Abstract
Thrombotic thrombocytopenic purpura (TTP), a rare and potentially life-threatening blood disorder, poses unique challenges during pregnancy. It is characterized by microangiopathic hemolytic anemia, severe thrombocytopenia, and microvascular thrombosis. Pregnancy induces significant changes in hemodynamics and coagulation factors, complicating the already complex pathophysiology and presentation of TTP. TTP during pregnancy may be clinically challenging due to overlapping features with other thrombotic microangiopathies. Here, we report a 34-year-old woman at 12 weeks of gestation who presented with sudden onset of altered sensorium and fever. Initial investigations revealed anemia, thrombocytopenia, and numerous schistocytes in the peripheral blood smear. She was diagnosed with TTP based on clinical symptoms and laboratory findings. Ensuring correct diagnosis and management is critical because of the impact on fetal and maternal outcomes.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare but severe complication during pregnancy that often occurs late in the third trimester or during puerperium. Before the development of therapeutic plasma exchange (PEX) and plasma infusion, pregnancy-related TTP caused significant maternal and fetal mortality rates as high as 80%. However, survival rates have dramatically improved after the development of these treatments1.
Case report
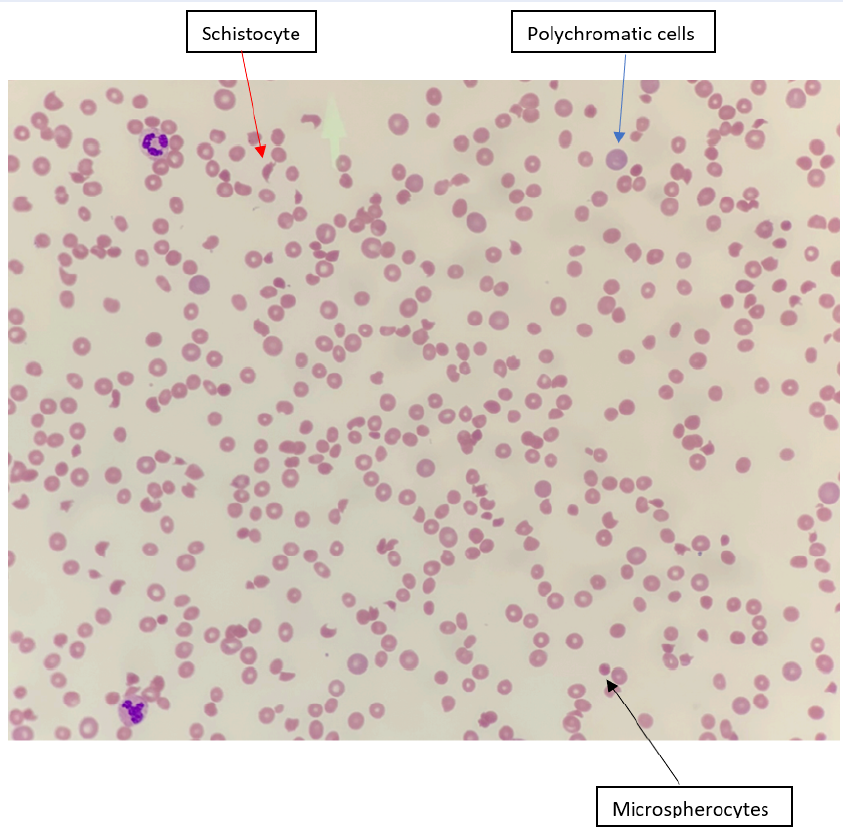
A 34-year-old woman, gravida 8 para 3+4, presented to the emergency department at 12 weeks gestation with sudden onset of altered sensorium and fever. On examination, she was pale, jaundiced, febrile, and tachycardic. Laboratory investigations showed anemia with hemoglobin of 6.0 g/dL, thrombocytopenia with a platelet count of 11.0 × 109/L, and numerous schistocytes on a peripheral blood smear (Figure 1). Her lactate dehydrogenase (LDH) plasma level was very high at 2767 U/L, and her reticulocyte count was 10%. The renal function test was slightly abnormal, with blood urea of 7.5 mmol/L, total bilirubin of 31 µmol/L, and indirect bilirubin of 23 µmol/L. Levels of transaminases, prothrombin time, activated partial thrombin time, and international normalized ratio were within normal limits. Based on her clinical symptoms and laboratory findings, she was diagnosed with TTP.
| Parameters | Day 1 (On admission) | Day 11 (After treatment with PEX and Rituximab) | Normal range |
|---|---|---|---|
| Hemoglobin (Hb) | 6.0 g/dL | 9.7 g/dL | 12.0 -15.0 g/dL |
| White blood cell | 10.1 x10 9 /L | 6.48 x10 9 /L | 4 - 10 x10 9 /L |
| Platelet | 11 x10 9 /L | 150 x10 9 /L | 150 - 410 x10 9 /L |
| Reticulocytes | 10.0% | 1.5% | 0.5 - 2.5% |
| Total bilirubin | 31 μ mol/L | 27 μ mol/L | 1 - 17 μ mol/L |
| Indirect bilirubin | 23 μ mol/L | 10 μ mol/L | < 7 μ mol/L |
| Blood urea nitrogen | 7.5 mmol | 6.8 mmol | 2.8 - 7.2 mmol/L |
| Creatinine | 66 | 32 | 45 - 84 umol/L |
| Lactate dehydrogenase (LDH) | 2767 U/L | - | 140 - 280 U/L |
| ADAM TS13 activity | - | 1% | 40-130% |
| ADAMTS13 antibody levels | - | 36.2 U/mL | Negative: < 12 U/mL Borderline:12-15 U/mL Positive: >15 U/mL |
She was immediately started on PEX with fresh frozen plasma at 30 cc/kg daily for three days. Methylprednisone (500 mg) was also administered intravenously for three days. Despite continued PEX, her platelet count and hemoglobin remained unchanged. A regimen of 500 mg rituximab weekly was initiated to improve the curative efficiency of PEX since she might be refractory to PEX. The PEX procedure was maintained according to the established protocol. After three days of rituximab administration, the patient responded favorably by regaining consciousness. The patient’s platelet count had returned to normal by the eleventh day. Disintegrin-like and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13) activity was assessed as part of the comprehensive diagnostic workup for the TTP episode at the referral laboratory. The results showed low ADAMTS13 activity (1%), well below the normal range of 40%–130%, and elevated ADAMTS13 antibody levels (36.2 U/mL), exceeding the positive range threshold of 15. All the laboratory investigations are shown in Table 1.
Discussion
TTP is a life-threatening disorder characterized by microangiopathic hemolytic anemia (MAHA), severe thrombocytopenia, and microvascular thrombosis2. It is caused by a deficiency of the ADAMTS13 enzyme, leading to the accumulation of ultra-large von Willebrand factor (vWF) molecules that bind to platelets and cause thrombotic occlusion in small blood vessels. TTP can be either acquired or congenital. Cases of acquired TTP show low ADAMTS13 activity and the presence of anti-ADAMTS13 antibodies. Cases of congenital TTP show low ADAMTS13 activity (<10%) and no detectable anti-ADAMTS13 antibodies3.
Pregnancy is one of the main triggers of acute TTP episodes, contributing to approximately 5% — 10% of all TTP cases in women2. The challenges in diagnosing and managing TPP during pregnancy are due to its atypical interaction with physiological changes. This correlation arises from the elevated levels of procoagulant factors, reduced fibrinolytic activity, diminished thrombomodulin function, loss of endothelial cell thrombomodulin, and a decline in ADAMTS13 activity accompanying pregnancy4. Pregnancy-related TTP has been associated with higher risks of maternal and fetal morbidity and mortality.
Clinical TPP presentations include thrombocytopenia, MAHA, altered renal function, fever, and neurological symptoms such as seizures5. Among patients, 24% have fever, 59% have renal abnormalities, and 63% have neurological signs. Renal involvement may present as proteinuria or increased serum creatinine levels. Neurological involvement may result in seizures, strokes, or confusion6.
TPP diagnosis requires the presence of both thrombocytopenia and hemolytic anemia. In the acute phase, the platelet count is often very low (< 30 × 109/L), and there are signs of mechanical hemolytic anemia, including the presence of schistocytes, reticulocytosis, high indirect serum bilirubin, low serum haptoglobin, and negative direct Coombs test. LDH is usually significantly elevated due to red cell destruction and tissue necrosis, and routine coagulation parameters are usually normal7. Testing for ADAMTS13 activity is essential since the prevailing mechanism thought to underlie TTP is associated with ADAMTS13 deficiency. The TTP diagnosis is confirmed if ADAMTS13 activity is <10%. The differential diagnosis of TTP during pregnancy includes other thrombotic microangiopathies such as acute fatty liver of pregnancy, preeclampsia, atypical hemolytic uremic syndrome, and HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome.
Plasma treatment should begin as soon as possible when TTP is suspected, regardless of ADAMTS13 activity8. PEX appears more successful than plasma infusion. PEX eliminates ultra-large vWF multimers, anti-ADAMTS13 antibodies, and ADAMTS13 immune complexes while restoring ADAMTS13 activity. There is no specific PEX regimen that works best. Our standard of care, which follows the guidelines set by the British Committee on Standards in Haematology, involves initiating PEX with 1.5 plasma volume (PV) exchanges for the first three days, followed by 1.0 PV exchange the next day. PEX is maintained until the platelet count reaches normal levels (≥ 150 × 109/L for two consecutive days). After attaining a steady response, many groups will discontinue PEX, while others suggest tapering the frequency of PEX to prevent exacerbations (recurrent TTP within 30 days of the last PEX)9.
Corticosteroids may be used in TTP to reduce the synthesis of ADAMTS13 inhibitors10. Rituximab (anti-membrane spanning 4-domains A1 [MS4A1/CD20]) also showed an excellent response with a reduced recurrence11, 12. In small case studies, other immunosuppressive drugs such as vincristine, cyclophosphamide, or cyclosporine were beneficial.
Conclusion
In our case, we had to administer rituximab as an immunosuppressive agent due to PEX refractoriness. This report highlights the complexities of managing TTP during pregnancy. Collaborative efforts are needed to refine treatment strategies, ultimately enhancing the prognosis for mothers and their infants in these challenging cases.
Abbreviations
ADAMTS13: A Disintegrin and Metallo‑proteinase with Thrombospondin type 1 motif, member 13; MAHA: microangiopathic haemolytic anaemia PEX: plasma exchange TPE: therapeutic plasma exchange; TTP: Thrombotic thrombocytopenic purpura
Acknowledgments
None
Author’s contributions
SA and NR are responsible for the writing of the article. NFFNMH and NASAS helped in data collection and interpretation. AD participated in sequence alignment. All authors read and approved the final manuscript.
Funding
None
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Soe
A.M.,
Tun
N.M.,
Guevara
E.,
Shulimovich
M.,
A case of thrombotic thrombocytopenic purpura in late pregnancy. Blood Research.
2016;
51
(3)
:
207-10
.
View Article PubMed Google Scholar -
Thomas
M.R.,
Robinson
S.,
Scully
M.A.,
How we manage thrombotic microangiopathies in pregnancy. British Journal of Haematology.
2016;
173
(6)
:
821-30
.
View Article PubMed Google Scholar -
Scully
M.,
Thomas
M.,
Underwood
M.,
Watson
H.,
Langley
K.,
Camilleri
R.S.,
collaborators of the UK TTP Registry
Thrombotic thrombocytopenic purpura and pregnancy: presentation, management, and subsequent pregnancy outcomes. Blood.
2014;
124
(2)
:
211-9
.
View Article PubMed Google Scholar -
Sikka
P.,
Chopra
S.,
Aggarwal
N.,
Suri
V.,
Chandrasekaran
A.,
Thrombotic thrombocytopenic purpura in the first trimester of pregnancy. Asian Journal of Transfusion Science.
2013;
7
(1)
:
79-80
.
View Article PubMed Google Scholar -
Basta
M.,
Thrombotic thrombocytopenic purpura during pregnancy and its overlap with the HELLP syndrome, a clinical dilemma: A case report and review of the literature. Journal of Obstetric Anaesthesia and Critical Care.
2019;
9
(1)
:
50
.
View Article Google Scholar -
Shaz BH, Hillyer CD, editors. Transfusion medicine and hemostasis: clinical and laboratory aspects. Newnes; 2013 May 13.Elsevier .
Google Scholar -
Rubia
J. de la,
Contreras
E.,
Río-Garma
J. Del,
[Thrombotic thrombocytopenic purpura]. Medicina Clínica.
2011;
136
(12)
:
534-40
.
View Article PubMed Google Scholar -
George
J.N.,
How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood.
2010;
116
(20)
:
4060-9
.
View Article PubMed Google Scholar -
Dane
K.,
Chaturvedi
S.,
Beyond plasma exchange: novel therapies for thrombotic thrombocytopenic purpura. Hematology 2014, the American Society of Hematology Education Program Book.
2018;
2018
(1)
:
539-47
.
View Article Google Scholar -
Balduini
C.L.,
Gugliotta
L.,
Luppi
M.,
Laurenti
L.,
Klersy
C.,
Pieresca
C.,
Italian TTP Study Group
High versus standard dose methylprednisolone in the acute phase of idiopathic thrombotic thrombocytopenic purpura: a randomized study. Annals of Hematology.
2010;
89
(6)
:
591-6
.
View Article PubMed Google Scholar -
Page
E.E.,
Kremer Hovinga
J.A.,
Terrell
D.R.,
Vesely
S.K.,
George
J.N.,
Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood.
2016;
127
(24)
:
3092-4
.
View Article PubMed Google Scholar -
Caramazza
D.,
Quintini
G.,
Abbene
I.,
Coco
L.L.,
Malato
A.,
Di Trapani
R.,
Rituximab for managing relapsing or refractory patients with idiopathic thrombotic thrombocytopenic purpura\textemdashhaemolytic uraemic syndrome. Blood Transfusion.
2010;
8
(3)
:
203-10
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 10 (2023)
Page No.: 5956-5959
Published on: 2023-10-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3607 times
- PDF downloaded - 1247 times
- XML downloaded - 190 times
 Biomedpress
Biomedpress



