Abstract
The present case report describes the uncommon and adverse plasmablastic transformation of multiple myeloma (MM) following autologous hematopoietic stem cell transplantation. To the best of our knowledge, this is the first case of plasmablastic myeloma (PBM) after an autologous hematopoietic transplant to be reported in Malaysia. A 41-year-old man initially diagnosed with MM IgG kappa reported lower back pain symptoms for a year, along with other associated symptoms. After receiving several lines of chemotherapy, the patient displayed a partial response (PR), and an autologous stem cell transplant (ASCT) was subsequently performed. Two months after the transplant, the patient showed signs of anemia, with a hemoglobin level of 8.0 g/dL. A peripheral blood film revealed the presence of a leucoerythroblastic blood film with normocytic normochromic red blood cells and rouleaux formation but no apparent plasma cells. The main infiltrating cells in the bone marrow aspirate (BMA) and trephine biopsy were plasmablasts with kappa light chain restriction. An increase in serum kappa free light chain (FLC), serum lambda FLC, and a low albumin/globulin (A/G) ratio were observed. In addition, serum protein electrophoresis showed an IgG kappa paraprotein band in the gamma region. Post-ASCT, the disease transformed into PBM, which conferred a poor prognosis on thepatient despite his post-transplant status. This case report highlights the diagnostic challenges of plasmablastic transformation in MM. Diagnosing PBM is thus crucial for the prompt and proper management of affected patients. Another consideration in the present case is whether the transplant procedure itself or the immunopathogenesis that took place after the ASCT resulted in the subsequent transformation into PBM.
Introduction
Multiple myeloma (MM), the second most frequently occurring hematologic malignancy, is a clonal plasma cell tumor that accounts for 1% to 8% of all cancers1. Plasmablastic transformation of myeloma in the bone marrow is rare and represents the extreme end of the MM morphologic continuum. Moreover, it is linked to a poor prognosis in patients with MM2, and formal diagnostic criteria for PBM is not currently reported in the literature2.
Most studies have adopted the definition of PBM proposed by Bartl et al. and Greipp et al., which is based on the cytomorphology of bone marrow aspirates in which plasmablasts must either be the predominant cell type or at least 2% of all cells by differential count3. In addition, plasmablastic morphology plays a significant predictive role in individuals with relapsed or primary refractory myeloma, including lower rates of overall survival and shorter progression-free survival (PFS)4. To our knowledge, the case presented in this report is the first case reported in Malaysia of plasmablastic transformation of MM post-autologous hematopoietic transplant and highlights the diagnostic challenges of plasmablastic transformation in MM.
CASE PRESENTATION
The patient, a 41-year-old man diagnosed with MM IgG kappa in 2016 at a different center, initially presented with a one-year history of lower back pain that was associated with loss of appetite and weight loss; otherwise, there was no history of trauma or fever, and the patient was referred to our center for further management.
We treated the patient with multiple lines of chemotherapy, including bortezomib-thalidomide-dexamethasone for eight cycles; lenalidomide and dexamethasone for four cycles; bortezomib, lenalidomide, and dexamethasone for eight cycles; daratumumab and dexamethasone for three cycles; dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide, bortezomib, and thalidomide for five cycles; and bortezomib, melphalan, and prednisolone for four cycles. In addition, the patient was treated with pomalidomide, cyclophosphamide, and dexamethasone for four cycles at another center. Subsequently, he achieved a PR, and received a tandem autologous stem cell transplant (ASCT). High-dose chemotherapy (melphalan) was administered to the patient prior to the ASCT, which was performed seven years after his initial diagnosis.
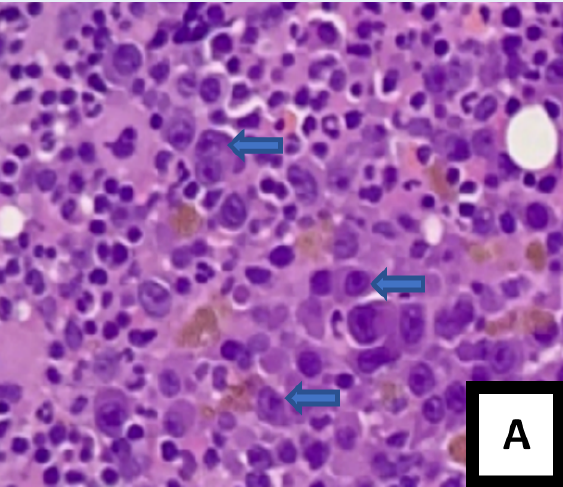
After two months, a post-transplant assessment was performed. A full blood count showed anemia with a hemoglobin level of 8.0 g/dL, while the peripheral blood film revealed the presence of leucoerythroblastic blood film with normocytic normochromic red blood cells, rouleaux formation, and no apparent plasma cells (Figure 1 A). The bone marrow aspirate (BMA) was hypercellular with some degree of hemodilution; both plasmablasts (8%) and abnormal plasma cells (14%) were easily observed (Figure 1 B and C). The trephine biopsy showed a homogenous population of plasma cells, consisting primarily of plasmablasts based on cytomorphological characteristics (Figure 2 A). These plasmablasts were positive for CD138 with kappa light chain restriction (Figure 2 B — D). The biochemical parameters showed an increase in serum kappa free light chain (FLC), serum lambda FLC, and a low A/G ratio. Serum protein electrophoresis showed IgG kappa paraprotein bands in the gamma region.
Unfortunately, the immunophenotyping was performed with severely hemodiluted samples and showed that only 0.6% of plasma cells expressed CD19 (heterogenous), CD138, and beta-2 microglobulin with inconclusive clonality but were negative for CD56 and CD117. Cytogenetically, no metaphase was available for analysis. The final diagnosis, based on the available data, was relapsed MM with plasmablastic transformation. The patient was offered the opportunity to undergo tandem ASCT but did not wish to receive the second transplant.
DISCUSSION
PBM is a subtype of MM consisting of immature plasma cells (i.e., plasmablasts) with distinct, round nuclei, nucleoli, and trace quantities of cytoplasm5. The median survival time for this rare and aggressive subtype of MM is ten months, and the prognosis is often extremely poor6, 7. This independent prognostic effect persists even after ASCT3. In the present case, the patient was initially diagnosed with MM. Following ASCT, however, the disease transformed into PBM, which conferred a poor prognosis on the patient despite his post-transplant status.
Immunophenotypically, PBM cells express plasma cell markers such as CD38, CD138, IRF/MUM 1, PRDM1/BLIMP1, and XBP1, and sometimes express cyclin D18. In the present case study, due to the severely hemodiluted bone marrow sample, only 0.6% of plasma cells could be retrieved with flow cytometry. While these cells expressed CD138 and CD19, clonality could not be determined. However, based on the immunohistochemistry, plasmablast morphology could be elucidated with a positivity toward CD138 and kappa light chain. Extramedullary involvement in MM has several characteristics, including the loss of CD56 expression9. In terms of immunophenotype in the present case, CD56 was negative, but no extramedullary site involvement was observed.
Identifying an appropriate regimen for managing PBM can be challenging due to the condition’s rarity. Nevertheless, novel induction regimens have greatly improved the treatment of MM in recent years. These regimens typically include a variety of proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies6. For example, the lenalidomide + bortezomib + dexamethasone regimen and the cyclophosphamide + bortezomib + dexamethasone (CyBorD) regimen have demonstrated promising results in MM and are currently considered the standard of care in the United States. Other combination regimens with monoclonal antibodies, such as daratumumab, have also shown favorable outcomes7. Clinical research has demonstrated that ASCT remains a successful treatment in eligible patients and increases PFS beyond novel treatments alone. Historically, myeloablative chemotherapy cytoreduction has been shown to improve PFS following ASCT10. In the present case, the patient was treated with multiple chemotherapy agents prior to his first ASCT. However, he developed relapsed MM with plasmablastic transformation post-transplant and was scheduled for tandem ASCT; however, the patient did not wish to undergo a subsequent transplantation.
Diagnosing PBM is therefore crucial for the prompt and proper management of susceptible patients. Despite improvements in our understanding of the disease process, however, long-term cures in most patients are not feasible, largely due to regionally scattered genetic and biological heterogeneity resulting from aggressive and therapy-resistant subclones11. Another aspect that requires consideration in the present case is whether the transplant process itself, or the immunopathogenesis that occurred post-ASCT, induced the transformation to PBM. Clinical evidence, along with recent compelling pre-clinical investigations in mice, support the hypothesis that T-cell exhaustion leads to immunological escape after ASCT10. Hence, the present case suggests that novel immunotherapy approaches are needed to halt myeloma progression after ASCT consolidation. Before introducing immunotherapeutic strategies into clinical practice, however, further research should be carried out on novel immunotherapies. Patients benefit most from immunotherapy when it is delivered at an earlier stage, given that the bulk of related studies have been conducted in relapsed or refractory patients12.
Conclusion
Transformation of MM to PBM post-autologous hematopoietic stem cell transplant conferred poor prognosis in a post ASCT patient. Thus, this entity need to be addressed clinically as to improve patient treatment and care. A close follow up is also crucial in this case.
Abbreviations
ASCT: Autologous stem cell transplant, BMA: Bone marrow aspirate, FLC: Free light chain, H&E: Haemotoxylin and eosin, MGG: May Grunwald Giemsa, MM: Multiple Myeloma, PBF: Peripheral blood film, PBM: Plasmablastic Myeloma, PFS: Progression-free survival, PR: Partial response, SPEP: Serum protein electrophoresis
Acknowledgments
We are grateful for our laboratory staff at the Department of Hematology, Hospital Universiti Sains Malaysia for their great assistance.
Author’s contributions
The initial concept is by Nurul Asyikin Nizam Akbar, Mohd Nazri Hassan. The first draft is wrote by Nurul Asyikin Nizam Akbar. Nur Ilyia Syazwani Saidin, Wardah Roslan, Nur Ain Izzati Abd Halim contributed to the full blood picture , bone marrow aspirate results and cytogenetic. Abu Dzarr Abdullah, Hany Hakimi Wan Hanafi contributed to the clinical history and management of this case. Nur Diyana Mohd Shukri and Sumaiyah Adzahar contributed to the discussion and conclusion. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Dimopoulos
M.A.,
Moreau
P.,
Terpos
E.,
Mateos
M.V.,
Zweegman
S.,
Cook
G.,
Committee
EHA Guidelines,
Committee
ESMO Guidelines,
Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology : Official Journal of the European Society for Medical Oncology.
2021;
32
(3)
:
309-22
.
View Article PubMed Google Scholar -
Liu
Y.,
Jelloul
F.,
Zhang
Y.,
Bhavsar
T.,
Ho
C.,
Rao
M.,
Genetic Basis of Extramedullary Plasmablastic Transformation of Multiple Myeloma. The American Journal of Surgical Pathology.
2020;
44
(6)
:
838-48
.
View Article PubMed Google Scholar -
Zriba
S.,
Kacem
K.,
Ghedira
H.,
Doghri
R.,
Hamida Marwa
B.,
Stambouli
H.,
PLASMABLASTIC MYELOMA: CASE REPORT. International Journal of Advanced Research.
2020;
8
(4)
:
737-41
.
View Article Google Scholar -
Rajkumar
S.V.,
Fonseca
R.,
Lacy
M.Q.,
Witzig
T.E.,
Therneau
T.M.,
Kyle
R.A.,
Plasmablastic morphology is an independent predictor of poor survival after autologous stem-cell transplantation for multiple myeloma. Journal of Clinical Oncology.
1999;
17
(5)
:
1551-7
.
View Article PubMed Google Scholar -
Ramadas
P.,
Williams
M.,
Duggan
D.B.,
Plasmablastic Lymphoma or Plasmablastic Myeloma: A Case of Post-Transplant Lymphoproliferative Disorder. Case Reports in Hematology.
2021;
2021
:
4354941
.
View Article PubMed Google Scholar -
Dah
K.,
Lavezo
J. L.,
Dihowm
F.,
Aggressive plasmablastic myeloma with extramedullary cord compression and hyperammonemic encephalopathy: Case report and literature review. Anticancer Research.
2021;
41
(11)
:
5839-5845
.
View Article Google Scholar -
Suarez-Londono
J. A.,
Rohatgi
A.,
Antoine-Pepeljugoski
C.,
Braunstein
M. J.,
Aggressive presentation of plasmablastic myeloma. BMJ Case Reports.
2020;
13
(4)
.
View Article Google Scholar -
Bailly
J.,
Jenkins
N.,
Chetty
D.,
Mohamed
Z.,
Verburgh
E. R.,
Opie
J. J.,
Plasmablastic lymphoma: An update. International Journal of Laboratory Hematology.
2022;
44
(S1)
:
54-63
.
View Article Google Scholar -
Bladé
J.,
Beksac
M.,
Caers
J.,
Jurczyszyn
A.,
Lilienfeld-Toal
M. von,
Moreau
P.,
Rasche
L.,
Rosiñol
L.,
Usmani
S. Z.,
Zamagni
E.,
Richardson
P.,
Extramedullary disease in multiple myeloma: a systematic literature review. Cancer Journal.
2022;
12
(3)
:
1-10
.
View Article Google Scholar -
Minnie
S.A.,
Hill
G.R.,
Autologous Stem Cell Transplantation for Myeloma: cytoreduction or an Immunotherapy?. Frontiers in Immunology.
2021;
12
:
651288
.
View Article PubMed Google Scholar -
Schürch
C. M.,
Rasche
L.,
Frauenfeld
L.,
Weinhold
N.,
Fend
F.,
A review on tumor heterogeneity and evolution in multiple myeloma: pathological, radiological, molecular genetics, and clinical integration. Virchows Archiv.
2020;
476
(3)
:
337-351
.
View Article Google Scholar -
Franssen
L. E.,
Mutis
T.,
Lokhorst
H. M.,
Donk
N. W. C. J. van de,
Immunotherapy in myeloma: how far have we come?. Therapeutic Advances in Hematology.
2019;
10
:
1-19
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 8 (2023)
Page No.: 5796-5800
Published on: 2023-08-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 4135 times
- PDF downloaded - 1417 times
- XML downloaded - 119 times
 Biomedpress
Biomedpress




