Abstract
Background: The angiotensin converting enzyme insertion-deletion (ACE I/D) polymorphism located on chromosome 17q23 (287 bp in intron 16) is associated with cardiovascular risk factors (CRFs), but results vary among populations, which is thought to be the cause of ethnic differences. This study explored the role of the ACE I/D polymorphism and its correlation with CRF determinants among urban and rural groups.
Methods: A total of 182 male and female participants were recruited in the age range of 20 ? 55 years for CRF determinant examination and ACE gene polymorphism (n = 140).
Results: Most samples examined for polymorphic ACE genes showed increased CRF determinants in the two groups. For genotype II and urban group ID, the risk was increased 5 ? 8 times for the CRF of obesity. The frequency of genotype II significantly increased the incidence of CRFs of smoking and sedentary by 1 ? 3 times in both groups.
Conclusions: The ACE I/D polymorphism has a differential effect on both urban and rural groups. Smoking, sedentary behavior, and obesity were risk factors for CRF in both groups. Therefore, an overall design strategy for health policies is needed to mitigate the burden of cardiovascular disease, which ends in death in both groups.
Introduction
The current public health challenge is the prevention and control of cardiovascular risk factors (CRFs)1. Coronary heart disease, often known as coronary artery disease (CAD), contributes to 31% of deaths worldwide. The trend of disease is increasing throughout Southeast Asia2. Coronary heart disease attacks people aged < 45 years, with an average age of 39 ± 6 years. Similar studies through retrospective studies have also reported that there were approximately 10,268 patients who underwent coronary surgery with age clusters in the range of < 45 to < 55 years (male; female)3. A paradigm shift in lifestyle increases the prevalence of this disease. Various lifestyle changes include unbalanced diets, excessive carbohydrate consumption, progressive economic growth, and urbanization4. Based on population analysis, it has been reported that on the Asian continent, including in Indonesia, this disease is increasing exponentially in the population, especially in vulnerable urban groups5.
The migration of several rural to urban community groups occurs due to employment factors that have effects on society and health. This is certainly detrimental to the population, including the potential to increase the incidence of CRF determinants6. One challenge that is very serious in developing countries, especially in Indonesia, is urban health issues, including CAD and CRF7.
One way CAD is affected in urban communities is via extreme lifestyle changes, including lack of physical activity, smoking, and unhealthy eating habits8.
Previous studies have reported the involvement of genes in various populations, particularly in urban and rural populations, but other studies have shown that urban groups are correlated with CRFs in contrast to rural groups9.
Other studies have focused on ethnicity and ethnic groups and their CRFs, such as nutritional deficits, obesity, and hypertension (HTN). Furthermore, Pena et al. (2016) studied the complex correlation between genes and the environment triggered by CRFs10.
Thus, it is important to trace the role of genetics in different urban and rural populations, especially gene polymorphisms of populations.
The angiotensin converting enzyme (ACE) gene, which is located on chromosome 17q23, contains many polymorphisms that are collections of different phenotypes composed of DNA strands. The insertion‒deletion (I/D) polymorphism consists of 287 bp in intron 1611. The ACE I/D polymorphism is one of the main enzymes that control the renin–angiotensin system (RAS) pathway. Some researchers have reported that this polymorphism is associated with several diseases, especially non-communicable and generative diseases, such as metabolic syndrome, HTN, dyslipidemia, diabetes, obesity, and hypertriglyceridemia12.
The population distribution among urban and rural populations has shown that they have the same risk of developing CRF diseases. However, no reports have compared the two populations. Thus, it is important to provide an overview of the population distribution for those at risk of CAD and CRFs and the role of genetics among urban and rural populations, especially gene polymorphisms in South Sulawesi populations.
Methods
Study design
A population-based design with a cross-sectional study approach was used in this study. Data were collected via a purposive random sampling method. The samples were from urban and rural community groups.
Sample size
In total, 182 participants were successfully recruited in the age range of 20 – 50 years and included both males and females. Of these, 42 subjects were excluded from the study because they refused to provide consent to participate in the study, even though the informed consent form had been explained in detail. The ACE I/D polymorphism was examined and identified, and samples were separated according to population location (n = 60 for the rural group and n = 80 for the urban group). CRF determinant tests included smoking, sedentary, obesity, HTN, abnormal heart rate, and poor VO2max capacity (n = 140) for both groups. We prepared anamnesis schedules and initial examinations to obtain basic participant data, including each participant’s name, age, gender, medical history, and occupation. Three milliliters of blood were collected using a standard biomedical laboratory protocol from the biomolecular Hasanuddin University - Medical Research Center (HUM-RC). All materials and tools used in this study were approved by the Health Ethics Committee of the Makassar Health Polytechnic.
Anthropometric measurement and CRF determinants
Anthropometric measurements included age (years), height (cm), weight (kg), body mass index (BMI = BB/TB2) in kg/m2, systolic blood pressure (sbp) in mmHg, diastolic blood pressure in mmHg, heart rate, and VO2max. Specific standardization of BMI refers to Southeast Asian populations according to WHO standards (2000)13. Blood pressure (BP) measurements were taken on the left arm of participants while they laid on the examination room bed using a BP digital instrument (OMROM-7080), with standardization of measurements according to JNC VII guidelines. Measurements were performed at one time to minimize measurement errors14.
Polymorphism and genotyping
DNA was extracted according to the Rigat et al. (1990) protocol, and we performed allele-specific polymerase chain reaction (PCR) of ACE I/D polymorphism using specific primer sequences via standardpro kol from Rigat et al., 199015.
Forward primer: 5′-CTGGAGACCACTCCCATCCTTTCT-3′ and
Reverse primer: 5′-GATGTGGCCATCACATTCGTCAGAT-3′
DNA was amplified for 30 cycles with denaturation at 94 °C for 1 min, annealing at 58 °C for 1 min, and extension at 72 °C for 2 min using an Applied Biosystems thermal cycler. Genotyping of the PCR products [in base pairs (bp): DD (190 bp), ID (490 bp, 190 bp), and II (490 bp)] was performed via 2% agarose gel electrophoresis. As per Perna et al. 1992 and Shanmugam et al. 1993, there is a possibility of ID genotypes being mistyped as DD. To avoid this, 51 samples with DD genotypes were reanalyzed using the protocol suggested by Shanmugam et al. 1993 in which 5% dimethyl sulfoxide (DMSO) was added to the PCR mixture16.
Statistical analysis
Using SPSS software application version 22.0, we conducted statistical testing using the chi-square (X2) test to identify general characteristics of the population and CRF among the urban and rural groups. We also used the chi-square test to determine the differences in genotype distributions according to the CRF determinants. Meanwhile, Hardy–Weinberg equilibrium (HWE) was carried out to observe the frequency distribution of ACE I/D polymorphism genotypes among the two population groups. Furthermore, the variables, including age, sex, education, occupation, smoking, and alcohol consumption, were adjusted to understand the role of the ACE I/D polymorphism in the CRF determinants using logistic regression and interaction tests (moderated regression analysis), with a significance of p < 0.05 (2-tailed).
| General characteristics | Urban | Rural | χ2 P |
|---|---|---|---|
| Age (years) | 39.04 ± 9.5 | 38.78 ± 9.4 | .025 (.875) |
| Sex | |||
| Female | 67 (83.75) | 30 (50) | 20.819 (.0001) |
| Male | 13 (16.25) | 30 (50) | |
| Education | |||
| Illiterate | 56 (72.73) | 32 (53.33) | 20.819 (.012) |
| literate | 21 (27.27) | 28 (46.67) | |
| Occupation | |||
| Sedentary | 52 (65) | 25 (41.67) | 7.858 (.006) |
| Active | 28 (35) | 35 (58.33) | |
| Alkohol | |||
| No | 67 (83.75) | 50 (83.33) | .004 (.948) |
| Yes | 13 (16.25) | 10 (16.67) | |
| Smoking | |||
| No | 53 (66.25) | 51 (85) | 6.514 (.012) |
| Yes | 27 (33.75) | 9 (15) |
| Cardiovascular risk factor | Normal range | Urban (± SD) | Rural (± SD) (%n = 60) | χ2 (P ) |
|---|---|---|---|---|
| Smoking | 6.31 (.012) | |||
| No | 53 (66.25) | 51 (85) | ||
| Yes | 27 (33.75) | 9 (15) | ||
| Occupation | 7.542 (.0006) | |||
| Sedentary | 52 (65) | 25 (41.67) | ||
| Active | 28 (35) | 35 (58.33) | ||
| BMI (kg/m 2 ) | 21.15 ± 3.25 | 22.99 ± 1.92 | 15.198 (.0001) | |
| Underweight (U) | < 18.5 | 25 (31.25) | 0 | |
| Normal (N) | ≥ 18.5 but < 23 | 32 (40) | 43 (71.67) | |
| Overweight (Ov) | ≥ 23 but < 25 | 10 (12.5) | 6 (10) | |
| Obese (Ob) | ≥ 25 | 13 (16.25) | 11 (18.33) | |
| BP (mmHg) | 127.63 ± 19.86; 85.94 ± 9.56 | 114.17 ± 17.92; 80.87 ± 9.78 | 16.675 (.000) 8.027 (.018) | |
| Normotension (Nr) | SBP < 120 and DBP < 80 | 6 (7.5) | 22 (36.67) | |
| Pre-hypertension (PrPHTN) | SBP 120 – 139 or DBP 80 – 89 | 35 (43.75) | 18 (30) | |
| Hypertension (HTN) | SBP ≥ 140 or DBP ≥ 90 | 39 (48.75) | 20 (33.33) | |
| HR (bpm) | 94.36 ± 10.097 | 90.73 ± 9.669 | 12.782 (.0001) | |
| Normal | 60 - 100 | 33 (41.25) | 43 (71.67) | |
| Abnormal | < 60 and > 100 | 47 (58.75) | 17 (28.33) | |
| VO 2max (ml/kg/min) | 41.92 ± 3.69 | 45.81 ± 4.15 | .277 (.0001) | |
| Good | 27 (36.99) | 40 (66.67) | ||
| Poor | 63 (63.01) | 20 (33.33) |
| Genotype | Urban total N = 80 (%) | Rural total N = 60 (%) |
|---|---|---|
| II | 21 (26.25) | 16 (26.67) |
| ID | 35 (43.75) | 17 28.33) |
| DD | 24 (30) | 27 (45) |
| I allele | 36 (45) | 24 (40) |
| D allele | 44 (55) | 36 (60) |
| HWE X 2 (P ) | X 2 = 24.177 (0.001) | X 2 = 20.292 (0.001) |
| N number of individuals P ≤ 0.05 significant | P = 0.038 | |
| Cardiovascular risk factor | Urban | OR (95%CI) P II versus ID + DD | |||
|---|---|---|---|---|---|
| II n (%) | ID n (%) | DD n (%) | X 2 (P) | ||
| Smoking | 1.547 (.219) | 1.026 (.357 - 2.947) .962 | |||
| No | 26 (70.27) | 36 (76.60) | 42 (82.35) | ||
| Yes | 11 (29.73) | 16 (23.40) | 9 (17.65) | ||
| Occupation | .750 (.476) | .833 (.297-2.340) .729 | |||
| Sedentary | 23 (62.16) | 26 (50) | 28 (54.90) | ||
| Active | 14 (37.84) | 26 (50) | 23 (45.10) | ||
| BMI (Kg/m 2 ) | 38.508 (.0001) | 5.250 (1.113 - 24.771) .036 | |||
| Underweight | 15 (71.43) | 0 | 10 (41.7) | ||
| Normal | 4 (19.05) | 17 (48.6) | 11 (45.8) | ||
| Overweight | 1 (4.76) | 9 (25.7) | 0 | ||
| Obese | 1 (4.76) | 9 (25.7) | 3 (12.5) | ||
| BP (mmHg) | 3.068 (.216) | .914 (.334-2.506) .862 | |||
| Normotensives | 6 (28.57) | 11 (31.43) | 6 (25) | ||
| Prehypertensives | 6 (28.57) | 13 (37.14) | 5 (20.83) | ||
| Hypertensives | 9 (42.86) | 11 (31.43) | 13 (54.17) | ||
| HR (bpm) | 1.030 (.362) | ||||
| Normal | 10 (59.46) | 16 (55.77) | 7 (49.02) | ||
| Abnormal | 11 (49.54) | 19 (44.23) | 17 (50.98) | ||
| VO 2max (ml/kg/min) | .475 (.623) | 1.296 (.460 - 3.652) .624 | |||
| Good | 19 (51.35) | 26 (50.00) | 22 (43.14) | ||
| Poor | 18 (48.65) | 26 (50.00) | 29 (56.86) | ||
| Cardiovascular risk factor | Rural | OR (95%CI) P II versus ID + DD | |||
|---|---|---|---|---|---|
| II n (%) | ID n (%) | DD n (%) | X 2 (p value) | ||
| Smoking | 1.169 (.318) | .385 (.089 - 1.665) .201 | |||
| No | 12 (75) | 16 (94.12) | 23 (85.10) | ||
| Yes | 4 (25) | 1 (5.88) | 4 (14.81) | ||
| Occupation | 2.115 (.130) | 3.222 (.982 - 10.578) .054 | |||
| Sedentary | 10 (71.43) | 5 (29.41) | 10 (37.04) | ||
| Active | 6 (28.57) | 12 (70.59) | 17 (62.96) | ||
| BMI (Kg/m 2 ) | 6.659 (.047) | 3.621 (.726 - 18.066) .117 | |||
| Normal | 14 (87.5) | 12 (70.59) | 17 (62.96) | ||
| Overweight | 2 (12.5) | 3 (17.65) | 1 (3.70) | ||
| Obese | 0 | 2 (11.76) | 9 (33.34) | ||
| BP (mmHg) | 6.807 (.146) | .568 (.141 - 2.280) .425 | |||
| Normal | |||||
| Abnormal | |||||
| Normotensives | 12 (57.14) | 24 (68.57) | 11 (45.83) | ||
| Prehypertensives | |||||
| Hypertensives | 9 (42.86) | 11 (31.43) | 13 (54.17) | ||
| HR (bpm) | .294 (.746) | 1.258 (.342 - 4.634) .730 | |||
| Normal | 12 (75%) | 13 (76.47) | 18 (66.67) | ||
| Abnormal | 4 (25%) | 4 (23.53) | 9 (33.33) | ||
| VO 2max (ml/kg/min) | .151 (.860) | 1.138 (.334 - 3.882) .837 | |||
| Good | 11 (68.75) | 12 (70.59) | 17 (62.96) | ||
| Poor | 5 (31.25) | 5 (29.41) | 10 (37.04) | ||
| Cardiovascular risk factors | Odds ratio (95%CI) [p value] (II genotype) | Odds ratio (95%CI) [p value] (ID genotype) | Odds ratio (95%CI) [p value] (DD genotype) | |||
|---|---|---|---|---|---|---|
| Urban | Rural | Urban | Rural | Urban | Rural | |
| Smoking | 1.026 (.357 - 2.947) .962 | .385 (.089 - 1.665) .201 | 2.062 (.805 - 5.283) .131 | 2.600 (.601 - 11.256) .201 | .407 (.000 - .000) .116 | .974 (.234 - 4.053) .971 |
| Sedentary | .833 (.297 - 2.340) .729 | 3.222 (.982 - 10.578) .054 | 1.476 (.585 - 3.723) .409 | .310 (.095 - 1.019) .054 | .515 (.000 - .000) .224 | 1.417 (.501 - 4.004) .511 |
| Obese | 5.250 (1.113 - 24.771) .036 | 3.621 (.726 - 18.066) .117 | 8.471 (2.704 - 26.533) .0001 | .395 (.000 - .000) .0001 | .257 (.068 - .970) .045 | 2.185(.697 - 6.853) .180 |
| Hypertension | .914 (.334 - 2.506) .862 | .568 (.141 - 2.280) .425 | .479 (.190 - 1.205).118 | .224 (.000 - .000) .0001 | 2.127 (.805 - 5.619) .128 | .391(.093 - 1.649) .201 |
| Abnormal HR | 1.423 (.522 - 3.882) .491 | 1.258 (.342 - 4.634) .730 | .721 (.294 - 1.769) .475 | .795 (.216 - .2.928) .730 | 2.105 (.755 - 5.866) .155 | 1.562 (.505 - 4.831) .438 |
| Poor VO 2max | 1.296 (.460 - 3.652) .624 | 1.138 (.334 - 3.882) .837 | .609 (.239 - 1.551) .299 | .879 (.258 - 2.998) .837 | 2.459 (.000 - .000) .116 | 1.353 (.461 - 3.975) .582 |
Results
Characteristics of urban and rural populations
Our study successfully recruited 140 participants, distributed in two population groups, namely, urban and rural, with average ages of 39.04±9.5 and 38.78±9.4 years, respectively. However, these subjects were similar in terms of gender, education, occupation, and alcohol and smoking consumption, but not age. Comparison of urban and rural groups based on general characteristics found significant differences, including differences in gender, education, occupation, and smoking, except for age and alcohol consumption (Table 1).
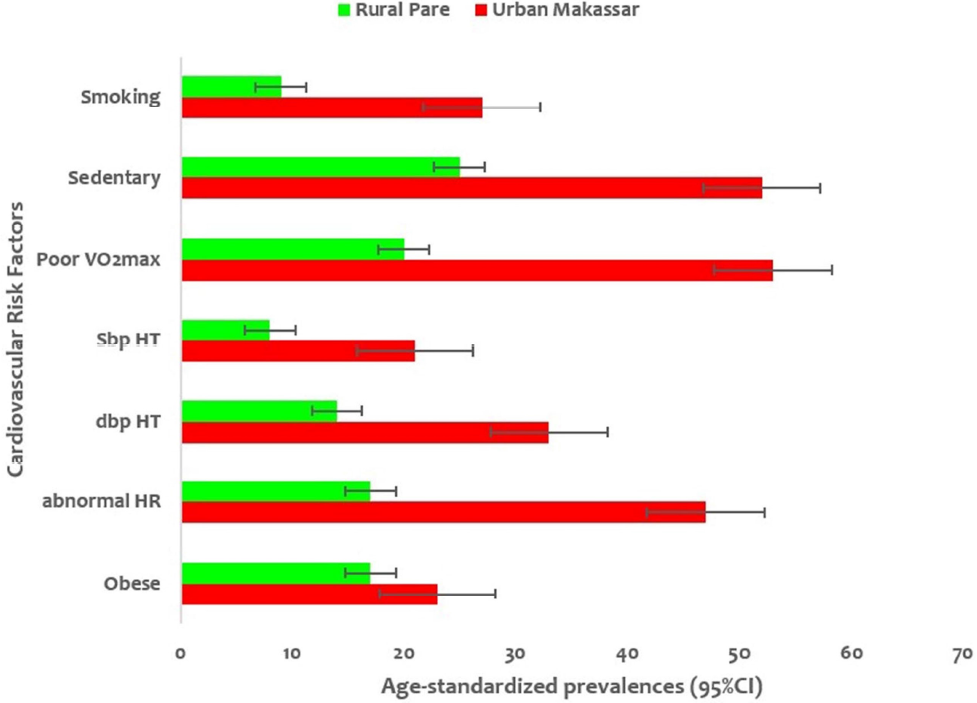
A significant proportion of the women and men were smokers, had poor VO2max capacity, and were obese (0.003, 0.002, and 0.032, respectively), which were increased CRFs compared with sedentary, systolic and diastolic HTN, and abnormal heart rate factors (Figure 1).
Influence of CRF determinants among urban and rural groups
The prevalence of CRF determinants among urban and rural groups is shown in Table 2. The CRF determinants that were similar included smoking and occupation. Other similar risk factors were BMI, BP, HR, and VO2max. Overall, the CRF determinants that were significantly (< 0.05) correlated with the urban and rural community groups included smoking, occupation, BMI, BP, HR, and VO2max (Table 2).
The comparison of adult, middle, and advanced ages showed that middle age people had a higher prevalence of CRF determinants than the other two age groups. Specifically, three factors included systolic and diastolic HT and abnormal HR (0.000, 0.000, and 0.009, respectively), which were the dominant CRFs (Figure 2).
Distribution of ACE I/D genotype and allele frequency among urban and rural groups
This study focused on the ACE I/D polymorphism among urban and rural groups. We observed Hardy–Weinberg equilibrium (HWE) in genotypes II, ID, and DD, and frequencies of alleles I and D were observed in the urban group (p = 0.0001). Both groups (urban and rural) showed significant HWE equilibrium (X2 = 24.177; 20,292, p = 0.0001) (Table 3). The distribution of genotypes II, ID, and DD in both urban and rural groups was evenly distributed, and there was no difference in genotype (0. 956, 0.062, 0.069, respectively) between the two groups (Figure 3).
Distribution of the ACE I/D genotype and its correlation with CRF determinants in urban and rural groups
The ACE I/D polymorphism examinations of the urban group showed differences in the CRF determinants. Genotype II in the urban groups showed smoking risk to be more prevalent than in ID and DD genotypes (29%, 23.40%, and 17.65%, respectively). The sedentary factor was higher than for DD and ID (62.16%, 50%, and 54.90%, respectively). The obese factor was higher in genotype ID than in II and DD (25.7%, 4.76%, and 12.5%, respectively). Moreover, the DD genotype showed a higher prevalence of HTN than the II and ID genotypes (54.17%, 42.86%, and 31.43%, respectively). The prevalence of abnormal HR was higher in DD than in II and ID (50.98%, 49.54%, and 44.23%, respectively), and poor VO2max capacity was higher in DD than in ID and II genotypes (56.86%, 50%, and 48.65%, respectively Their respective odds ratio values (1.026, 0.833, 5.250, 0.914, and 1.296) showed the incidence of CRF determinants in the future (Table 4).
Similarly, the rural group showed differences in almost all CRF determinants. In genotype II, smoking and sedentary factors were higher than in the DD and ID genotypes (25%, 14.81%, 5.88%, 71.43%, 14.81%, and 5.88%, respectively). The DD genotype was dominant for obesity (33.34% and 11.76%), HTN (54.17%, 42.86%, and 31.43%), abnormal HR (33.33%, 25.00%, and 23.53%), and poor VO2max capacity (37.04%, 31.25%, and 29.41%) compared with those in genotypes II and ID. Thus, genotypes II and DD in the rural group have increased potential for CRF determinants compared with the ID genotype group, with their respective odds ratios (1.026, 0.833, 5.250, 0.914, and 1.296) increasing the CRF determinants (Table 5).
Interaction of ACE I/D genotype with CRF determinants in urban and rural groups
We analyzed the interactions of the ACE I/D polymorphism among the three genotypes, II, ID, and DD, with the CRF determinants in the urban and rural groups. Individuals in the rural group who had genotype ID had an 8-fold increased risk of obesity, a 2-fold increased risk of smoking, and a 1-fold increased risk of being sedentary, with the remaining risks below a 1-fold increase (HTN, abnormal heart rate, and poor VO2max capacity). In genotype II, the risk increased five times for the incidence of obesity, and we observed a 1-fold increase in the incidence of smoking, abnormal heart rate, and poor VO2max capacity. The sedentary behavior and HTN risks were below a 1-fold increase in incidence. The DD genotype resulted in a 2-fold increased risk of obesity and a 1-fold increased risk of sedentary behavior and abnormal heart rate. It showed a less than 1-fold increase in the incidence of smoking. In the rural group, those who had genotype II had a 3-fold increased risk of sedentary behavior and obesity and a 1-fold increased risk of abnormal heart rate and poor VO2max capacity. This genotype also resulted in below a 1-fold increase in the incidences of smoking and HTN. In genotype ID, the risk was twice the incidence of smoking, and the remaining risks were below a 1-fold increase, including the incidence of sedentary behavior, obesity, HTN, abnormal heart rate, and poor VO2max capacity. Finally, in the DD genotype, there was a 2-fold increased incidence of obesity, and a 1-fold increased incidence in sedentary behavior, abnormal heart rate, and poor VO2max capacity. The remaining risks were below a 1-fold increased incidence, including smoking (Table 6).
Discussion
Our study provides an important description of different ways in which ACE I/D polymorphism affects different environmental dimensions, such as urban and rural communities. Urbanization is one of the main biomarkers for the occurrence of CRF determinants, and this is the main demographic change occurring in developing countries that have large populations17.
Sociodemographic data of urban and rural groups with CRF determinants
Almost all sociodemographic data between these two groups were similar for the study subjects in terms of sex, education, occupation, alcohol, and smoking, except for age. This study assessed two main populations: urban and rural groups.
The CRFs that were determinants in this study included smoking, sedentary lifestyle, obesity, HTN, abnormal heart rate, and poor VO2max capacity. Almost all CRF determinants observed were high in the urban group. However, only one CRF determinant, obesity, was found in the rural group. Thus, the main sociodemographic finding was that almost all CRF determinants were higher in the urban group than in the rural group (Table 2). The findings challenge the previous paradigm that CRF determinants are evenly distributed upon migration in urban and rural groups. However, a meta-analysis study from Gupta and Hernández et al. reported that urban populations have the potential for increased CRF determinants18.
ACE I/D genotype in urban and rural groups
The separation between the urban and rural groups took place over a long time, namely, during the development of the new Indonesian government. Previously, the rural population was dominant, and even when they separated into two different groups, their genetics did not change. With the discovery of sequencing technology for the ACE I/D polymorphism, these two groups were found to be different in terms of their HWE scales. Genotypes II and ID are dominant in the urban group, while DD genotypes are dominant in the rural group with a statistical significance (p = 0.038).
Differences in the genotypes of the two groups in the ACE I/D polymorphism occur specifically, where rural groups have a high number of homozygotes, and urban groups have a higher number of heterozygotes (Table 3).
The shift or change in homozygous traits owing to the erosion of the influence of urbanization indicates that the DD genotype from homozygotes was more dominant in the rural group, whereas the DD genotype in the urban group showed homozygous relaxation19.
With the evolution of humans in the past, many genes have undergone positive selection, e.g., changes in viral pathogenic genes (APOBEC3G) to prevent the body's immune response (PRM1 evolution of male reproductive genes and evolution (FOXP2) for HIV infection resistance (CCR5delta32)). Interestingly, when physical fitness (VO2max) was measured in both groups, a higher level of physical fitness (VO2max) was found for all genotypes, II, ID, and DD, in the rural group than in the urban group20.
Interaction of the ACE I/D genotype with CRF determinants
Our study has shown that the ACE I/D polymorphism is directly correlated to CRF determinants with variable results9, 21, 22. The determinants that cause this variability in the results are demographic, geographical, and ethnic factors. One important finding of our study was that the urban and rural groups were in the same Mendelian family, namely, ethnic Malayu, where allele D was found to have a 1,227-fold increase in the urban group compared with the rural group (Table 4). Similar research has been conducted previously23.
The CRF determinants showed an increased correlation with the ACE I/D polymorphism of the D allele in the urban group. Comparison of HWE equilibrium and decreased function of the DD genotype in the rural group revealed new data related to loss of positive selection sensitivity in this group.
However, the analysis of the odds ratio in the urban groups that had genotype II and ID showed a 5-8-fold increased risk of obesity compared with the DD genotype. Thus, urban groups have protection against obesity. However, this is not entirely true because genotypes II and ID and obesity may have a negative selection, which leads to the omission of genotype DD as a marker; thus, the odds ratio results could have been skewed (Table 6).
Some researchers have found that obesity and dyslipidemia are causative factors of cardiovascular diseases and are considered adverse CFR determinant phenotypes24.
Other studies have revealed positive interactions of the ACE I/D polymorphism with smokers, which is likely because ACE is the main enzyme of the renin–angiotensin system (RAS) that plays a role in regulating the mechanism of cardiorespiratory homeostasis25. In addition, one CAD and CVD trigger is the ACE gene. Previous researchers from Northern Ireland have found that the ACE I/D polymorphism is directly related to the incidence of smoking. Researchers from Turkey also reported their findings on the relationship between ACE I/D polymorphism and obesity and found that the incidence of obesity was significantly higher than that in controls (21.4%), which was dominant in women. Similarly, in another study that sampled the Tibetan population, researchers reported that the D allele of the ACE gene contributed to obesity in women. However, in Tibetan men, this allele was not identified. Concerning rural population groups, one study also reported that ACE I/D polymorphism does not cause primary HTN. Our research found the presence of the DD genotype in urban and rural groups and the DD genotype in rural groups leads to the increased CRF of obesity.
CONCLUSIONS
In this study, we found that almost all CRF determinants that activate ACE I/D polymorphism were higher in the urban group than in the rural group. The urban and rural groups were in the same Mendelian family, namely, ethnic Melayu, where allele D was found to have a 1,227-fold increase in urban groups compared with in rural groups. CRF determinants showed a high correlation with ACE I/D polymorphism in both groups with a high incidence of cardiovascular disease, resulting in death. Therefore, an overall design strategy for health policies is needed to mitigate the burden of cardiovascular disease, which ends in death in both groups.
Abbreviations
ACE: angiotensin-converting enzyme, I/D: insertion-deletion, CRF: cardiovascular risk factor, II: insertion insertion, ID: insertion deletion, DD: deletion deletion, CAD: coronary arteries deseases, HTN: hypertension, RAS: renin–angiotensin system, HUM-RC: Hasanuddin University — Medical Research Center, BMI: body mass index, SBP: Sistolic blood pressure, DBP: diastolic blood pressure, BP: blood pressure, DMSO: dimethyl sulfoxide, HWE: hardy–weinberg equilibrium, VO2max: volume oxygen maximum.
Acknowledgments
Thank you to all components involved in this research and members of HUM-RC Makassar Laboratory Team.
Author’s contributions
I.I: Constructing an idea or hypothesis for research and/or manuscript, Taking responsibility in the construction of the whole or body of the manuscript, reviewing the article before submission not only for spelling and grammar but also for its intellectual content; M.B: Organising and supervising the course of the project or the article and taking the responsibility; MM: Planning methodology to reach the conclusion, Taking responsibility in this necessary function, taking responsibility in logical interpretation and presentation of the results; A.N: Providing personnel, environmental and financial for the support and tools and instruments that are vital project, biological materials, reagents and referred patient. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
This research data is available not for dissemination but stored for the benefit of future research.
Ethics approval and consent to participate
The research ethics protocol has been approved by the Health Research Ethics Commission of the Makassar Health Polytechnic with number 0232/O/KEPK-PTKMS/III/2023. Participants involved in this study have signed letters of consent to become respondents in the researcher.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Mishra
D.,
Naorem
K.,
Saraswathy
K.N.,
Angiotensin-Converting Enzyme Gene Insertion/Deletion Polymorphism and Cardiometabolic Risk Factors: A Study Among Bhil Tribal Population from Two Environmental Settings. Biochemical Genetics.
2018;
56
(4)
:
295-314
.
View Article PubMed Google Scholar -
Hoo
F.K.,
Foo
Y.L.,
Lim
S.M.,
Ching
S.M.,
Boo
Y.L.,
Acute coronary syndrome in young adults from a Malaysian tertiary care centre. Pakistan Journal of Medical Sciences.
2016;
32
(4)
:
841-5
.
PubMed Google Scholar -
Zuhdi
A.S.,
Mariapun
J.,
Mohd Hairi
N.N.,
Wan Ahmad
W.A.,
Abidin
I.Z.,
Undok
A.W.,
Young coronary artery disease in patients undergoing percutaneous coronary intervention. Annals of Saudi Medicine.
2013;
33
(6)
:
572-8
.
View Article PubMed Google Scholar -
Coffey
S.,
Williams
M.J.,
Cox
B.,
Letter by Coffey et al regarding article: Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement\textquotedblright. Circulation.
2013;
128
(6)
:
e84-84
.
View Article PubMed Google Scholar -
Bhatti
G.K.,
Bhatti
J.S.,
Vijayvergiya
R.,
Singh
B.,
Implications of ACE (I/D) Gene Variants to the Genetic Susceptibility of Coronary Artery Disease in Asian Indians. Indian Journal of Clinical Biochemistry.
2017;
32
(2)
:
163-70
.
View Article PubMed Google Scholar -
Fu
Y.,
Lin
W.,
Yang
Y.,
Du
R.,
Gao
D.,
Analysis of diverse factors influencing the health status as well as medical and health service utilization in the floating elderly of China. BMC Health Services Research.
2021;
21
(1)
:
438
.
View Article PubMed Google Scholar -
Chen
J.,
Chronic conditions and receipt of treatment among urbanized rural residents in China. BioMed research international.
2013;
2013
:
568959
.
View Article Google Scholar -
Goff
D.C.,
Lloyd-Jones
D.M.,
Bennett
G.,
Coady
S.,
D'Agostino
R.B.,
Gibbons
R.,
2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology.
2014;
63
(25)
:
2935-59
.
View Article PubMed Google Scholar -
Kumari
S.,
Sharma
N.,
Thakur
S.,
Mondal
P.R.,
Saraswathy
K.N.,
Beneficial role of D allele in controlling ACE levels: a study among Brahmins of north India. Journal of Genetics.
2016;
95
(2)
:
291-5
.
View Article PubMed Google Scholar -
Kshatriya
G.K.,
Acharya
S.K.,
Triple burden of obesity, undernutrition, and cardiovascular disease risk among Indian tribes. PLoS One.
2016;
11
(1)
:
e0147934
.
View Article PubMed Google Scholar -
Dhar
S.,
Ray
S.,
Dutta
A.,
Sengupta
B.,
Chakrabarti
S.,
Polymorphism of ACE gene as the genetic predisposition of coronary artery disease in Eastern India. Indian Heart Journal.
2012;
64
(6)
:
576-81
.
View Article PubMed Google Scholar -
Vilela-Martin
J.F.,
Vaz-de-Melo
R.O.,
Cosenso-Martin
L.N.,
Kuniyoshi
C.H.,
Yugar-Toledo
J.C.,
Pinhel
M.A.,
Renin angiotensin system blockage associates with insertion/deletion polymorphism of angiotensin-converting enzyme in patients with hypertensive emergency. DNA and Cell Biology.
2013;
32
(9)
:
541-8
.
View Article PubMed Google Scholar -
World Health Organization. Western Pacifc Region, International Association for the Study of Obesity, International Obesity Task Force (2000) Asia Pacifc perspective: redefning obesity and its treatment [Internet]. who.int. Sydney : Health Communications Australia; 2021 [cited 2023 Apr 8]. Available from: https://apps.who.int/iris/handle/10665/206936. 2021
.
-
Mitka
M.,
Blood pressure, cholesterol guidelines face more delays. JAMA.
2013;
310
(6)
:
568-9
.
View Article Google Scholar -
Rigat
B.,
Hubert
C.,
Alhenc-Gelas
F.,
Cambien
F.,
Corvol
P.,
Soubrier
F.,
An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. The Journal of Clinical Investigation.
1990;
86
(4)
:
1343-6
.
View Article PubMed Google Scholar -
Perna
N.T.,
Batzer
M.A.,
Deininger
P.L.,
Stoneking
M.,
Alu insertion polymorphism: a new type of marker for human population studies. Human Biology.
1992;
64
(5)
:
641-8
.
PubMed Google Scholar -
Joshi
S.R.,
Anjana
R.M.,
Deepa
M.,
Pradeepa
R.,
Bhansali
A.,
Dhandania
V.K.,
Collaborative Study Group
ICMR-INDIAB,
Prevalence of dyslipidemia in urban and rural India: the ICMR-INDIAB study. PLoS One.
2014;
9
(5)
:
e96808
.
View Article PubMed Google Scholar -
Hernández
A.V.,
Pasupuleti
V.,
Deshpande
A.,
Bernabé-Ortiz
A.,
Miranda
J.J.,
Effect of rural-to-urban within-country migration on cardiovascular risk factors in low- and middle-income countries: a systematic review. Heart (British Cardiac Society).
2012;
98
(3)
:
185-94
.
View Article PubMed Google Scholar -
Sia
R.K.,
Ryan
D.S.,
Rivers
B.A.,
Logan
L.A.,
Eaddy
J.B.,
Peppers
L.,
Vision-Related Quality of Life and Perception of Military Readiness and Capabilities Following Refractive Surgery Among Active Duty U.S. Military Service Members. Journal of Refractive Surgery (Thorofare, N.J.).
2018;
34
(9)
:
597-603
.
View Article PubMed Google Scholar -
Goodwin
Z.A.,
de Guzman Strong
C.,
Recent Positive Selection in Genes of the Mammalian Epidermal Differentiation Complex Locus. Frontiers in Genetics.
2017;
7
:
227
.
View Article PubMed Google Scholar -
Choudhury
I.,
Jothimalar
R.,
Patra
A.K.,
Angiotensin Converting Enzyme Gene Polymorphism and its Association with Hypertension in South Indian Population. Indian Journal of Clinical Biochemistry.
2012;
27
(3)
:
265-9
.
View Article PubMed Google Scholar -
Krishnan
R.,
Sekar
D.,
Karunanithy
S.,
Subramanium
S.,
Association of angiotensin converting enzyme gene insertion/deletion polymorphism with essential hypertension in south Indian population. Genes & Diseases.
2016;
3
(2)
:
159-63
.
View Article PubMed Google Scholar -
Jellinger
P.S.,
Handelsman
Y.,
Rosenblit
P.D.,
Bloomgarden
Z.T.,
Fonseca
V.A.,
Garber
A.J.,
American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocrine Practice.
2017;
23
:
1-87
.
View Article PubMed Google Scholar -
Anari
R.,
Amani
R.,
Latifi
S.M.,
Veissi
M.,
Shahbazian
H.,
Association of obesity with hypertension and dyslipidemia in type 2 diabetes mellitus subjects. Diabetes & Metabolic Syndrome.
2017;
11
(1)
:
37-41
.
View Article PubMed Google Scholar -
Singh
M.,
Singh
A.K.,
Singh
S.,
Pandey
P.,
Chandra
S.,
Gambhir
I.S.,
Angiotensin-converting enzyme gene I/D polymorphism increases the susceptibility to hypertension and additive diseases: A study on North Indian patients. Clinical and Experimental Hypertension (New York, N.Y.).
2016;
38
(3)
:
305-11
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 8 (2023)
Page No.: 5831-5842
Published on: 2023-08-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3722 times
- PDF downloaded - 1169 times
- XML downloaded - 92 times
 Biomedpress
Biomedpress





