Abstract
Faced with an enlarged lymph node, many pathologists are puzzled by the question of whether it is benign or malignant. Our practical experience has shown that alone histological knowledge of the lymph node is not sufficient to diagnose their pathology. Recognizing the extremely subtle histological changes of reactive lymph nodes is decisive in the indication of other complementary research methods, such as immunohistochemistry and possibly molecular biology, to distinguish between benign and malignant lymph nodes. Immunostaining is also essential to detect the misdiagnosis pitfalls that often emerge in benign reactive proliferative lymph nodes. This review summarizes malignancy-like subtle histological changes of reactive enlarged lymph nodes, the misdiagnosis pitfalls, and experience in using several immunohistochemical markers to distinguish these pitfalls.
Introduction
The histopathological features of the lymphoproliferative status are complicated by the overlapping histological features of reactive and neoplastic lymphoproliferation. This complexity is being gradually mitigated by new insights into histopathology, immunohistochemistry, and genetics that are often updated. Since 2002, the World Health Organization (WHO) has published four publications on the classification of tumors of the hematopoietic system, including new data on immunohistochemistry and molecular biology of many hematopoietic tumors1, 2, 3, 4. As a result, the diagnosis of hematopoietic tumors, especially common B-cell lymphomas, has become more consistent between histopathology and clinical course, reaching about 90–98%5, 6.
During life, most lymph nodes are exposed to various levels of many types of antigens. Consequently, most of them show variable degrees of reactive proliferation. So at any age, reactive lymph node proliferation is common7, 8. The most critical aspect of lymph node evaluation is to distinguish reactive lymph nodes from their neoplasia. Accurate diagnosis of a histological type and the mutation status of lymphoma cells will greatly affect the treatment of patients. Faced with this situation, it is essential, in addition to a thorough understanding of the structure of the normal lymph node, to avoid confusing benign changes in the reactive lymph node and the overlapping histological features of neoplastic and reactive lymphoproliferation. This review summarizes malignancy-like subtle histological changes of enlarged lymph nodes, the misdiagnosis pitfalls, and experience in using several immunohistochemical markers to distinguish these pitfalls.
MALIGNANCY-LIKE SUBTLE HISTOLOGICAL CHANGES
To facilitate the identification of abnormal histological features, hyperplastic lymph nodes have been classified into histological patterns.
Common reactive follicular hyperplasia patterns
Reactive follicular hyperplasia (RFH) is defined as >90% of lymphatic follicles exhibiting germinal center response and is the phenomenon in which B cells are stimulated to proliferate in their compartment9. It is caused by a proliferation of the secondary follicles and occurs mainly in the cortex without infiltrating the capsule of the lymph node10. In contrast to follicular lymphoma (FL), B cells of reactive germinal centers (GCs) are negative for Bcl-211, 12. Under reactive conditions, the follicles can become very large due to fusion. Their composition and morphology can also vary somewhat; however, these abnormal changes usually involve only a few follicles at once, so the presence of normal follicles nearby means that the altered follicles are not neoplastic13. It should be noted that none of the histological features is pathognomonic. Therefore, based on a set of features, even a medical history can be helpful. Older patients are more susceptible to FL3, 13.
Enlarged follicles are of various sizes and shapes and can coalesce and show variable structures with dominant GCs and a distinct mantle zone. GCs show response patterns of B cells and mixed large and small lymphocytes consisting of centrocytes and centroblasts. The mitotic figure is common. Large numbers of tingible body macrophages are characteristic features of RFH. Surface immunoglobulins of lymphocytes are polyclonal. GCs are negative for Bcl-2.
Variations of the reactive follicular pattern
Progressive transformation of germinal center
The characteristic of progressive transformation of the GC (PTGC) is the expansion of the mantle lymphocytes into the surrounding compartments and GC. Some follicles may lose part of their mantle cells. The GCs became more sparse as mantle-derived lymphocytes were occupied. The B lymphocytes component is much more dominant than the T cells in these GCs. These cells with high density are often arranged in plaques or clumps, with dark nuclei resembling mantle cells. The involved follicles become very enlarged, with a diameter usually 3–5-times larger than that of adjacent normal follicles14, 15 (Figure 1).
At the end of the lymph node response, gradual elimination of the GC occurred by most mantle cells and few T cells migrating into the GC, with subsequent disintegration of these GCs into foci of centrocytes, centroblasts, and follicular dendritic cells (FDCs)16 (Figure 1). Therefore, PTGC can be commonly confused with nodal lymphocyte-predominant Hodgkin lymphoma (NLPHL) and floral FL2, 3, 17. Of note, PTGC is often associated with marginal lymphoma in children18. Indeed, this presents a diagnostic challenge in histopathology. In general, PTGC is usually expressed in only a small part of the lymph nodes; The lesional follicles are interspersed with benign secondary follicles and are devoid of small foci of epithelial macrophages, and lack the characteristic lymphocyte-predominant cells. Further immunostaining with Bcl-2− and Ki67+ reinforces the differential diagnosis. Larger CD10+ blasts in PTGC are usually absent outside of the nodules; they neither form rosettes with CD57+ T cells nor are they positive for epithelial membrane antigen13.
Giant reactive follicles
Reactive giant follicles can be tens of times larger in size than normal follicles, often taking on a zigzag appearance due to fusion of adjacent follicles (Figure 1). If this is the first case for the pathologist, this can easily be misdiagnosed as FL. Although these follicles are gigantic, the structure of the GC, mantle zone, and sometimes marginal zone is completely clear13, 15, 19.
However, in this case, there is often a combination of giant follicular hyperplasia with paracortical hyperplasia. The paracortical region at high magnification contains many small to medium lymphocytes in size (transformed lymphocytes), immunoblasts, rare neutrophils, and epithelioid-like histiocytes13, 19. There may be some giant cells that resemble Reed–Sternberg (Figure 2). Small lymphocytes often have a uniformly rounded nucleus, whereas medium-sized cells sometimes have an irregular nucleus with an angular shape. Individual cell necrosis (apoptotic or karyorrhectic) of the GC is common13.
Florid reactive follicles
Follicles with a predominance of centroblasts were involved in lacking or significantly reducing the mantle zone. This is a common feature of florid RFH and is common in infections with certain viruses, especially human immunodeficiency virus11, 20, 21. Occasionally, the proliferative mantle zone invaginates into the GC, resulting in disruption of the GC. This phenomenon is called ‘follicular lysis’20, 21, 22. Recognizing the floral variant of FL is essential (Figure 1).
Bcl-2 immunostaining is always the most useful to distinguish RFH from FL. More than 85% of FLs were positive for Bcl-223. In addition, other features of RFH are often overexpression of light chains by immunostaining, as well as by flow cytometry, no immunoglobulin rearrangement, and lack of t(14;18)2, 23.
Marginal zone hyperplasia
This involves monomorphic proliferation of small lymphocytes resembling monocytes with clear cytoplasm and rounded nuclei. Neutrophils are scattered. No pericapsular infiltrates and scattered reactive follicles are noted. Unlike early marginal lymphoma and marginal lymphoma, marginal zone hyperplasia has a limited extent, obvious marginal zone, high prevalence of Ki67 in follicles, no CD43 expression, and no follicular colonization.
Reactive paracortical hyperplasia
Reactive paracortical hyperplasia (RPH) is a phenomenon in which T cells are preferentially stimulated to proliferate in their compartment. It is caused by abnormal enlargement of the interfollicular zone but is confined to the nodal capsule. The cellular composition of this compartment is cytologically diverse. RPH is often accompanied by vascular hyperplasia – high endothelial veins (Figure 2, Figure 3). It is difficult to find a concrete etiology in most cases of RPH and is also not easy to distinguish from nodal neoplasia because of the overlap in histology such as peripheral T-cell lymphoma and nodal lymphocyte predominant Hodgkin lymphoma (NLPHL). Currently, according to the WHO 2022 classification, NLPHL has been renamed as ‘nodal lymphocyte-predominant B-cell lymphoma’ due to its close association with histocyte/T-cell rich large B-cell lymphoma12.
Unlike peripheral T-cell lymphoma, RPH often shows a diversity of B-cell numbers, degrees of differentiation, and of cellular morphology and size (Figure 2). The richer this diversity, the higher the probability of diagnosing RPH. Furthermore, under normal conditions, the number of T cells in this compartment predominates over that of B cells; however, the most important criterion of T-cell lymphoma is the presence of atypical numerous T cells. Immunohistochemistry has revealed immunophenotypic abnormalities of T-cell lymphoma including loss of expression of at least one pan-T cell marker or their aberrant expression. Finally, T-cell receptor gene rearrangements identified by genetic analysis may be invoked in some cases where it is difficult to differentiate benign from neoplastic lymph nodes.
As B cells and immunoblasts are present both in RPH and in histiocytic/T-cell rich large B-cell lymphoma (THRLBCL), their differentiation has always been problematic for the pathologist. Neoplastic B cells of diffuse large B-cell lymphoma are uniform in size with nuclear polymorphism, coarse chromatin, and irregularity of the nuclear margin. As these lymphoma B cells are dispersed in dense regions of T cells and reactive histiocytes that form the background of the slide, immunoexpression for CD20 is necessary to recognize the presence of neoplastic B cells. An abnormal nuclear feature of these lymphoma cells is one of the basic criteria for an accurate diagnosis. The lack of an FDC network is an indispensable feature of THRLBCL. Aberrant immunoexpression of lymphoma B cells for CD43 (compared with CD3 and CD20 stains) is another useful clue to avoid misdiagnosis despite the CD43+ rate of THRLBCL being 26–30%15, 23.
Diffuse paracortical hyperplasia
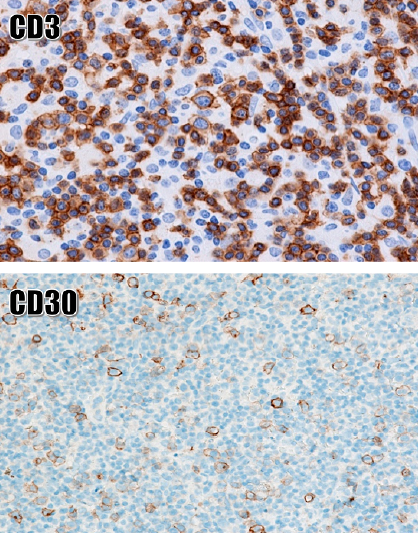
During inflammation, the paracortical compartment may increase in size due to inflammatory stimuli that induce an immune response by T-cell proliferation. The characteristic histological feature of RPH is an enlargement of T-cell-rich regions by increasing the diversity of the cell population including small lymphocytes, immunoblasts, dendritic cells, and histocytes. Such a diverse mixture of cells is described as ‘speckled’ or ‘moth-eaten’ at low magnification24 (Figure 2). Misdiagnosis of T-cell neoplasia can be avoided due to the heterogeneous (weak or negative) immunoexpression pattern of immunoblasts for CD30 (Figure 2).
Nodular paracortical hyperplasia
Cellular proliferation results in a distinctive nodular or spherical pattern. This is the most common hyperplastic growth pattern in the reactive lymph nodes. These round or spherical nodules are the result of an assemblage of cells unlike those that make up the background structure of the node, consisting of a mixture of cells. Vague nodular patterns may also be common due to the proliferation of the paracortical compartment. Antigen presentation that occurs between macrophages of interfollicular dendritic cell origin and virgin lymphocytes promotes to form of specific immunity. This can be seen in dermatology lymphadenopathy and drug-induced lymphadenopathy25.
Mixed hyperplasia
The cellular diversity of the paracortex is abundant with numerous small to medium lymphocytes in size, immunoblasts, a few neutrophils in blood vessels, histiocytes, and a few Reed-Sternberg-like cells26 (Figure 2). Individual cell death (apoptotic or karyorrhectic) may be present in many cases but not patchy necrosis in this hyperplastic pattern24, 27. The following clinicopathological features suggest reactive lymph node hyperplasia28: patient's young age, sparse traces of normal lymph node, some large polymorphic cells, and continuous maturation from plasmablasts to plasma cells. Here, there are no abnormally large cells or discontinuous maturation of lymphoid line, but the presence of both B and T cells to a fairly similar extent.
PITFALLS OF MISDIAGNOSIS
Abnormal location of follicles
Neoplastic follicles tend to be diffusely distributed in the lymph nodes, reaching structures such as the cortex and medulla, and spreading into the tissue around a lymph node and hilar adipose tissue. They are quite similar in size and morphology and are located next to each other. The follicles usually exhibit an incomplete layer of mantle cells surrounding them. Furthermore, their mitotic and apoptotic activities are not as dominant as the reactive follicles. Neoplastic follicles were positive for Bcl-2 while reactive hyperplastic follicles exhibited the opposite reaction. The mantle cells of reactive follicles are usually mildly or moderately positive for Bcl-2, further highlighting the negative GC of these follicles.
High follicular density (follicular to interfollicular zone ratio)
The most useful histological feature in follicular patterns is follicular density. The higher the follicular density the higher the likelihood of lymph node neoplasia.
Approximately 75% of FL has a high follicular density, whereas this is only seen in the most complex cases of RFH24. High numbers of Bcl2+ helper T cells may be present in RFH.11 This pitfall can be recognized by comparison with the CD3 stain, which shows that Bcl-2+ cells are not completely centroblasts (B cells) (Figure 2). In reactive lymph nodes, the CD4:CD8 rate is 3:1.27
Expression of Bcl-2
Bcl-2+ is present in FL and primary follicles. In this case, comparisons with other markers, including the Bcl-6, CD10 markers of GCs, or the IgD marker of primary follicular cells, were highly effective. Primary follicles do not express Bcl-6, CD10 in contrast to FL.
Bcl-2+ in the PTGC can be caused by mantle cells invaginating into GCs. In this case, the Bcl-2+ pattern is patchy or focal (Figure 3). However, the high density of mantle cells in PTGC can be confused with FL because the Bcl-2+ pattern of mantle cells resembles that of neoplastic follicular cells. In this case, given the immunophenotypic context and the structural and cytological features of the respective H&E-stained tissue sections, this misdiagnosis pitfall could have been avoided. Furthermore, the GC in this condition is weakly to moderately positive for CD5 due to its origin in the mantle zone cells.
Diffuse CD23 expression of lymphoproliferation
With this expression pattern, confusion between reactive lymph nodal and small lymphocytic lymphoma (SLL) should be avoided18, 29, 30. In chronic lymphocytic leukemia (CLL)/SLL, a diffuse expression for CD23 was found in 94% of cases, and for CD5 in 92% of cases31, 32. CLL/SLL is involved in memory B cells rather than naive or B-CD5+ cells33. Confusion should also be avoided with a new subtype of BCL-2− rearrangement (BCL2-R−), where CD23+ follicular center lymphoma, low-grade, and often diffuse growth patterns predominate. This variant usually presents with focal inguinal lymph node involvement12. B-CD5+ cells are formed from B-CD5+ cells of pre-GC accounting for 15–25% of B cells in mature secondary lymphoid organs. B-CD5+ cells show an increased tendency for malignant transformation33.
Abnormal follicular patterns
PTGC is a benign process of the lymph node with a disintegrated or twisted FDC network resembling lymphoid neoplasia due to PTGC lysis that has occurred during the healing GC, resulting in an abnormal image of the FDC network14, 16. The FDC network in PTGC is one of the pitfalls of overdiagnosing lymphoid neoplasia (Figure 1, Figure 3). On detailed analysis, benign features of PTGC can be detected, such as a preserved mantle zone, although irregular with small clusters of mantle cells invaginated into the GC, while macrophages and mitosis are still present in the GC. In these GCs, Bcl-2 staining is a mixed pattern consisting of negativity for cells of the GC and positivity for mantle zone cells inserted in the GC. The staining patterns of CD21 and CD23 have some differences, although both are used for the detection of FDC networks. The CD21 stain is more sensitive for the loose FDC networks than the CD23 stain, and conversely, the CD23 stain is more sensitive for small cell lymphoma, particularly mantle cell lymphoma (MCL), than the CD21 stain10, 16. Therefore, the CD21 stain is more useful than the CD23 stain in cases of mucosal-associated lymphoid tissue lymphoma and angioimmunoblastic T-cell lymphoma because the FDC networks are loose and sporadic in the paracortical and interfollicular areas.
PTGC can be confused with ‘floral’ follicular center lymphoma (FCL) with a nodular variant. The key to identifying FCL is the predominance of centrocytes in ‘floral’ FCL and the number of centroblasts is highly variable. Both of these cells are derived from B cells. Furthermore, the GC in this case is weakly to moderately positive for CD5 due to its origin in mantle zone cells34.
Giant GCs with high numbers of tingible body macrophages are easily confused with Burkitt lymphoma. Our experience, in this case, is that a careful review of the overall lymph node structure and features of GCs may be useful in determining the benign nature of the lymph node with ‘floral’ reactive follicles, giant reactive follicles, and PTGC, such as a distinct mantle cell rim, a limit of large lymphoid cells (centrocytes and centroblasts), increased mitotic activity, and visible polarization of the GC. Confirmation of FL by Bcl2 stain is crucial.
Atypical GCs for Bcl-2- usually have only clusters of large centroblasts with aberrant mitosis and are not expressed for Bcl-2 by immunostaining35. Several studies have shown that lymphoid clones can still be detected even in the context of normal reactive GC and benign hyperplastic marginal regions by immunoglobulin gene rearrangement36, 37, 38, 39. As such, to avoid overdiagnosing lymphoma in this setting, immunostaining to detect follicular dendritic cell network and GC origin of B cells is indispensable. Evaluation of immunoexpression for IRF4/MUM1, Bcl-2 and c-MYC is essential for prognosis and treatment. In addition, immunofluorescence in situ hybridization should be used to analyze IRF4/MUM1, BCL-2, and c-MYC gene rearrangements12.
Some large cells in size in reactive lymph nodes
The various number of immunoblasts in the paracortex can also be a pitfall of misdiagnosis with lymph node neoplasia; however, if the immunoblasts have both T and B cells with variable sizes (i.e., continuous differentiation of the T cell line and the B cell line) the possibility of RPH is higher (Figure 2).
Activated T and B immunoblasts are positive for CD30 at their surface. These immunoblasts may be large and multinuclear, resembling Hodgkin cells and Reed–Sternberg (HRS) cells of classic Hodgkin lymphoma (CHL) (Figure 2). The differential diagnosis of lymph node neoplasia by CD20, CD3 and CD30 immunostaining is useful because negative or weakly positive staining for CD30 is common in reactive lymphoproliferative conditions26, 39. In T cells, the CD30 receptor increases cytokine production, and in B cells it is detected during cell differentiation and antibody production40, 41. In reactive immunoblasts, CD30 staining is weak or negative and is sometimes moderate but rarely a paranuclear coarse lump41, 42 (Figure 3).
Other abnormal expression of immunohistochemical markers
Many diseases are associated with CD43, such as peripheral blood B cells concurrently expressing CD43 and CD20 are implicated in hematopoietic neoplasm. CD43-positive was approximately 29.4–33% of diffuse large B-cell lymphoma (DLBCL), whereas non-neoplastic mature B cells were negative for CD4343, 44, 45. CD43 and CD5 are simultaneously expressed in peripheral blood T cells that are involved in the hematopoietic malignancies of the T cell line30, 46.
CD15+ or CD30+ in some non-neoplastic diseases such as Kimura disease47. The lymph nodes in this disease also have follicular proliferation involved in paracortical proliferation with eosinophilic infiltrates in the paracortical compartment, sometimes with eosinophilic microabscesses. The hyperplastic GC usually has a pink protein fluid of an IgE nature. The lymphoblasts of the GC are positive for IgE13, 47.
Missing lymph node neoplasms such as FL, peripheral T cell lymphoma, and angioimmunoblastic T cell lymphoma should be avoided in cases where Bcl-6 expression is focal or diffuse. Diagnosis is supported by the presence of centroblasts and/or centrocytes and Bcl-2+ for FL; by the negative of one of the T cell markers and GATA3+ for peripheral T cell lymphoma, and finally, immunoexpression of follicular helper T cells (Bcl-6, ICOS, CXLC-13, PD-1, and CD10) and diffuse network of FDCs (CD21+) for angioimmunoblastic T cell lymphoma.
Follicular hyperplasia relevant to neoplasm
Follicular neoplasia in situ (FNIS) is defined as incomplete invasion of GCs by BCL-2-transforming clonal B cells of FL in a single lymph node with an preserved structure48, 49. FNIS relates to ‘follicular lymphoma-like B cells of uncertain significance’ (FLBUS). Flow cytometry results demonstrated that FLBUS was not misinterpreted as it could only reflect B cells similar to FL. Recently, FLBUS has been proposed to show that these cases are not lymphomas but only look like lymphomas31, 35. Consistent with concerns that overdiagnosis of lymphoma leads to a patient's pessimism and may result in unnecessary treatment. Several studies have shown that the frequency of FLBUS is much higher than that of FL (2.3% vs. 0.03%)31, 50.
Partially involved FL (PFL) should be distinguished from FLBUS because PFL often has partial structural changes with an aggregation of abnormal follicles that are usually larger and have more centroblasts than FLBUS. Compared with FLBUS, PFL has less clear boundaries between GC and mantle zones, thinner mantle zones, higher intensity of Bcl-2 and CD10 immunostaining, and possibly a few CD10+, Bcl-2+ neoplastic cells outside the follicles. A CD10+ stain is also a clue for abnormal follicles because their staining intensity can be higher than that of hyperplastic GCs. Like FL, FLBUS tends to express CD10 more strongly than RFH51.
Several studies of FLBUS have shown FL or DLBCL-like lesions in approximately 18–38% of patients, of whom 15–23% subsequently develop FL or DLBCL52, 53. A clonal relationship between FL and CHL, FL and mantle cell lymphoma (MCL) was detected in the same patient, so it is still possible that a clonal association exists between FLBUS and other types of lymphoma54, 55.
Distinguishing FNIS from RFH is also a pitfall of misdiagnosis. FNIS may be associated with various lymphomas, most often B-cell lymphoma, and possibly with reactive lymph node proliferation. Characterization of FNIS showed an intact follicular structure, normal follicular size, enlarged involved follicles, intact cuff with a sharp edge of the GC, restriction of the GC of atypical B cells, almost pure centrocytes, and overexpression of Bcl-2 and CD103, 4, 12, 56.
Pattern of FDC networks
Patterns of FDC networks are commonly recognized in reactive follicles with well-arranged dendritic networks consisting of six patterns: (1) a typical tight ‘spherical’ network pattern; (2) a pattern of polarization network; (3) a pattern of network expansion, spreading into mantle zones; (4) a contracted network pattern; (5) a distorted/decayed network pattern; and (6) a virtually absent network pattern10 (Figure 1, Figure 3). FDC network patterns are not a specific criterion for lymphatic neoplasia or lymphoma; however, the high degree of dispersion of the FDC network should be carefully studied to avoid missing tumor lymphocytes interspersed with this network. The detection of abnormal cells residing in the FDC network is one of the diagnostic features of some lymphoid neoplasia such as popcorn cells in NLPHL, often residing in nodules with deformed and disintegrated FDC network structures2, 49. Partial or complete loss of the network of FDCs often favors lymph node neoplasia.
Indolent T-cell lymphoproliferative disorder of the gastrointestinal tract
This is a lymphoproliferative type of the gastrointestinal tract with small, benign-looking lymphocytes with mild polymorphism. These lymphocytes reside in the lamina propria and can displace mucosal glands but do not invade or destroy blood vessels and are EBV-negative. Lamina propria T-CD4 and T-CD8 cells are the sources of this proliferative disorder. In this case, the clinical presentation is benign with individual lesions that usually resolve spontaneously in a short time, but sometimes they can become chronic with new lesions over many years. Before 2022, this process was considered benign; however, recent research has identified somatic mutations in many genes, including JAK3 recurrent mutations. The WHO histological classification of tumors of the hematopoietic system in 2022 used a new nomenclature for this entity called "indolent T cell lymphoma of the gastrointestinal tract".4, 57
CONCLUSION
One of the difficult areas to solve for the pathologist is the histological evaluation of reactive lymphoproliferation, yet it is the most reliable evidence among all other evidence in medicine. Current medicine tends to use less invasive exploratory techniques. The core biopsy technique of the lymph node has been used in the hope of being able to replace lymph node surgery in pathological diagnosis. However, the limitation of this method is that the material is both small and difficult to obtain representative tissue of lesion. Tissue microarray technique has been used to be able to overcome the limitations of core biopsies. However, regardless of the method, the histopathological outcome is completely dependent on whether the biopsy core has captured the damaged tissue or not. Identifying malignancy-like subtle histological changes and misdiagnosis pitfalls in nonspecific reactive hyperplastic lymph nodes by comprehensive analysis of their cytological and histological features is an essential skill for the pathologist, and at the same time, appropriate application of immunohistochemical markers for B cells (CD20, CD19 and CD79a), T cells (CD3, CD2, CD4, CD5, CD7 and CD8) and the dendritic cell network (CD21 and CD23) can limit misdiagnosis, thus also avoiding overtreatment of patients presenting with reactive enlarged lymph nodes.
Abbreviations
Bcl-2: B-cell lymphoma 2; Bcl-6: B-cell lymphoma 6; CD2: Cluster of differentiation 2; CD3: Cluster of differentiation 3; CD4: Cluster of differentiation 4; CD5: Cluster of differentiation 5; CD7: Cluster of differentiation 7; CD8: Cluster of differentiation 8; CD10: Cluster of differentiation 10; CD20: Cluster of differentiation 20; CD19: Cluster of differentiation 19; CD22: Cluster of differentiation 22; CD24: Cluster of differentiation 24; CD79a: Cluster of differentiation 79a; PAX5: Paired box 5; CD38: Cluster of differentiation 38; CD138: Cluster of differentiation 138; CD43: Cluster of differentiation 43; CD21: Cluster of differentiation 21; CD23: Cluster of differentiation 23; CD57: Cluster of differentiation 57; CD68: Cluster of differentiation 68; CD163: Cluster of differentiation 163; S100: Protein S100; CHL: classic Hodgkin lymphoma; CLL/SLL: small lymphocytic lymphoma; CD45 (LCA): Cluster of differentiation 45; c-MYC: multifunctional transcription factor; DLBCL: diffuse large B-cell lymphoma; FDC: Follicular dendritic cells; FISH: Fluorescence in situ hybridization; FL: follicular lymphoma; FLBUS: Follicular lymphoma-like B cells of uncertain significance; FLC: “floral” follicle center lymphoma; FNIS: Follicular neoplasia in situ; GCs: germinal centers; HEVs: high endothelial veins; HRS- like: Hodgkin and Reed-Sternberg – like; ICOS: Integrated Carbon Observation System; IDCs: interdigitating dendritic cells; IRF4/MUM1: Multiple myeloma oncogene-1; LP: lymphocyte predominant cells; MBL: monocytoid B-lymphocyte; MCL: mantle cell lymphoma; MZL: Marginal zone lymphoma; NLPHL: nodal lymphocyte predominant Hodgkin lymphoma; PD1: Programmed Cell Death Protein 1; PFL: Partial-involved FL; PTGC: Progressive transformation of the germinal center; RFH: Reactive follicular hyperplasia; RPH: Reactive paracortical hyperplasia; THRLBCL: histiocytic/T-cell rich large B-cell lymphoma.
Acknowledgments
We would like to thank the University for the study’s approval.
Author’s contributions
NVH, NTT, TNM: Conceptualization, Methodology, Writing-Original draft preparation; NTT, TNM: Visualization, Methodology, Software; TVC, DMK, DTL : Data curation, Writing-Original draft preparation; DTL, HTNM: Validation, investigation, Supervision. All authors read and approved the final manuscript.
Funding
None
Availability of data and materials
None
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Slater
D.N.,
The new World Health Organization classification of haematopoietic and lymphoid tumours: a dermatopathological perspective. British Journal of Dermatology.
2002;
147
(4)
:
633-9
.
View Article PubMed Google Scholar -
Swerdlow
S.H.,
Campo
E.,
Harris
N.L.,
WHO Classification of Tumours of Haematopoietic and Lymphoid TissuesInternational Agency for Research on Cancer 2008.
Google Scholar -
Swerdlow
S.H.,
Campo
E.,
Harris
N.L.,
WHO Classification of Tumours of Haematopoietic and Lymphoid TissuesInternational Agency for Research on Cancer 2017.
Google Scholar -
Alaggio
R.,
Amador
C.,
Anagnostopoulos
I.,
The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia.
2022;
36
(7)
:
1720-48
.
View Article Google Scholar -
LaCasce
A.S.,
Kho
M.E.,
Friedberg
J.W.,
Niland
J.C.,
Abel
G.A.,
Rodriguez
M.A.,
Comparison of referring and final pathology for patients with non-Hodgkin's lymphoma in the National Comprehensive Cancer Network. Journal of Clinical Oncology.
2008;
26
(31)
:
5107-12
.
View Article PubMed Google Scholar -
Morton
L.M.,
Wang
S.S.,
Devesa
S.S.,
Hartge
P.,
Weisenburger
D.D.,
Linet
M.S.,
Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood.
2006;
107
(1)
:
265-76
.
View Article PubMed Google Scholar -
Moore
S.W.,
Schneider
J.W.,
Schaaf
H.S.,
Diagnostic aspects of cervical lymphadenopathy in children in the developing world: a study of 1,877 surgical specimens. Pediatric Surgery International.
2003;
19
(4)
:
240-4
.
View Article PubMed Google Scholar -
Karadeniz
C.,
Oguz
A.,
Ezer
U.,
The etiology of peripheral lymphadenopathy in children. Pediatric hematology and oncology.
1999;
16
(6)
:
525-31
.
View Article Google Scholar -
Sevilla
D.W.,
Murty
V V.,
Sun
X.-L.,
Nandula
S V.,
Mansukhani
M.M.,
Cytogenetic abnormalities in reactive lymphoid hyperplasia: byproducts of the germinal centre reaction or indicators of lymphoma?. Hematological oncology.
2011;
29
(2)
:
81-90
.
View Article Google Scholar -
Rezk
S.A.,
Nathwani
B.N.,
Zhao
X.,
Weiss
L.M.,
Follicular dendritic cells: origin, function, and different disease-associated patterns. Human pathology.
2013;
44
(6)
:
937-50
.
View Article Google Scholar -
Weiss
L.M.,
O'Malley
D.,
Benign lymphadenopathies. Modern Pathology.
2013;
26
:
88-96
.
View Article PubMed Google Scholar -
Campo
E.,
Jaffe
E.S.,
Cook
J.R.,
The international consensus classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood, The Journal of the American Society of Hematology.
2022;
140
(11)
:
1229-53
.
View Article Google Scholar -
Gatter
K.C.,
Delsol
G.,
Warnke
R.A.,
Pezzella
F.,
The Diagnosis of Lymphoproliferative Diseases. 2th ed. A John Wiley & Sons, Ltd., Publication; 2012. 2012.
Google Scholar -
Hicks
J.,
Flaitz
C.,
Progressive transformation of germinal centers: review of histopathologic and clinical features. International Journal of Pediatric Otorhinolaryngology.
2002;
65
(3)
:
195-202
.
View Article PubMed Google Scholar -
Leong
A.S.,
Pattern Approach to Lymph Node DiagnosisSpringer 2011.
View Article Google Scholar -
Nybakken
G.E.,
Bala
R.,
Gratzinger
D.,
Jones
C.D.,
Zehnder
J.L.,
Bangs
C.D.,
Isolated follicles enriched for centroblasts and lacking t(14;18)/BCL2 in lymphoid tissue: diagnostic and clinical implications. PLoS One.
2016;
11
(3)
:
e0151735
.
View Article PubMed Google Scholar -
Chang
C.-C.,
Osipov
V.,
Wheaton
S.,
Tripp
S.,
Perkins
S.L.,
Follicular hyperplasia, follicular lysis, and progressive transformation of germinal centers: A sequential spectrum of morphologic evolution in lymphoid hyperplasia. American journal of clinical pathology.
2003;
120
(3)
:
322-6
.
View Article Google Scholar -
van den Brand
M.,
van Krieken
J.H.,
Recognizing nodal marginal zone lymphoma: recent advances and pitfalls. A systematic review. Haematologica.
2013;
98
(7)
:
1003-13
.
View Article PubMed Google Scholar -
Osborne
B.M.,
Butler
J.J.,
Variakojis
D.,
Kott
M.,
Reactive lymph node hyperplasia with giant follicles. American Journal of Clinical Pathology.
1982;
78
(4)
:
493-9
.
View Article Google Scholar -
Policepatil
S.M.,
Go
R.S.,
Zeng
G.G.,
Floral variant of follicular lymphoma. American Journal of Hematology.
2011;
86
(6)
:
503-503
.
View Article PubMed Google Scholar -
Karube
K.,
Ohshima
K.,
Tsuchiya
T.,
Yamaguchi
T.,
Kawano
R.,
Suzumiya
J.,
A right variant of nodal marginal zone lymphoma. Human Pathology.
2005;
36
(2)
:
202-6
.
View Article PubMed Google Scholar -
Goates
J.J.,
Kamel
O.W.,
LeBrun
D.P.,
Floral variant of follicular lymphoma. Immunological and molecular studies support a neoplastic process. The American journal of surgical pathology.
1994;
18
(1)
:
37-47
.
View Article Google Scholar -
Gaulard
P.,
d'Agay
M.F.,
Peuchmaur
M.,
Brousse
N.,
Gisselbrecht
C.,
Solal-Celigny
P.,
Expression of the bcl-2 gene product in follicular lymphoma. American Journal of Pathology.
1992;
140
(5)
:
1089-95
.
PubMed Google Scholar -
Willard-Mack
C.L.,
Normal structure, function, and histology of lymph nodes. Toxicologic pathology.
2006;
34
(5)
:
409-24
.
View Article Google Scholar -
Stebegg
M.,
Kumar
S.D.,
Silva-Cayetano
A.,
Fonseca
V.R.,
Linterman
M.A.,
Graca
L.,
Regulation of the Germinal Center Response. Frontiers in Immunology.
2018;
9
(October)
:
2469
.
View Article PubMed Google Scholar -
Chetty
R.,
Biddolph
S.,
Gatter
K.,
An immunohistochemical analysis of reedsternberg-like cells in posttransplantation lymphoproliferative disorders: The possible pathogenetic relationship to Reed-Sternberg cells in Hodgkin's disease and reedsternberg-like cells in non-Hodgkin's lymphomas and reactive conditions. Human pathology.
1997;
28
(4)
:
493-8
.
View Article Google Scholar -
Mills
S.E.,
Histology for PathologistsLIPPINCOTT WILLIAMS & WILKINS 2012.
Google Scholar -
Chan
J.K.,
Kwong
Y.L.,
Common misdiagnoses in lymphomas and avoidance strategies. The Lancet. Oncology.
2010;
11
(6)
:
579-88
.
View Article PubMed Google Scholar -
Disanto
M.G.,
Ambrosio
M.R.,
Rocca
B.J.,
Ibrahim
H.A.H.,
Leoncini
L.,
Optimal minimal panels of immunohistochemistry for diagnosis of B-cell lymphoma for application in countries with limited resources and for triaging cases before referral to specialist centers. American Journal of Clinical Pathology.
2016 ;
145
(5)
:
687-95
.
View Article Google Scholar -
Yoshino
T.,
Tanaka
T.,
Sato
Y.,
Differential diagnosis of chronic lymphocytic leukemia/small lymphocytic lymphoma and other indolent lymphomas, including mantle cell lymphoma. Journal of clinical and experimental hematopathology.
2020;
60
(4)
:
124-9
.
View Article Google Scholar -
Pillai
R.K.,
Surti
U.,
Swerdlow
S.H.,
Follicular lymphoma-like B cells of uncertain significance (in situ follicular lymphoma) may infrequently progress, but precedes follicular lymphoma, is associated with other overt lymphomas and mimics follicular lymphoma in flow cytometric studies. Haematologica.
2013;
98
(10)
:
1571
.
View Article Google Scholar -
Bagdi
E.,
Krenacs
L.,
Krenacs
T.,
Miller
K.,
Isaacson
P.G.,
Follicular dendritic cells in reactive and neoplastic lymphoid tissues: a reevaluation of staining patterns of CD21, CD23, and CD35 antibodies in paraffin sections after wet heat-induced epitope retrieval. Applied Immunohistochemistry & Molecular Morphology.
2001;
9
(2)
:
117-24
.
View Article Google Scholar -
Dono
M.,
Cerruti
G.,
Zupo
S.,
The CD5+ B-cell. The international journal of biochemistry & cell biology.
2004;
36
(11)
:
2105-11
.
View Article Google Scholar -
Tiesinga
J.J.,
Wu
C.D.,
Inghirami
G.,
CD5+ follicle center lymphoma. Immunophenotyping detects a unique subset of follicular lymphoma. American Journal of Clinical Pathology.
2000;
114
(6)
:
912-21
.
View Article PubMed Google Scholar -
Stefano
G. Di,
Magnoli
F.,
Granai
M.,
B Lymphoproliferative Neoplasms of Uncertain Biological Significance: Report from the IV Workshop of the Italian Group of Hematopathology and Review of the Literature. Hemato.
2022;
3
(4)
:
634-49
.
View Article Google Scholar -
Nam-Cha
S.H.,
San-Milla
B.,
Mollejo
M.,
Light‐chain‐restricted germinal centres in reactive lymphadenitis: report of eight cases. Histopathology.
2008 ;
52
(4)
:
436-44
.
View Article Google Scholar -
Kussick
S.J.,
Kalnoski
M.,
Braziel
R.M.,
Wood
B.L.,
Prominent clonal B-cell populations identified by flow cytometry in histologically reactive lymphoid proliferations. American Journal of Clinical Pathology.
2004;
121
(4)
:
464-72
.
View Article PubMed Google Scholar -
Iijima
T.,
Inadome
Y.,
Noguchi
M.,
Clonal proliferation of B lymphocytes in the germinal centers of human reactive lymph nodes: possibility of overdiagnosis of B cell clonal proliferation. Diagnostic Molecular Pathology.
2000;
9
(3)
:
132-6
.
View Article Google Scholar -
Greer
R.O.,
Marx
R.E.,
Prok
S.,
Said
D.,
L. Pediatric Head and Neck PathologyCambridge University Press 2017.
Google Scholar -
Kennedy
M.K.,
Willis
C.R.,
Armitage
R.J.,
Deciphering CD30 ligand biology and its role in humoral immunity. Immunology.
2006;
118
(2)
:
143-52
.
View Article PubMed Google Scholar -
Xu
M.L.,
Gabali
A.,
Hsi
E.D.,
Fedoriw
Y.,
Vij
K.,
Practical approaches on CD30 detection and reporting in lymphoma diagnosis. The American Journal of Surgical Pathology.
2020;
44
(2)
:
e1-4
.
View Article Google Scholar -
Lu
J.,
Chang
K.L.,
Practical immunohistochemistry in hematopathology: a review of useful antibodies for diagnosis. Advances in Anatomic Pathology.
2011;
18
(2)
:
133-51
.
View Article Google Scholar -
Xiao-Bo
M.,
Zheng
Y.,
Yuan
H.,
Jiang
Jing,
Wang
Y.-P.,
CD43 expression in diffuse large B-cell lymphoma, not otherwise specified: CD43 is a marker of adverse prognosis. Human pathology.
2015;
46
(4)
:
593-9
.
View Article Google Scholar -
Naresh
K.N.,
Nodal marginal zone B‐cell lymphoma with prominent follicular colonization–difficulties in diagnosis: a study of 15 cases. Histopathology.
2008;
52
(3)
:
331-9
.
View Article Google Scholar -
Pedraza-Alva
G.,
Rosenstein
Y.,
CD43–One molecule, many tales to recount. Signal Transduction.
2007;
7
(5-6)
:
372-85
.
View Article Google Scholar -
Dogan
A.,
Modern histological classification of low grade B-cell lymphomas. Best Practice & Research Clinical Haematology.
2005;
18
(1)
:
11-26
.
View Article Google Scholar -
Rastogi
N.,
Kimura’s disease: an extremely rare case report in an elderly male, followed by Hodgkin’s disease. International Journal of Science and Research Archive.
2021;
2
(1)
:
156-9
.
View Article Google Scholar -
Cong
P.,
Raffeld
M.,
Teruya-Feldstein
J.,
Sorbara
L.,
Pittaluga
S.,
Jaffe
E.S.,
In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood.
2002;
99
(9)
:
3376-82
.
View Article PubMed Google Scholar -
Carbone
A.,
Santoro
A.,
How I treat: diagnosing and managing “in situ” lymphoma. Blood, The Journal of the American Society of Hematology.
2011;
117
(15)
:
3954-60
.
View Article Google Scholar -
Henopp
T.,
Quintanilla-Martínez
L.,
Fend
F.,
Adam
P.,
Prevalence of follicular lymphoma in situ in consecutively analysed reactive lymph nodes. Histopathology.
2011;
59
(1)
:
139-42
.
View Article PubMed Google Scholar -
Ray
S.,
Craig
F.E.,
Swerdlow
S.H.,
Abnormal patterns of antigenic expression in follicular lymphoma: a flow cytometric study. American journal of clinical pathology.
2005;
124
(4)
:
576-83
.
View Article Google Scholar -
Fend
F.,
Cabecadas
J.,
Gaulard
P.,
Jaffe
E.S.,
Kluin
P.,
Early lesions in lymphoid neoplasia: Conclusions based on the Workshop of the XV. meeting of the European Association of Hematopathology and the Society of Hematopathology in Uppsala, Sweden. Journal of hematopatholog.
2012;
5
:
169-99
.
View Article Google Scholar -
Montes-Moreno
S.,
Castro
Y.,
Rodríguez-Pinilla
S.M.,
García
J.F.,
Mollejo
M.,
Castillo
M.E.,
Intrafollicular neoplasia/in situ follicular lymphoma: review of a series of 13 cases. Histopathology.
2010;
56
(5)
:
658-62
.
View Article PubMed Google Scholar -
Yoshida
M.,
Ichikawa
A.,
Miyoshi
H.,
Takeuchi
M.,
Kimura
Y.,
High frequency of t (14; 18) in Hodgkin's lymphoma associated with follicular lymphoma. Pathology International.
2012;
62
(8)
:
518-24
.
View Article Google Scholar -
Tsang
P.,
Pan
L.,
Cesarman
E.,
Tepler
J.,
Knowles
D.M.,
A distinctive composite lymphoma consisting of clonally related mantle cell lymphoma and follicle center cell lymphoma. Human Pathology.
1999;
30
(8)
:
988-92
.
View Article PubMed Google Scholar -
Jegalian
A.G.,
Eberle
F.C.,
Pack
S.D.,
Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood, The Journal of the American Society of Hematology.
2011;
118
(11)
:
2976-84
.
View Article Google Scholar -
Soderquist
C.R.,
Bhagat
G.,
Indolent T-and NK-cell lymphoproliferative disorders of the gastrointestinal tract: current understanding and outstanding questions. Hemato.
2022 ;
3
(1)
:
219-31
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 10 (2023)
Page No.: 5960-5971
Published on: 2023-10-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5581 times
- PDF downloaded - 1375 times
- XML downloaded - 163 times
 Biomedpress
Biomedpress




