Abstract
Introduction: This study was designed to evaluate the relationship between taking sodium valproate (VPA) and the onset of polycystic ovarian syndrome (PCOS) in women with epilepsy (WWE) and analyze the biochemical factors and expression levels of three miRNAs as diagnostic or predictive biomarkers. These miRNAs target numerous genes and molecular pathways involved in hyperandrogenism, insulin resistance, and obesity in PCOS patients.
Methods: The study was conducted on 120 WWE aged 18?35 years before and after monotherapy with VPA and 120 women with PCOS (WWP). After collecting the plasma samples of patients, the total RNA was extracted. Then, the miRNA-218, miRNA-222, and miRNA-146 expression levels were determined via qRT? PCR.
Results: In this study, the relative expression levels of miRNA-146 and miRNA-218 showed a significant increase in the WWP and treated epileptic patients (P < 0.01). However, the expression level of miRNA-222 significantly (P0.05) reduced. Our finding showed a significant increase in the concentrations of anti-M?llerian hormone (AMH), free testosterone, and estradiol and an increased LH/FSH ratio after treatment compared with pre-treatment with VPA (P < 0.05).
Conclusion: Significant changes were observed in the expression of the examined miRNAs after receiving VPA, especially miRNA-218. In addition, a significant correlation was found between PCOS and AMH, free testosterone, estradiol, and the LH/FSH ratio. Therefore, the miRNA-218 expression and these biochemical factors are valuable biomarkers for predicting PCOS symptoms. They are cost-effective for controlling side effect and timely medication adjustments in patients receiving VPA.
Introduction
Epilepsy is a common neurological disease affecting both genders, and it is estimated that approximately 50 million individuals are afflicted with this disorder worldwide1. According to epidemiological studies conducted on Iranian epileptic patients, the incidence of epilepsy is likely 5 per 1000 people2, and its prevalence is higher in women than men3. Epilepsy has been linked to several reproductive endocrine disorders depending on the type, duration, and dose of anticonvulsant drugs. Hormonal changes, irregular menstrual cycles, and symptoms resembling polycystic ovarian syndrome (PCOS) are some of the most frequently described signs4. There are various antiepileptic medicines (AEDs) available for therapy, but valproate (VPA) is regarded as the top option5. VPA has mood-stabilizing properties and is prescribed for treating focal and generalized epilepsy. It is also effective for partial seizures, bipolar disorder, migraines, and neuralgia6. It has been shown that VPA inhibits liver enzymes7 and can increase the concentration of sex hormone-binding globulin (SHBG) in both sexes. It has been associated with an increased free androgen index and increased levels of dehydroepiandrosterone sulfate, androstenedione, and testosterone, as well as the development of ovarian cysts, sexual problems, subfertility, and irregularities in the menstrual cycle8. Finally, a higher prevalence of PCOS-like symptoms in epileptic women who use VPA indicates that it may impair androgen synthesis and ovarian function. This is probably due to various effects on the hypothalamic–pituitary–ovarian axis9. Recent research has shown that using AEDs while having epilepsy causes several issues, with PCOS and its consequences having been considered10.
MicroRNAs (miRNAs) are small, non-coding RNAs with a length of 18 to 25 nucleotides that significantly affect cell development, proliferation, differentiation, inflammation, apoptosis, stress response, and the spread of malignant tumors via post-transcriptional suppression. Circulating miRNA has recently been linked to the development of cardiovascular disorders, endometriosis, poor ovarian response, and cervical cancer11. Recent research demonstrates that the miRNA expression is altered in the serum, plasma, follicular fluid, and granulosa cells of PCOS patients compared with healthy controls, making them potential biomarkers for diagnosing, monitoring, and treating this condition12, 13. Furthermore, miRNAs may indicate PCOS-related aberrant metabolism, impaired oocyte quality, and decreased endometrial receptivity. PCOS patients have considerably higher levels of miR-146a and miR-222 than healthy women14. In addition, miR-146a is differentially expressed in PCOS ovarian tissue. Bioinformatics research has revealed that miR-146a and miR-222 target genes are implicated in the cell cycle, apoptosis, and endocrine pathways, such as Wnt, MAPK, and Jak-STAT signaling. These findings suggest that miR-222 and miR-146a may play a role in the etiology of PCOS15. Mature miR-218 has been identified to regulate mRNA expression through more than 900 target genes, including RICTOR, LAMB3, BIRC5, and ROBO1, which may be crucial in the development of cervical cancer, according to the Microcosm Targets software program (http://www.ebi.ac.uk/enright-srv/microcosm/). Several of these genes are involved in various cancer signaling pathways, including the Wnt/β-catenin, ERK/MAPK, and Notch pathways16. One study showed that higher miRNA-218 levels are related to adiponectin suppression in obesity and PCOS by binding to AMP-activated protein kinase and p38 mitogen-activated protein kinase and acting on the adiponectin receptor (AdipoR2)17. Numerous studies have linked the markers miR-135b, miR-190, miR-217, miR-218, miR-299, and miR-342 to the presence of estrogen receptors in breast cancer18. Notably, the expression of these miRNAs regulated by estrogen receptors (ERs) exerts indirect steroidal effects on follicular cells or directly on the androgen receptor pathway to modulate androgen metabolism and related disorders, such as breast cancer and PCOS19, 20. Because PCOS is diagnosed early, the severity and progression of its problems can be treated2. As a result, this study was established to identify a valid and non-invasive biological marker for PCOS prediction. Some miRNAs regulated in PCOS have been confirmed, while others have been only predicted21. In this study, miR-146a, miR-222, and miR-218 expression levels were investigated as potential indicators for developing PCOS-like symptoms in epileptic patients following VPA administration.
Methods
Patient groups and study design
This study was performed at the Abortion Research Center, Yazd Reproductive Sciences Institute of Shahid Sadoughi University of Medical Sciences, Yazd, Iran, with the approval of the local ethics committee. All participants signed informed consent forms to use both their samples and information documented in their medical records.
All epileptic patients were referred from the neurology and epilepsy clinics of Maseh Epilepsy Clinic Isfahan and Isfahan Epilepsy Society. An expert neurologist diagnosed all epileptic patients (generalized or focal epilepsy) according to the criteria of the International League Against Epilepsy (ILAE) (2017). Samples from PCOS women were collected from the Abortion Research Center, Yazd Reproductive Sciences Institute of Shahid Sadoughi University of Medical Sciences, Iran, and the diagnosis was made by a gynecologist according to Rotterdam criteria. In the epileptic group, patients with a history of endocrine disorders, diabetes, liver disease, kidney disease, pituitary abnormality, depression, or any CNS disease were excluded. Other exclusion criteria were abnormal thyroid function tests and body mass index (BMI) > 30 kg/m2 in women with epilepsy before VPA treatment (WWEb). Patients were asked to report any unwanted pregnancies or if they discontinued treatment and were excluded from the study. Patients with excessive body hair, acne, or lactation problems were screened thoroughly. The study also included 120 Iranian women with PCOS aged 18 to 35 years (WWP) with exclusion and inclusion criteria similar to the epileptic group. None in the WWP group had a history of seizures, neurologic or other endocrine disorders. All WWP and WWE patients were newly diagnosed or untreated.
Drug dosage and sample collection
All epileptic patients received VPA (RAHA DRUG) in a dosage of 500 mg/day (250 mg twice a day) for the first week, which was increased to 1000 mg/day the following week. The target maintenance dosage for VPA was 1000–2000 mg/day for at least 3 months. In this study, 5 mL of peripheral blood was drawn from each patient before and after treatment in the epileptic women. The whole blood was centrifuged twice: once at 4000 rpm for 10 min and then for 15 min at 1200 rpm to remove cell particles completely. Then, the serum was stored at −80°C for further analysis.
Demographic and hormonal information
Some of the required information for the study was extracted from patient records or questionnaires filled out by specialists. The demographic information and clinical findings included BMI, age, irregular menstruation, and hirsutism (F–G score). Furthermore, some information about the classification of epilepsy was recorded: length, frequency, family history, and age at symptom onset of seizures. Moreover, information on hormonal and biochemical parameters was extracted from the medical records. These parameters included luteinizing hormone (LH), follicle-stimulating hormone (FSH), LH/FSH ratio, anti-Müllerian hormone (AMH), estradiol (E), free testosterone, dehydroepiandrosterone (DHEA-S), estradiol, hydroxyl progesterone, prolactin, SHBG, fasting insulin, and fasting glucose levels.
| miRNA | Tm | Primer (stem loop) (5'-3') |
|---|---|---|
| hsa-mir-222-F | 58/5 | AACTACATCTGGCTACTGGGT |
| hsa- snord47-F | 60 | ATC ACT GTA AAA CCG TTC CA |
| hsa-miR-218-5p-F | 58 | TTG GGC TTG ATC TAA CCA T |
| hsa-miR-146a-F | 58/3 | TGG AAG GTT GAG AAC TGA AT |
| WWEb 1 (n = 120) (mean ± std. Error)* | WWEa 2 (n = 120) (mean ± std. Error)* | WWP 3 (n = 120) (mean ± Std. Error)* | 𝑃 value (between groups) | |||
|---|---|---|---|---|---|---|
| 1-2 | 2-3 | 1-3 | ||||
| BMI (kg/m 2 ) | 24.64 ± 0.580 | 25.70 ± 0.499 | 27.90 ± 0.53 | 0.51 | 0.35 | 0.18 |
| Age (years) | 26.5 ± 0.950 | 26.5 ± 0.950 | 27.5 ± 0.80 | - | - | 0.81 |
| % Irregular menstrual | 20.50 ± 0.450 | 67 ± 1.801 | 80.5 ± 1.03 | 0.03** | 0.087 | 0.02** |
| Hirsutism (G-F score) | 5.83 ± 1.314 | 15.06 ± 0.980 | 19.13 ± 1.07 | 0.00** | 0.021** | 0.00** |
| Variables | WWEb 1 (n = 120) (mean ± std. Error)* | WWEa 2 (n = 120) (mean ± std. Error)* | WWP 3 (n = 120) (mean ± Std. Error)* | 𝑃 value** (between groups) | ||
|---|---|---|---|---|---|---|
| 1-2 | 2-3 | 1-3 | ||||
| Free Testosterone (pg/mL) | 8.373 ± 1.390 | 16.02 ± 0. 864 | 19.51 ± 1.00 | 0.01** | 0.038** | 0.005** |
| DHEA-S (𝜇g/dL) | 268.80 ± 8.18 | 413.15 ± 2.78 | 432.37 ± 4.019 | 0.003** | 0.043** | 0.00** |
| Estradiol (pg/mL) | 308.00 ± 26.710 | 430.42 ± 19.047 | 528.19 ± 13.55 | 0.004** | 0.01** | 0.00** |
| Hydroxy progesterone (ng/dL) | 356.53 ± 4.557 | 394.36 ± 8.922 | 451.40 ± 5.349 | 0.018** | 0.06 | 0.005** |
| Prolactin (ng/mL) | 17.466 ± 0.709 | 39.966 ± 1.899 | 50.633 ± 1.324 | 0.010** | 0.056 | 0.006** |
| SHBG (nmol/L) | 51.05 ± 1.707 | 24.03 ± 1.47 | 18.86 ± 0.814 | 0.005** | 0.06 | 0.001** |
| Fasting Insulin (𝜇Iu/mL) | 11.32 ± 3.390 | 19.283 ±1.864 | 19.70 ± 1.442 | 0.04** | 0.877 | 0.012** |
| Fasting glucose(mmol/dL) | 4.353 ± 1.703 | 5.373 ± 1.150 | 6.02 ± 1. 903 | 0.03** | 0.042** | 0.01** |
| HOMA-IR | 2.21 ± 0.631 | 4.63 ± 0.532 | 5.31 ± 0. 72 | 0.005** | 0.023** | 0.004** |
| AMH (ng/mL) | 2.86 ± 1.721 | 7.50 ± 3.59 | 7.91 ± 4.609 | 0.005** | 0.809 | 0.003** |
| FSH (mIu/mL) | 8.55 ± 3.971 | 9.05 ± 2.911 | 11.24 ± 2.719 | 0.52 | 0.00** | 0.001** |
| LH (mIu/mL) | 10.86 ±2.479 | 25.59 ± 1.322 | 33.89 ± 2.934 | 0.00** | 0.00** | 0.00** |
| LH/FSH ratio | 1.26 ± 0.832 | 3.00 ± 0.914 | 3.09 ± 0.742 | 0.002** | 0.899 | 0.001** |
| miRNA Expression | WWEb 1 (n = 120) (mean ± std. Error) | WWEa 2 (n = 120) (mean ± std. Error) | WWP 3 (n = 120) (mean ± Std. Error) | 𝑃 value (between groups) | ||
|---|---|---|---|---|---|---|
| 1-2 | 2-3 | 1-3 | ||||
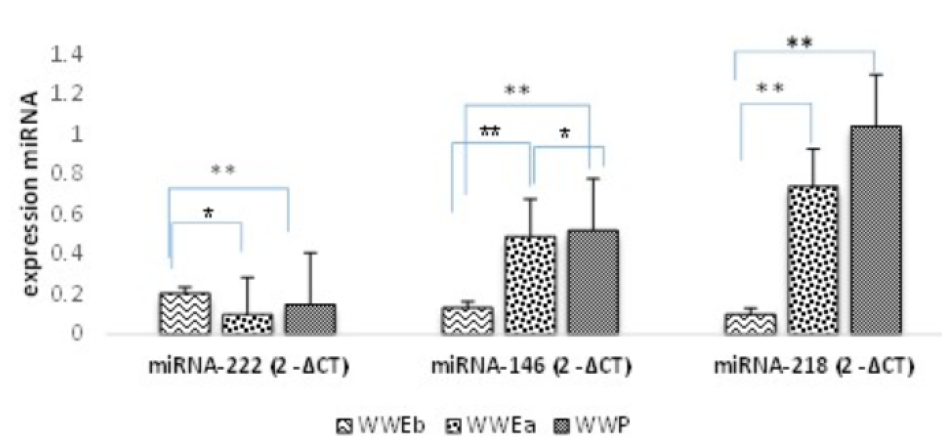
| miRNA-222 (2 -ΔCT ) | 0.206 ± 0.74 | 0.099 ± 0.89 | 0.15 ± 0.71 | 0.013** | 0.072 | 0.00*** |
| miRNA-146 (2 -ΔCT ) | 0.133 ± 0.685 | 0.49 ± 0.718 | 0.52 ± 0.885 | 0.001*** | 0.02** | 0.00*** |
| miRNA-218 (2 -ΔCT ) | 0.10 ± 0.936 | 0.74 ± 0.831 | 1.04 ± 0.903 | 0.001*** | 0.503 | 0.00*** |
microRNA extraction and quantification
Circulating miRNA from plasma was extracted using a miRNA extraction kit (Roche) according to the manufacturer's instructions. A NanoDrop 2000c from Thermo Scientific was used to evaluate the concentration of the extracted RNA and its purity by determining the A260/A280 ratio, and then, it was stored at −80°C. cDNA synthesis was achieved using the BON-miR 1st-strand cDNA synthesis miRNA kit (catalog #BON209001). qRT–PCR was performed using RealQ Plus 2x Master Mix Green with high ROX™ (Ampliqon, cat. No: A325402) and StepOne software v2.3 on the qRT–PCR ABI system (Applied Biosystems, Carlsbad, CA, USA). SNORD was used as an endogenous control due to its stable expression in all samples in the profiling experiments for all miR-146, miR-218, and miR-222. qRT–PCR was performed to evaluate the expressions of the miRNAs using a specific primer (Table 1) in a total volume of 25 µL by following the thermal profile of initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 1 min, and 72°C for 45 s. All PCR experiments were performed in duplicate. The relative expression values of all miRNAs were evaluated using the relative quantification 2-∆CT method.
Statistical analysis
Data processing and statistical analysis were performed using the SPSS software version 26. The parameters are reported as means ± SD between the PCOS and epileptic patients before and after treatment. Multiple comparisons were made using Pearson correlations and ANOVA test, discrete variables were compared with Pearson's test, and P ˂ 0.05 was considered significant. For comparing the mean differences in miRNA-146, miRNA-222, and miRNA-218 expression levels between epileptic patients before and after treatment, P ˂ 0.01 was considered significant.
Results
A total of 120 women (mean age, 26.5 years; range, 18–35 years) before and after monotherapy with VPA were compared to women who only had PCOS (mean age, 27.5 years; range, 18–35 years). There was no significant difference in BMI between groups 1 and 2, but women in the VPA-treated group became more obese (BMI > 25) after treatment (Table 2). The hirsutism score was significantly different between groups (P ≤ 0.05) with an average of 5.83 ± 0.314 in WWEb, 15.06 ± 0.480 in WWEa, and 19.13 ± 0.980 in WWP (F–G score in Table 2). The information about clinical menstrual disturbances summarized in Table 1 includes irregular menstruation (amenorrhea, dysmenorrhea, and oligomenorrhea) or the time of irregular menses. The pattern of menstrual cycle irregularities in WWEa was very similar to that of WWP and significantly different from WWEb.
The comparison of biochemical parameters, including target hormones, between the three groups, WWEa, WWEb, and WWP, is summarized in Table 3. The women had significantly lower free testosterone levels before taking the VPA than the control (WWP) and VPA groups (WWEb) (P ≤ 0.05). Next, we examined DHEA-S, and its serum level increased after treatment. However, the DHEA-S mean was not significantly different after VPA treatment (P ≤ 0.05). Concerning PCOS, serum levels of LH, FSH, and AMH are important for both early diagnosis and prognosis. Our findings showed increased levels of all three hormones after VPA treatment (P ≤ 0.05). The increases in serum levels of AMH and LH were significant, and the increase in the serum level of AMH was high. No difference was observed compared to the WWP group (P > 0.05). However, in the case of FSH, the increase showed an insignificant difference after treatment. Moreover, the LH/FSH ratio significantly increased after treatment, reaching the amount found in the PCOS patients (WWP) (Table 3).
Regarding the increase in estradiol hormone, there should be a significant difference after treatment with VPA, but not to the extent that it reaches the WWP group level. In contrast to the increasing trend of estradiol, a significant decrease in the serum level of SHBG was observed after treatment (Table 3). Moreover, in the women after VPA treatment, higher serum levels of PRL and hydroxy progesterone than in the WWEb group were significantly observed (P ≤ 0.05), but not to the extent that it reached the WWP group level. In addition to the mentioned hormones, fasting insulin and glucose levels were also assessed. Our results showed increased fasting insulin and glucose levels after treatment (P ≤ 0.05). Finally, during the treatment, the mean HOMA-IR values were significantly increased from 2.21 to 4.63 in women treated with VPA (Table 3). Thus, insulin resistance (HOMA-IR > 2.5) developed during therapy among the VPA-treated patients. For the women who took VPA, those who had proximate insulin resistance levels before starting therapy became obviously insulin resistant during therapy. In addition, serum levels of AMH and LH and the LH/FSH ratio increased significantly after treatment (Table 3).
Linear regression analysis was performed to determine the role of demographic information and hormonal and biochemical parameters on the miRNA expression in the WWEa, WWEb, and WWP groups. In the linear regression analysis, the hirsutism and AMH levels were correlated with the miRNA-222 expression level (b = 0.01; P = 0.000) (b = 0.0209; P = 0.049) (Table 1S in the Appendix). Additionally, significant correlations were observed between parameters, such as hydroxprogesterone (b = 0.273; P= 0.02), fasting G (b = 0.453; P = 0.00), AMH (b = -0.282; P = 0.014), LH/FSH ratio (b = −0.217; P = 0.049), free testosterone (b = 0.207; P = 0.05), and estradiol (b = 0.628; P = 0.05), LH (b = 0.094; P = 0.05) with the miRNA-218 expression level (Table 1S in the Appendix). Moreover, significant correlations were shown between variables of DHEA-S (b = 0.51; P = 0.00), estradiol (b = 0.345; P = 0.00), SHBG (b = −0.004; P = 0.01), FSH (b = 0.187; P = 0.017), prolactin (b = 0.362; P = 0.002), LH (b = 0.359; P = 0.003), AMH (b = −0.201; P = 0.05) and the miRNA-146 expression level (Table 2S in the Appendix). Additionally, significant correlations were observed between some hormones (associated with PCOS and VPA treatment) and the miRNA expressions. A significant difference was found among PCOS phenotypes in terms of the expression of the three miRNAs and AMH level.
This study examined the expression of three miRNAs related to PCOS with the aim of early diagnosis of this syndrome and its complications during treatment with VPA. The mean expressions of miRNA-146 and miRNA-218 were significantly higher after treatment. Despite the significant increase in these two miRNAs in the WWEa group compared with the WWEb group (P ≤ 0.001), the increase in miRNA-218 is not comparable to its level in WWP (P = 0.503). In contrast, miRNA-222 expression reduced significantly after treatment with VPA (P = 0.013) (Table 4) (Figure 1).
Discussion
There are two types of anti-epileptic drugs: drugs that induce the liver enzymes, such as phenytoin, carbamazepine, and phenobarbital, and those that do not induce the liver enzymes, such as VPA22. There is mounting evidence that VPA can disrupt ovarian function and androgen production, presumably due to numerous effects on the hypothalamic–pituitary–ovarian axis, based on the emergence of PCOS-like symptoms in patients who use this medication. Accordingly, it has been shown that VPA alters numerous signaling pathways in the hypothalamic–pituitary–ovarian axis. Some of the most significant signaling cascades are involved in the functionality of this drug and its consequent symptoms. It has been indicated that VPA can increase GABA (an inhibitory neurotransmitter) signaling in GnRH neurons. Several studies demonstrated that this pathway might be responsible for activating the GnRH/LH system and correlate with downstream consequences in PCOS patients. Studies have reported that the GABA level is considerably higher in the cerebrospinal fluid of PCOS patients than in healthy women.
Clinical data have revealed that VPA treatment is directly linked to the development of PCOS symptoms in women. According to the results of previous studies and this study, an increase in the pulse frequency of LH leads to increased LH/FSH ratios in epileptic patients treated with VPA and in women with PCOS23. These pathological phenomena affect the ovarian pathogenesis of PCOS, including hyperplasia of theca cells, increased concentration of androgens, the altered response of LH to the stimulation of GnRH, and elevated serum prolactin levels.
Some studies have shown that women with PCOS require higher levels of exogenous progesterone and estradiol to diminish the high and frequent pulsed release of LH24. The impact of anti-epileptic drugs on the concentrations of reproductive hormones could indicate an association between the occurrence of reproductive disorders and the consumption of these medications. These correlations in epileptic patients are primarily based on several studies showing that alterations in SHBG are linked with sexual dysfunction25. Evidence shows that VPA effectively contributes to the metabolic pathways of estrogens and androgens. The considerable increase in the biochemical factors mentioned above following VPA treatment in this study also confirmed previous investigations.
The impact of VPA on the serum concentrations of sex hormones is complicated, and some studies have indicated increased estrogen and testosterone in patients who received VPA26. The development of hyperandrogenism and PCOS following the use of VPA has been extensively addressed in the literature. Several studies have demonstrated that VPA affects the hepatic cytochrome P450 enzyme system (another metabolizing system of VPA). This might result in elevated levels of biologically active and free forms of testosterone due to aromatase activity27. Gustavsen et al. showed that the treatment of human adrenal carcinoma cells, as a cell-line model of steroidogenesis, with VPA led to the downregulation of genes in response to some miRNAs regulating the early steps of steroidogenesis28.
In this study, we found a marked reduction in the serum levels of SHBG in the WWEa group. According to previous studies and our results, VPA increases the risk of developing PCOS in women29, 30. Heterogeneous conditions exist for diagnostic criteria of PCOS, including chronic anovulation or menstrual disorders, ovarian cyst morphology, hyperandrogenism, and hirsutism. In addition, PCOS has been associated with co-morbidities, such as type 2 diabetes, dyslipidemia, hyperinsulinemia, insulin resistance, obesity, infertility, and galactorrhea31. A study on Finnish women demonstrated that approximately 60% of individuals who received VPA exhibited biochemical and clinical changes related to PCOS32. One common side effect of PCOS is weight gain that could result from mitochondrial inhibition in response to oxidative metabolites33. In our study, no significant difference was detected in the weights of patients before and after VPA treatment. One randomized prospective study on women treated with lamotrigine and VPA reported serum concentrations of free and total testosterone following 6–12 months of treatment30. Our findings showed a significant increase in the serum levels of free testosterone in women after VPA treatment. Studies have assessed the correlation between PCOS-like symptoms and insulin resistance and obesity among patients who received VPA. VPA can alter some intracellular signaling cascades, such as mitogen-activated protein kinases (MAPK), serine/threonine protein kinase GDP (Akt), and protein kinase C (PKC)34. DeGrave and colleagues analyzed the effect of different doses of VPA on the activities of steroidogenic enzymes in thecal cell cultures isolated after hysterectomy from individuals with normal ovaries and PCOS patients. VPA (up to 500 µM) increased the production of DHEA, androstenedione, and progesterone in ovarian theca cells, thus increasing the expression levels of CYP17 and CYP11 α genes35. However, multiple investigations have found that individuals who received VPA exhibited hyperleptinemia and resistance to elevated leptin levels, mainly in those who are obese. A novel discovery concerning the hidden pharmacological effects of VPA was that it suppresses the amount of leptin mRNA in adipocytes in a dose-dependent manner, considerably reducing hormone production36. In this study, the serum concentrations of HOMA-IR and insulin were remarkably higher in epileptic patients after treatment with VPA than in those who did not receive VPA. Nisha and colleagues confirmed that individuals who received VPA had significantly higher insulin and HOMA-IR values than newly diagnosed patients. However, BMI did not change between the treated and untreated individuals37.
Recent studies have shown a correlation between the serum concentration of AMH, which belongs to the TGF-β family, and the incidence of PCOS. Thus, they introduced this hormone as a valuable and powerful diagnostic marker in the pathogenesis of PCOS. The role of AMH during folliculogenesis is to act as a regulator, avoiding FSH overuse and maintaining homeostasis in the ovaries38. In the clinical assessment and intervention of PCOS, two factors, AMH levels and insulin resistance, are significant. Insulin resistance is clinically correlated with hyperandrogenism and increased AMH serum levels. Nevertheless, there is contradictory information regarding the association between high AMH levels and insulin resistance and other metabolic symptoms of PCOS39. Furthermore, most research has involved few participants. This prompted us to examine the blood AMH levels in the epileptic women before and after VPA treatment40.
Biomarker identification for the early detection of PCOS-like symptoms in patients using VPA has shown considerable promise in both fundamental research and clinical applications, owing to the involvement of several signaling pathways associated with PCOS. MiRNAs should receive greater attention as a PCOS biomarker due to their widespread occurrence and strong link with endocrine abnormalities and inflammatory disorders. Numerous studies have shown that dysregulation of miRNAs participates in the initiation, development, and progression of various illnesses, including PCOS41. In this study, three miRNAs, including miRNA-218, miRNA-222, and miRNA-146, were chosen based on the literature and bioinformatics analysis for the following reasons: recent research has shown that these miRNAs are increased in the oocytes and undergo substantial variations at the gene expression levels during maturation of oocytes13, 42, 43. These miRNAs target many of the genes and signaling pathways involved in hyperandrogenism, insulin resistance, and obesity, as with PCOS. By constructing the miRNA–mRNA network, we demonstrated that miRNA-218, miRNA-222, and miRNA-146 correlate with PCOS.
Similar to in PCOS, many of the genes and signaling pathways associated with insulin resistance, hyperandrogenism, and obesity are targeted by these miRNAs. By establishing an miRNA–mRNA network, we showed that miRNA-218, miRNA-222, and miRNA-146 are significantly linked with PCOS incidence. Two of the three miRNAs are of particular interest as biomarkers of PCOS. It has been reported that miRNA-222 is positively associated with insulin resistance, while miRNA-146 is inversely linked with the testosterone level44, 45. It is now known that the insulin transport pathway is one of the most significant molecular pathways in developing PCOS, activating many detrimental pathways. The activation of PTPN1, targeted by miRNA-146, acts as an inhibitor in this pathway. Several lines of evidence indicate that miRNA-146 expression is elevated, while PTPN1 expression is considerably reduced in PCOS patients46. The RAS pathway could initiate insulin resistance or activation of detrimental molecular cascades, subsequently activating the MAPK pathway by phosphorylation of the RAS protein. Activating this pathway could result in the conversion of MEK to ERK, leading to increased production of androgens47. Of note, the fas gene, targeted by miRNA-146, can induce apoptosis in oocytes, thereby promoting folliculogenesis48. It has been reported that the miRNA-222 family is one of the major regulators of the expression of this protein. As shown in Figure 2, this miRNA is elevated in PCOS patients. It inhibits PTPN1 expression, thus stimulating the activation of the MAP kinase pathway, androgen production, and the emergence of disease complications49.
Notably, increased expression of miRNA-218 is correlated with the inhibition of adiponectin, which is secreted by differentiated adipose tissues and is abundantly present in the blood. However, its expression is substantially diminished in those with PCOS and obesity. The MEKKs/TAK1 pathway regulates the molecular mechanism of adiponectin production in muscle. miRNA-218 inhibits the adiponectin receptor (AdipoR2), which interacts with AMP-activated protein kinase and p38 mitogen-activated protein kinase50.
Conclusions
To identify biomarkers for early detection of PCOS-like symptoms in patients taking VPA, the expressions of miRNA-218 and miRNA-146 were evaluated. As previous evidence on PCOS-signaling pathways in valperat users has described, many genes involved in these pathways are targets of these miRNAs. According to our findings, the significant change in the expressions of these miRNAs and the results reported in previous articles on their target genes can be used for diagnostic or therapeutic purposes in patients taking VPA. Concerning the development of PCOS, serum levels of LH, FSH, and AMH are important for both early diagnosis and prognosis. Moreover, we found that serum AMH levels significantly differed among women before and after VPA treatment. However, positive correlations were observed between the other hormones that link PCOS and VPA metabolism and the expressions of the studied miRNAs.
Abbreviations
AdipoR2: receptor adiponectin; AEDs: antiepileptic drugs; Akt/ PBK: The serine / threonine protein kinase GDP also known as the Akt; AMH: Anti-Müllerian Hormone; BMI: body mass index; CNS disease: Central Nervous System disease; DHEA-S: dehydroepiandrosterone sulfate; E: estradiol; EIAEDs: liver enzyme inducing antiepileptic drugs; Ers: estrogen receptors; FSH: follicle stimulating hormone; GABA: inhibitory neurotransmitter; HOMA-IR: homeostasis model assessment-estimated insulin resistance; ILAE: International League Against Epilepsy; Jak-STAT: Janus Kinase and Signal Transducer and Activator of Transcription; LH: luteinizing hormone; LH/FSH ratio: luteinizing hormone/ follicle stimulating hormone ratio; MAPK: mitogen-activated protein kinase; miRNA: microRNA; non-EIAED: none liver enzyme inducing antiepileptic drugs; PCK: Protein kinase C; PCOS: polycystic ovarian syndrome; PCR: polymerase chain reaction; PTEN: Phosphatase and tensin homolog deleted on chromosome 10; PTENP1: Phosphatase and tension homolog Pseudogene 1; PTPN1: Protein Tyrosine Phosphatase Non-Receptor Type 1; qRT-PCR: quantitative real time polymerase chain reaction; SHBG: sex hormone binding globulin; VPA: Valproate; Wnt: canonical pathway; WWEa: women with epilepsy after treatment with VPA; WWEb: women with epilepsy before treatment with VPA; WWP: women with PCOS
Acknowledgments
This research is the product of a research project (code: 5374) and financial support of abortion research center, Yazd Reproductive Sciences Research Institute. I would like to thank the group of specialists and experts who took part of their precious time to share their experiences and knowledge in meetings. This group includes: Dr. Seyed Mehdi Kalantar and the Supervisor of the Genetics Laboratory of the Abortion Research Center) Dr. Fatemeh Montazeri ( , also neurologists (Dr. Seyed Massoud Etemadifar, Dr. Mohsen Beshtam) from the Epilepsy Center, and (Ms. Dr. Mitra Poursirfi). I would like to Special thank Dr. Mansour Salehi and the Genetics Laboratory of Al-Zahra Hospital in Isfahan for their cooperation in sampling. I am also grateful to everyone.
Author’s contributions
All the authors have somehow played a role in the implementation of this project, including: Mahya Rajabi and Fateme Montazeri: study design, literature review, doing laboratory works including miRNA extracting, qRT-PCR, data analysis, article writing; Seyed-Mehdi Kalantar: supervision, study design, literature review
Funding
This research is extracted from a research project (code: 5374) and financial support from the Abortion Research Center, Yazd Reproductive Sciences Institute.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Informed consent form was achieved from all participants in this research. All procedures performed in studies involving participants were accordance with the approved standards of Yazd Reproductive Sciences Institute Ethics committee (Ethical code: IR.SSU.RSI.REC.1396.8) and the ethical standards of 1964 Helsinki declaration and its later amendments comparable standards.We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Also none of the authors has any conflict of interest to disclose.
References
-
Santos-Peyret
A.,
Durón
R.M.,
Sebastian-Diaz
M.A.,
Crail-Meléndez
D.,
Gomez-Ventura
S.,
Briceño-González
E.,
Rito
Y. M.-J.,
E-health tools to overcome the gap in epilepsy care before, during and after COVID-19 pandemics. Revista de Neurologia.
;
70
(9)
:
323-8
.
View Article Google Scholar -
Sheikhalishahi
A.,
Jahdi
F.,
Haghani
H.,
Gharlipour
Z.,
The relationship between sexual health and personality type in women with epilepsy. Journal of Education and Health Promotion.
2021;
10
(January)
:
257
.
PubMed Google Scholar -
Beghi
E.,
The Epidemiology of Epilepsy. Neuroepidemiology.
2020;
54
(2)
:
185-91
.
View Article PubMed Google Scholar -
Bui
E.,
Harden
C.L.,
Scenario
C.,
Infertility and Menstrual Disorders : Seizure Medications vs . Seizures. In: Controversies in Caring for Women with Epilepsy. 2016. p. 87–97. . 2016
.
-
Giri
V.P.,
Giri
O.P.,
Khan
F.A.,
Kumar
N.,
Kumar
A.,
Haque
A.,
Valproic acid versus lamotrigine as first-line monotherapy in newly diagnosed idiopathic generalized tonic \textemdashClonic seizures in adults \textemdash A randomized controlled trial. Journal of Clinical and Diagnostic Research : JCDR.
2016;
10
(7)
:
01-04
.
View Article PubMed Google Scholar -
Ghasemian
M.,
Owlia
M.B.,
Mosaddegh
M.H.,
Nejad
M.N.,
Sohrevardi
S.M.,
Evaluation of sodium valproate low dose efficacy in radicular pain management and it's relation with pharmacokinetics parameters. Biomedicine (Taipei).
2020;
10
(3)
:
33-40
.
View Article PubMed Google Scholar -
Lipska
K.,
Gumieniczek
A.,
Filip
A.A.,
Anticonvulsant valproic acid and other short-chain fatty acids as novel anticancer therapeutics: possibilities and challenges. Acta Pharmaceutica (Zagreb, Croatia).
2020;
70
(3)
:
291-301
.
View Article PubMed Google Scholar -
Yasmeen
R.,
Mobeen
N.,
Khan
M.A.,
Aslam
I.,
Chaudhry
S.,
Intake of Anti-Epileptic Drugs and their Influences on Sexual Dysfunctions. Pakistan Biomed J..
2021;
3
(2)
:
3-9
.
View Article Google Scholar -
Ogunjimi
L.,
Yaria
J.,
Makanjuola
A.,
Alabi
A.,
Osalusi
B.,
Oboh
D.,
Polycystic ovarian syndrome in Nigerian women with epilepsy on carbamazepine/levetiracetam monotherapy. Acta Neurologica Scandinavica.
2021;
143
(2)
:
146-53
.
View Article PubMed Google Scholar -
Taub∅ll
E.,
Isojärvi
J.I.,
Herzog
A.G.,
The interactions between reproductive hormones and epilepsy. Handbook of Clinical Neurology.
2021;
182
:
155-74
.
View Article PubMed Google Scholar -
Chen
Z.,
Ou
H.,
Wu
H.,
Wu
P.,
Mo
Z.,
Society
A.E.,
Role of microRNA in the pathogenesis of polycystic ovary syndrome. DNA and cell biology.
2019;
38
(8)
:
754-62
.
View Article Google Scholar -
Liu
L.,
Wang
X.,
How close are we to diagnosing Polycystic ovary syndrome with miRNAs: A Meta-analysis and review.. ResearchSquare.
;
:
[Preprint]
.
View Article Google Scholar -
Mu
L.,
Sun
X.,
Tu
M.,
Zhang
D.,
Non-coding RNAs in polycystic ovary syndrome: a systematic review and meta-analysis. Reproductive Biology and Endocrinology.
2021;
19
(1)
:
1-8
.
View Article Google Scholar -
W
Long,
C
Zhao,
C
Ji,
H
Ding,
Y
Cui,
X
Guo,
R
Shen,
J
Liu,
Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkers. Cellular Physiology and Biochemistry.
2014;
33
(5)
:
1304-15
.
View Article Google Scholar -
Xue
Y.,
Lv
J.,
Xu
P.,
Gu
L.,
Cao
J.,
Xu
L.,
Identification of microRNAs and genes associated with hyperandrogenism in the follicular fluid of women with polycystic ovary syndrome. Journal of Cellular Biochemistry.
2018;
119
(5)
:
3913-21
.
View Article PubMed Google Scholar -
Sharma
P.C.,
Gupta
A.,
MicroRNAs: potential biomarkers for diagnosis and prognosis of different cancers. Translational Cancer Research.
2020;
9
(9)
:
5798-818
.
View Article PubMed Google Scholar -
Du
H.,
Fu
Z.,
He
G.,
Wang
Y.,
Xia
G.,
Fang
M.,
MicroRNA-218 targets adiponectin receptor 2 to regulate adiponectin signaling. Molecular Medicine Reports.
2015;
11
(6)
:
4701-5
.
View Article PubMed Google Scholar -
Zhang
Y.,
Xia
F.,
Zhang
F.,
Cui
Y.,
Wang
Q.,
Liu
H.,
miR-135b-5p enhances doxorubicin-sensitivity of breast cancer cells through targeting anterior gradient 2. Journal of Experimental & Clinical Cancer Research.
2019;
38
(1)
:
26
.
View Article PubMed Google Scholar -
Hossain
M.M.,
Cao
M.,
Wang
Q.,
Kim
J.Y.,
Schellander
K.,
Tesfaye
D.,
Altered expression of miRNAs in a dihydrotestosterone-induced rat PCOS model. Journal of Ovarian Research.
2013;
6
(1)
:
36
.
View Article PubMed Google Scholar -
I. Sidorkiewicz,
M. Jóźwik,
M. Niemira,
A. Krętowski,
Insulin Resistance and Endometrial Cancer: Emerging Role for microRNA. Cancers.
2020;
12
(9)
:
2559-14
.
View Article Google Scholar -
Branavan
U.,
Wijesundera
S.,
Chandrasekharan
V.,
Wijeyaratne
C.,
Potential Genetic Polymorphisms Predicting Polycystic Ovary Syndrome (PCOS) in Sri Lankan Women: Comparison with Different Ethnicity. Adv Technol..
2021;
1
(1)
:
65-88
.
View Article Google Scholar -
Li
S.,
A Meta-Analysis of PCOS-Related Reproductive Abnormalities in Women Taking Valproate for Epilepsy. ResearchSquare.
2021;
:
[Preprint]
.
View Article Google Scholar -
Ruddenklau
A.,
Campbell
R.E.,
Neuroendocrine impairments of polycystic ovary syndrome. Endocrinology.
2019;
160
(10)
:
2230-42
.
-
Shaaban
Z.,
Khoradmehr
A.,
Reza
M.,
Shirazi
J.,
Tamadon
A.,
Pathophysiological mechanisms of gonadotropins–and steroid hormones–related genes in etiology of polycystic ovary syndrome. Iranian journal of basic medical sciences..
2018;
22
(1)
:
3
.
View Article Google Scholar -
Santos
A.M.,
Filho
H. Castro Lima,
Siquara
G.M.,
Lopes
J.M.,
Bastos
C.G.,
Brito
M.B.,
Sexual function in women of fertile age with epilepsy. Epilepsy & Behavior.
2021;
125
:
108399
.
View Article PubMed Google Scholar -
Taub∅ll
E.,
Isojärvi
J.I.,
Herzog
A.G.,
The interactions between reproductive hormones and epilepsy. Handbook of Clinical Neurology.
2021;
182
:
155-74
.
View Article PubMed Google Scholar -
Praharaj
S.K.,
Munoli
R.N.,
Udupa
S.T.,
Vaidyanathan
S.,
Valproate‐associated hair abnormalities: Pathophysiology and management strategies. Human Psychopharmacology: Clinical and Experimental.
2022;
37
(1)
:
e2814
.
View Article Google Scholar -
Gustavsen
W.M.,
Reproductive endocrine side effects of antiepileptic drugs. 2008 Jun 15 [cited 2022 Jan 18]; Available from: https://www.duo.uio.no/handle/10852/29594. 2008
.
-
Qu
X.,
Donnelly
R.,
Sex Hormone-Binding Globulin (SHBG) as an Early Biomarker and Therapeutic Target in Polycystic Ovary Syndrome. Int J Mol Sci 2020, Vol 21, Page 8191 [Internet]. 2020 Nov 1 [cited 2022 Jan 19];21(21):8191. Available from: https://www.mdpi.com/1422-0067/21/21/8191/htm. 2020
.
-
Sidhu
H.S.,
Srinivasa
R.,
Sadhotra
A.,
Evaluate the effects of antiepileptic drugs on reproductive endocrine system in newly diagnosed female epileptic patients receiving either Valproate or Lamotrigine monotherapy: A prospective study. Epilepsy Research.
2018;
139
:
20-7
.
View Article PubMed Google Scholar -
Karakas
S.E.,
Evaluate the effects of antiepileptic drugs on reproductive endocrine system in newly diagnosed female epileptic patients receiving either Valproate or Lamotrigine monotherapy: A prospective study. Epilepsy research.
2022;
139
:
20-7
.
View Article Google Scholar -
Bayat
M.,
Frequency of metabolic syndrome and insulin resistance in epileptic patients treated with sodium valproate or carbamazepine monotherapy : A Case-Control Study. ResearchSquare.
2021;
:
[Preprint]
.
View Article Google Scholar -
Xu
S.,
Chen
Y.,
Ma
Y.,
Liu
T.,
Zhao
M.,
Wang
Z.,
Lipidomic Profiling Reveals Disruption of Lipid Metabolism in Valproic Acid-Induced Hepatotoxicity. Frontiers in Pharmacology.
2019;
10
:
819
.
View Article PubMed Google Scholar -
Li
S.,
Qi
J.,
Sun
Y.,
Gao
X.,
Ma
J.,
Zhao
S.,
An integrated RNA-Seq and network study reveals that valproate inhibited progesterone production in human granulosa cells. The Journal of Steroid Biochemistry and Molecular Biology.
2021;
214
:
105991
.
View Article PubMed Google Scholar -
Taub∅ll
E.,
Heuser
K.,
Sveberg
L.,
Svalheim
S.,
Experimental models for the study of hormonal changes in epilepsy. Zeitschrift für Epileptologie.
2015;
28
(4)
:
246-53
.
View Article Google Scholar -
Singh
D.,
Gupta
S.,
Verma
I.,
Morsy
M.A.,
Nair
A.B.,
Ahmed
A.F.,
Hidden pharmacological activities of valproic acid: A new insight. Biomedicine and Pharmacotherapy.
2021;
142
(July)
:
112021
.
View Article PubMed Google Scholar -
Nisha
Y.,
Bobby
Z.,
Wadwekar
V.,
Biochemical derangements related to metabolic syndrome in epileptic patients on treatment with valproic acid. Seizure.
2018;
60
:
57-60
.
View Article PubMed Google Scholar -
Quinn
M.,
Cedars
M.I.,
Huddleston
H.G.,
Santoro
N.,
Antimüllerian hormone use and misuse in current reproductive medicine practice: a clinically oriented review. F&S Reviews.
2021;
3
(1)
:
1-0
.
View Article Google Scholar -
Wiweko
B.,
Indra
I.,
Susanto
C.,
Natadisastra
M.,
Hestiantoro
A.,
The correlation between serum AMH and HOMA-IR among PCOS phenotypes. BMC research notes.
2018;
11
(1)
:
1-6
.
View Article Google Scholar -
Rajabi
M.,
Miresmaili
S.M.,
Montazri
F.,
Nasresfahani
M.,
Zieai
S.J.,
Kalantar
S.M.,
Evaluating Mirna-222 Expression Level and Its Association with AMH for Early Diagnosis of Pcos-Like Symptoms in Epileptic Patients Plasma Treated with Sodium Valproate: A Case – Control Study. Journal of Shahid Sadoughi University of Medical Sciences.
2021;
29
(10)
:
4198-4208
.
View Article Google Scholar -
Markoula
S.,
Siarava
E.,
Keramida
A.,
Chatzistefanidis
D.,
Zikopoulos
A.,
Kyritsis
A.P.,
Reproductive health in patients with epilepsy. Epilepsy {&}amp; Behavior.
2020;
113
:
107563
.
View Article PubMed Google Scholar -
Mafi
A.,
Mirhosseini
N.,
Aghadavod
E.,
Jahanshahi
M.,
Asemi
Z.,
Association between miRNAs Expression and Signaling Pathways of Oxidative Stress in Polycystic Ovary Syndrome. Critical Reviews in Eukaryotic Gene Expression.
2020;
30
(4)
:
359-68
.
View Article PubMed Google Scholar -
Qasemi
M.,
Amidi
F.,
Extracellular microRNA profiling in human follicular fluid: new biomarkers in female reproductive potential. Journal of Assisted Reproduction and Genetics.
2020;
37
:
1769-80
.
View Article Google Scholar -
Ebrahimi
S.O.,
Reiisi
S.,
Parchami Barjui
S.,
Increased risk of polycystic ovary syndrome (PCOS) associated with CC genotype of miR-146a gene variation. Gynecological Endocrinology.
2018;
34
(9)
:
793-7
.
View Article PubMed Google Scholar -
He
C.,
Huang
W.,
Zhou
F.,
Wang
J.,
Correlation between serum microRNA-222and metabolism of glucose and lipid in women with polycystic ovary syndrome. Chinese J Postgraduates Med [Internet].
2021;
2021
:
533-7
.
View Article Google Scholar -
Cirillo
F.,
Catellani
C.,
Lazzeroni
P.,
Sartori
C.,
Nicoli
A.,
Amarri
S.,
MiRNAs Regulating Insulin Sensitivity Are Dysregulated in Polycystic Ovary Syndrome (PCOS) Ovaries and Are Associated With Markers of Inflammation and Insulin Sensitivity. Frontiers in Endocrinology (Lausanne).
2019;
10
:
879
.
View Article PubMed Google Scholar -
Ye
W.,
Xie
T.,
Song
Y.,
Zhou
L.,
The role of androgen and its related signals in PCOS. Journal of Cellular and Molecular Medicine.
2021;
25
(4)
:
1825-37
.
View Article PubMed Google Scholar -
Gong
Z.,
Yang
J.,
Bai
S.,
Wei
S.,
MicroRNAs regulate granulosa cells apoptosis and follicular development — A review. Asian-Australasian Journal of Animal Sciences.
2022;
33
(11)
:
1714
.
-
Ye
H.,
Liu
X.J.,
Hui
Y.,
Liang
Y.H.,
Li
C.H.,
Wan
Q.,
Downregulation of MicroRNA-222 Reduces Insulin Resistance in Rats with PCOS by Inhibiting Activation of the MAPK/ERK Pathway via Pten. Molecular Therapy. Nucleic Acids.
2020;
22
(183)
:
733-41
.
View Article PubMed Google Scholar -
Du
H.,
Fu
Z.,
He
G.,
Wang
Y.,
Xia
G.,
Fang
M.,
MicroRNA-218 targets adiponectin receptor 2 to regulate adiponectin signaling. Molecular Medicine Reports.
2015;
11
(6)
:
4701-5
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 10 No 9 (2023)
Page No.: 5884-5895
Published on: 2023-09-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4109 times
- XML downloaded - 100 times
- PDF downloaded - 1170 times
- Supplement downloaded - 975 times
 Biomedpress
Biomedpress



