scholar -
Qian
L.,
Zhu
K.,
Lin
Y.,
An
L.,
Huang
F.,
Yao
Y.,
Insulin secretion impairment induced by rosuvastatin partly though autophagy in INS-1E cells. Cell Biology International.
2020;
44
(1)
:
127-36
.
View Article PubMed Google Scholar -
Otani
M.,
Yamamoto
M.,
Harada
M.,
Otsuki
M.,
Effect of long- and short-term treatments with pravastatin on diabetes mellitus and pancreatic fibrosis in the Otsuka-Long-Evans-Tokushima fatty rat. British Journal of Pharmacology.
2010;
159
(2)
:
462-73
.
View Article PubMed Google Scholar -
Wei
L.,
Yamamoto
M.,
Harada
M.,
Otsuki
M.,
Treatment with atorvastatin attenuates progression of insulin resistance and pancreatic fibrosis in the Otsuka Long-Evans Tokushima fatty rats. Metabolism: Clinical and Experimental.
2016;
65
(2)
:
41-53
.
View Article PubMed Google Scholar -
Xia
M.F.,
Bian
H.,
Gao
X.,
NAFLD and diabetes: two sides of the same coin? Rationale for gene-based personalized NAFLD treatment. Frontiers in Pharmacology.
2019;
10
:
877
.
View Article PubMed Google Scholar -
Cho
Y.,
Lee
H.,
Park
H.K.,
Choe
E.Y.,
Wang
H.J.,
Kim
R.H.,
Differential Diabetogenic Effect of Pitavastatin and Rosuvastatin, in vitro and in vivo. Journal of Atherosclerosis and Thrombosis.
2020;
27
(5)
:
429-40
.
View Article PubMed Google Scholar -
Li
W.,
Liang
X.,
Zeng
Z.,
Yu
K.,
Zhan
S.,
Su
Q.,
Simvastatin inhibits glucose uptake activity and GLUT4 translocation through suppression of the IR/IRS-1/Akt signaling in C2C12 myotubes. Biomedicine and Pharmacotherapy.
2016;
83
:
194-200
.
View Article PubMed Google Scholar -
Sun
B.,
Zhong
Z.,
Wang
F.,
Xu
J.,
Xu
F.,
Kong
W.,
Atorvastatin impaired glucose metabolism in C2C12 cells partly via inhibiting cholesterol-dependent glucose transporter 4 translocation. Biochemical Pharmacology.
2018;
150
:
108-19
.
View Article PubMed Google Scholar -
de las Heras
N.,
Valero-Muñoz
M.,
Ballesteros
S.,
Gómez-Hernández
A.,
Martín-Fernández
B.,
Blanco-Rivero
J.,
Factors involved in rosuvastatin induction of insulin sensitization in rats fed a high fat diet. Nutrition, Metabolism, and Cardiovascular Diseases.
2013;
23
(11)
:
1107-14
.
View Article PubMed Google Scholar -
Valero-Muñoz
M.,
Martín-Fernández
B.,
Ballesteros
S.,
Cachofeiro
V.,
Lahera
V.,
Heras
N. de Las,
[Rosuvastatin improves insulin sensitivity in overweight rats induced by high fat diet. Role of SIRT1 in adipose tissue]. Sociedad Espanola de Arteriosclerosis.
2014;
26
(4)
:
161-7
.
View Article PubMed Google Scholar -
Bellia
A.,
Rizza
S.,
Lombardo
M.F.,
Donadel
G.,
Fabiano
R.,
Andreadi
K.,
Deterioration of glucose homeostasis in type 2 diabetic patients one year after beginning of statins therapy. Atherosclerosis.
2012;
223
(1)
:
197-203
.
View Article PubMed Google Scholar -
Matafome
P.,
Louro
T.,
Rodrigues
L.,
Crisóstomo
J.,
Nunes
E.,
Amaral
C.,
Metformin and atorvastatin combination further protect the liver in type 2 diabetes with hyperlipidaemia. Diabetes/Metabolism Research and Reviews.
2011;
27
(1)
:
54-62
.
View Article PubMed Google Scholar -
Buldak
L.,
Dulawa-Buldak
A.,
Labuzek
K.,
Okopien
B.,
Effects of 90-day hypolipidemic treatment on insulin resistance, adipokines and proinflammatory cytokines in patients with mixed hyperlipidemia and impaired fasting glucose. International Journal of Clinical Pharmacology and Therapeutics.
2012;
50
(11)
:
805-13
.
View Article PubMed Google Scholar -
Krysiak
R.,
Gdula-Dymek
A.,
Okopien
B.,
The effect of fenofibrate on lymphocyte release of proinflammatory cytokines and systemic inflammation in simvastatin-treated patients with atherosclerosis and early glucose metabolism disturbances. Basic {&}amp; Clinical Pharmacology {&}amp; Toxicology.
2013;
112
(3)
:
198-202
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 11 (2023)
Page No.: 6035-6048
Published on: 2023-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 3409 times
- PDF downloaded - 1282 times
- XML downloaded - 119 times
 Biomedpress
Biomedpress






