Abstract
Pulmonary nocardiosis commonly presents with respiratory symptoms resembling those observed in community-acquired pneumonia caused by other agents. Nocardia was previously considered to primarily affect immunocompromised populations, particularly transplant patients. However, recent studies have indicated a rise in the incidence of pulmonary nocardiosis among non-immunocompromised patients. In this report, we present the cases of three nonimmunocompromised patients with pulmonary nocardiosis with a subacute clinical presentation who showed cavitation on chest computed tomography. Our reports emphasize the potential diagnostic clues documented in the literature that may help pulmonary nocardiosis diagnostic investigations. The development of a high index of suspicion for pulmonary nocardiosis remains crucial.
Introduction
Nocardiosis is a rare but potentially fatal infection that can be difficult to treat. Although a disseminated Nocardia infection can impact various organs, the respiratory system is the most prevalent site of infection since the respiratory tract is the bacteria's main transmission pathway1. Nocardiosis is a relatively rare cause of community-acquired pneumonia (CAP), and, notably, over a third of patients with this disease are not immunocompromised1, 2. Chronic lung disease is an increasingly recognized risk factor for Nocardia infection2, 3, 4, 5. This report describes three clinical cases of adult non-immunocompromised chronic lung disease patients with pulmonary nocardiosis presenting as CAP with subacute symptoms. These patients were observed at Cho Ray Hospital in southern Viet Nam.
Case presentation
Case 1
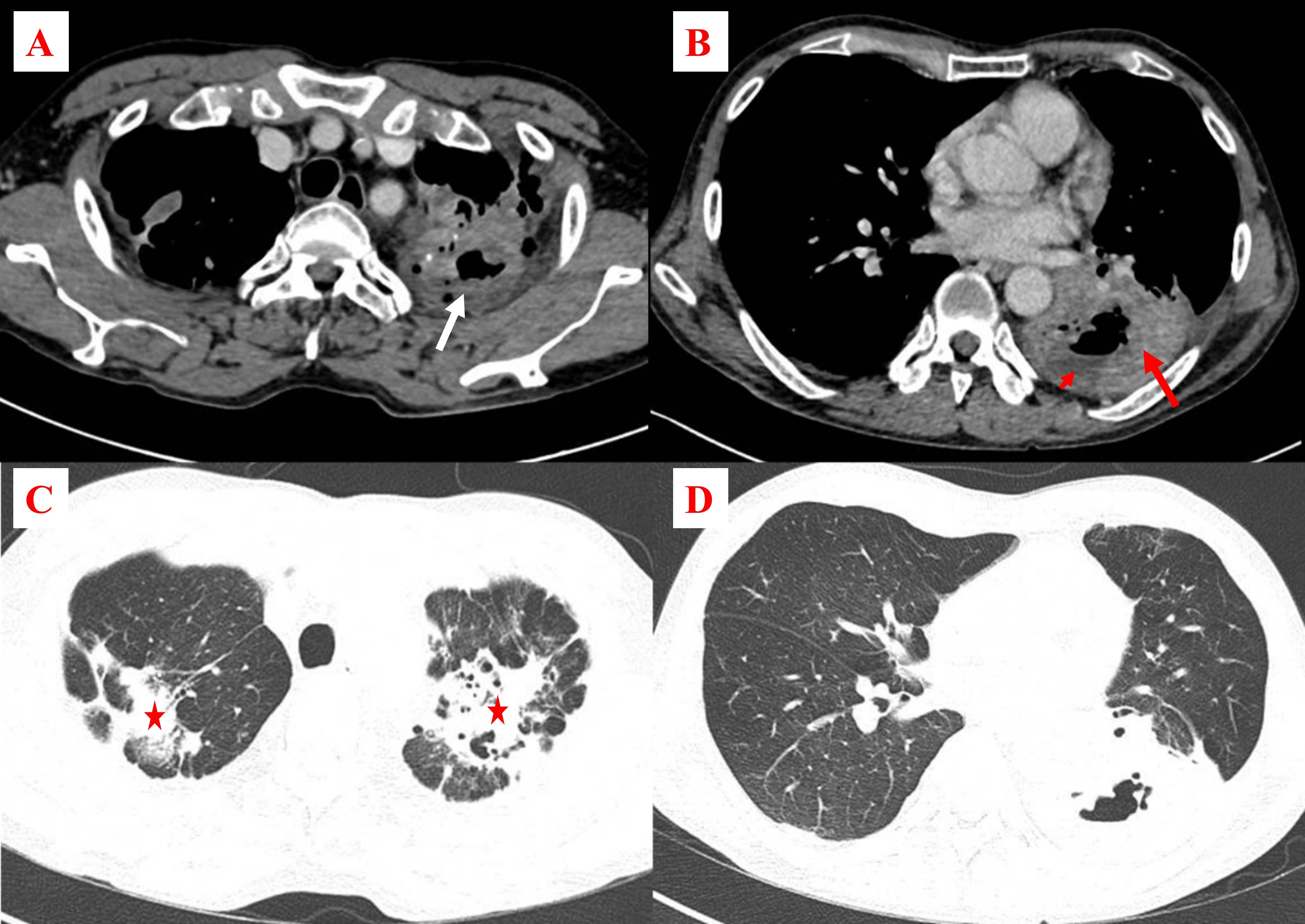
A 60-year-old male patient with type 2 diabetes and well-controlled chronic obstructive pulmonary disease (COPD) on two puffs of budesonide/formoterol (160/4.5 mcg) twice daily was admitted to the hospital due to shortness of breath, pleuritic left chest pain, and green sputum cough that started two weeks prior to hospitalization. At the time of admission to Cho Ray Hospital, the patient's vital signs were normal, and respiratory failure was absent. A clinical examination found no localized skin lesions or neurological signs; however, the lungs demonstrated sporadic crackles on auscultation. The patient was negative for human immunodeficiency virus (HIV) antibodies. The laboratory analysis revealed a C-reactive protein (CRP) level of 61 mg/l, a white blood cell (WBC) count of 17.71 G/L, and a neutrophil percentage of 95%. Blood cultures were not initially performed, and sputum cultures did not reveal any pathogens. The chest computed tomography (CT) showed bilateral patchy ground-glass opacities in the lungs and a cavitary nodule 2.5 cm in size with an even border in the left upper lobe adjacent to the chest wall, which was suspected to be malignant (as shown in Figure 1). Consequently, the patient underwent bronchoscopy, during which yellow, viscous sputum was observed exuding from the left lobe (as shown in Figure 2A). Bronchoalveolar lavage fluid (BALF) acid-fast bacillus (AFB), fungal culture, and Xpert MTB/RIF test results were all negative, as were the tuberculosis polymerase chain reaction (PCR) assay results. After three days, Nocardia beijingensis was discovered in the BALF using the Matrix Assisted Laser Desorption Ionization–Time of Flight (MALDI–TOF) technique; however, the antibiotic susceptibility pattern could not be determined. The transbronchial lung biopsy specimen analysis revealed no malignant cells. The therapy was altered from intravenous cefoperazone and ciprofloxacin (which yielded no improvement) to trimethoprim–sulfamethoxazole (TMP–SMX) after the pathogen was identified as Nocardia beijingensis; a six-month oral course was prescribed after ruling out pathogen-associated brain abscesses via CT. The patient showed no evidence of recurrence after one year of follow-up.
Case 2
A 62-year-old male patient with a 30 pack-year smoking history presented with a 15-day history of purulent cough, left chest pain, fever, and dyspnea. Initial treatment at a lower-level hospital included a combination of intravenous meropenem, moxifloxacin, and oral fluconazole. Unfortunately, the patient developed severe respiratory failure after three days of treatment, forcing transfer to Cho Ray Hospital. The patient's vital signs on arrival were as follows: blood pressure of 130/80 mmHg, pulse rate of 80 beats per minute, and oxygen administration through a cannula at a rate of 6 liters per minute to maintain SpO2 of 94%. The patient had no skin lesions or specific neurological impairments on clinical examination; however, tachypnea, accessory respiratory muscle usage, pyrexia, and bilateral lung crackles were recorded. The initial laboratory analysis showed a WBC count of 7.75 G/L, a neutrophil percentage of 82.7%, negative HIV antibody test results, a CRP level of 157 mg/L, and negative sputum and blood bacterial cultures. The patient's fever and respiratory failure persisted after six days of therapy with intravenous imipenem, linezolid, and oral fluconazole. Chest CT revealed two cavitary lesions. The first lesion, measuring 4.6 cm in diameter, was located in the S1 and S2 segments of the left lung. The second lesion, with maximum dimensions of 9 cm and 6 cm in diameter, was in the lower left lobe. The second lesion contained dense fluid that showed signs of necrosis or an abscess. The CT also demonstrated bronchiectasis, emphysema, and calcification, primarily in the upper lobes of the lungs (as shown in Figure 2). During the bronchoscopy, a significant volume of purulent sputum from the left segment S6 was discovered (as shown in Figure 3B). In the microbiological analysis of the BALF, the AFB, fungal culture, Xpert MTB/RIF, and tuberculosis PCR test results were all negative. Nocardia otitidiscaviarum was discovered on further investigation of the BALF by MALDI-TOF MS. Hence, the patient's antibiotic regimen was modified to include oral TMP–SMX in addition to intravenous meropenem and amikacin despite an unavailable antibiotic susceptibility pattern. No lesions were observed on brain CT, and the patient received ongoing therapy for an additional 14 days, resulting in improvements in fever and respiratory failure. A subsequent six-month oral course of TMP–SMX was prescribed, during which the patient remained asymptomatic and did not experience infection recurrence.
Case 3
A 65-year-old male patient with a medical history of untreated COPD was hospitalized at Cho Ray Hospital after experiencing a persistent fever and productive cough for ten days before admission. The patient's blood pressure was 140/80 mmHg, his heart rate was 106 beats per minute, and his oxygen saturation was 96% when breathing room air. No skin lesions or localized neurological deficits were observed during the clinical examination; however, both lung fields had crackles. Upon examination, the patient's test results showed elevated levels of CRP at 110 mg/L, a WBC count of 9.15 G/L, and a neutrophil percentage of 94.7%. HIV antibody testing yielded negative results. Additionally, arterial blood gas analysis recorded a PaO2 of 78 mmHg, PaCO2 of 46.1 mmHg, and HCO3 of 21.4 mmHg. A chest x-ray revealed diffuse alveolar damage affecting both lung fields, accompanied by multilobar cavitation (as shown in Figure 4). The chest CT demonstrated multiple lung consolidations containing dense fluid with cavitating features suggestive of necrosis or abscesses. The largest of these cavities, with maximum dimensions of 8 cm and 6 cm in diameter, was located in the right lower lobe. Additionally, bilateral patchy ground-glass opacifications were present in the lungs, and signs of emphysema were also observed (as shown in Figure 5). The patient’s initial treatment regimen consisted of intravenous administration of cefoperazone/sulbactam, levofloxacin, and clindamycin. The blood cultures were negative for bacteria. After three days of treatment, sputum cultures were assessed by MALDI–TOF, revealing Nocarida sp. as the causative agent. Accordingly, the patient's antibiotic therapy was modified to include meropenem, amikacin, and TMP–SMX. The patient's condition deteriorated after five days despite treatment with antibiotics specifically targeting Nocardia, with increasing respiratory failure despite the lack of COPD symptom exacerbation. A bronchoscopy revealed purulent sputum in both lungs (as shown in Figure 2C). BALF specimens were assessed by AFB, fungal culture, Xpert MTB/RIF, and tuberculosis PCR assays, all yielding negative results. However, microbiological testing revealed multidrug-resistant Acinetobacter baumannii. Imipenem, TMP–SMX, and colistin were administered; however, the patient's condition quickly deteriorated, resulting in respiratory failure and death.
Discussion
Prompt and accurate diagnosis of the causative agent is crucial for successfully managing nocardiosis. Pulmonary nocardiosis should be considered as a potential diagnosis in patients with chronic lung disease presenting with cavitary lesions on imaging and negative results for tuberculosis. When a patient with CAP does not respond sufficiently to initial broad-spectrum antibiotic therapy, clinicians should consider investigating for less common pathogens, such as Nocardia. While examining the patient's immunodeficiency status is essential, it is not mandatory for diagnosing nocardiosis. In the context of the three clinical cases we observed, identifying Nocardia was crucial in guiding treatment decisions, resulting in favorable outcomes for the first two patients. Co-infection with multidrug-resistant Acinetobacter baumannii may have contributed to disease progression and eventual mortality in the third patient.
Pulmonary nocardiosis can present with acute, subacute, or chronic symptoms, but subacute manifestations are more common. In a study of 51 transplant recipients, Hemmersbach revealed that pulmonary nocardiosis diagnosis is frequently delayed by one month from the onset of symptoms6. Similarly, Martínez reported a median time to diagnosis of 42 days in a report describing 31 patients with pulmonary nocardiosis7. Symptoms of Nocardia infection can be comparable to those observed with CAP caused by other agents8, such as shortness of breath, a dry or productive cough, hemoptysis, fever, weight loss, and weakness8, 9. The three patients in our study had symptoms for 10 to 15 days prior to admission, which is in line with the typical subacute presentation of pulmonary nocardiosis.
Pulmonary nocardiosis is characterized by various chest CT imaging patterns, including single or multiple nodular lesions with regular borders, diffuse consolidation, interstitial infiltrates, pleural effusions, or cavitary lesions9, 10. Kevin studied 53 patients with pulmonary nocardiosis and found that homogeneous consolidation was the most common lesion, observed in 64.2% of cases, followed by nodular lesions (56.6%) and nodular or mass lesions with cavitation (40%–60%)11. Baoliang reported 9 cases of pulmonary nocardiosis and found that consolidation and nodule/mass were the most typical findings12. All three patients reported here presented cavitation, and two-thirds exhibited diffuse consolidations. Hence, physicians should consider Nocardia as a potential causative organism when CAP imaging findings reveal cavitation and the patient’s tuberculosis test results are negative. The differential diagnosis should include pulmonary tuberculosis, aspergillus infection, and lung cancer8. Providing relevant clinical information to the Microbiology Department is important for accurately identifying Nocardia bacteria. Reports indicate that the growth of Nocardia on culture media, such as blood agar, chocolate agar, buffered charcoal-yeast extract (BCYE) agar, and sabouraud agar, can take from 1 to 2 weeks1, 9. Additionally, the MALDI–TOF MS method should be used for bacterial identification in suspected cases of pulmonary nocardiosis due to its ability to accurately identify the majority of commonly encountered Nocardia species1, 9.
Non-immunocompromised patients account for approximately one-third of nocardiosis cases1. The definition of immunodeficiency differs among studies; however, our report utilized the definition of immunodeficiency in the Treatment of Community-Acquired Pneumonia in Immunocompromised Adults guidelines of the American College of Chest Physicians, issued in 202013. Although all three patients in our report were not immunocompromised, they shared the common feature of chronic lung disease, particularly COPD, with one case prescribed inhaled corticosteroids. Many studies have linked chronic lung conditions, such as cystic fibrosis, bronchiectasis, chronic bronchitis, asthma, sarcoidosis, and COPD, to pulmonary nocardiosis. These conditions may account for 21.6% to 39.7% of nocardiosis patients7, 8, 14, 15, 16. Furthermore, pulmonary nocardiosis presenting as an exacerbation of chronic pulmonary disease has been reported17. Thus, chronic lung disease may be a risk factor for Nocardia infection. Specifically, case series and reports have indicated that COPD is commonly considered an isolated risk factor for pulmonary nocardiosis, even in immunocompetent patients18. Catellana et al. described pulmonary nocardiosis in an immunocompetent patient with COPD who had never undergone systemic or inhaled steroid therapy and did not have a respiratory failure or comorbidities leading to immunodepression18.
TMP–SMX is the cornerstone treatment for nocardiosis, either as monotherapy or as part of an antibiotic combination regimen. Although no consensus exists on managing nocardiosis, several regimens have been proposed1, 9, 18. In the first clinical case presented in our report, we utilized TMP–SMX monotherapy for non-severe pulmonary nocardiosis for six months and achieved favorable outcomes. We initially administered intravenous antibiotics for two weeks to the second patient, who was diagnosed with isolated pulmonary nocardiosis with respiratory failure. Subsequently, we switched to oral TMP–SMX for 6 months, resulting in positive outcomes. As most strains of Nocardia remain susceptible to TMP–SMX, it is a viable option for empiric treatment in resource-limited healthcare facilities19.
Conclusions
While Nocardia is an uncommon etiology of community-acquired pneumonia, patients with subacute symptoms, cavitary lesions on imaging, and underlying chronic lung disease should raise suspicion for Nocardia infection. Trimethoprim–sulfamethoxazole can be considered an empirical therapy for non-severe cases of pulmonary nocardiosis.
Abbreviations
CAP: Community-acquired pneumonia, COPD: Chronic obstructive pulmonary diseaseHIV: Human immunodeficiency virus, CRP: C-reactive protein, WBC: White blood cell, CT: Computed tomography, AFB: Acid-fast bacillus, PCR: Polymerase chain reaction, BALF: Bronchoalveolar lavage fluid, MALDI-TOF: Matrix assisted laser desorption ionization-time of flight, TMP-SMX: trimethoprim-sulfamethoxazole.
Acknowledgments
None.
Author’s contributions
NDK, LTV, NHL, TNN: Conceptualization, Methodology, Writing original draft preparation; NDK, TNN: Visualization, Methodology, Software; LTV, NHL: Data curation; NDK, LTV, NHL, TNN: Validation, Investigation; LTV: Supervision. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Written informed consent was obtained from the patient for publication of this Case series and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Margalit
I.,
Lebeaux
D.,
Tishler
O.,
Goldberg
E.,
Bishara
J.,
Yahav
D.,
How do I manage nocardiosis?. Clinical Microbiology and Infection.
2021;
27
(4)
:
550-8
.
View Article Google Scholar -
McGuinness
S.L.,
Whiting
S.E.,
Baird
R.,
Currie
B.J.,
Ralph
A.P.,
Anstey
N.M.,
Nocardiosis in the Tropical Northern Territory of Australia, 1997-2014. Open Forum Infectious Diseases.
2016;
3
(4)
.
View Article Google Scholar -
Ott
S.R.,
Meier
N.,
Kolditz
M.,
Bauer
T.T.,
Rohde
G.,
Presterl
E.,
Group
OPINION Study,
Pulmonary nocardiosis in Western Europe-Clinical evaluation of 43 patients and population-based estimates of hospitalization rates. International Journal of Infectious Diseases.
2019;
81
:
140-8
.
View Article Google Scholar -
Chen
J.,
Zhou
H.,
Xu
P.,
Zhang
P.,
Ma
S.,
Zhou
J.,
Clinical and radiographic characteristics of pulmonary nocardiosis: clues to earlier diagnosis. PLoS One.
2014;
9
(3)
:
e90724
.
View Article Google Scholar -
Paige
E.K.,
Spelman
D.,
Nocardiosis: 7-year experience at an Australian tertiary hospital. Internal Medicine Journal.
2019;
49
(3)
:
373-9
.
View Article Google Scholar -
Hemmersbach-Miller
M.,
Stout
J.E.,
Woodworth
M.H.,
Cox
G.M.,
Saullo
J.L.,
Nocardia infections in the transplanted host. Transplant Infectious Disease.
2018;
20
(4)
:
e12902
.
View Article Google Scholar -
Tomás
R. Martínez,
Villanueva
R. Menéndez,
Calzada
S. Reyes,
Durantez
M. Santos,
Tarazona
J.M. Vallés,
Alapont
M. Modesto,
Pulmonary nocardiosis: risk factors and outcomes. Respirology (Carlton, Vic.).
2007;
12
(3)
:
394-400
.
View Article Google Scholar -
Brown-Elliott
B.A.,
Brown
J.M.,
Conville
P.S.,
Wallace
R.J.,
Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clinical Microbiology Reviews.
2006;
19
(2)
:
259-82
.
View Article Google Scholar -
Restrepo
A.,
Clark
N.M.,
Transplantation
undefined Infectious Diseases Community of Practice of the American Society of,
Nocardia infections in solid organ transplantation: Guidelines from the Infectious Diseases Community of Practice of the American Society of Transplantation. Clinical Transplantation.
2019;
33
(9)
:
e13509
.
View Article Google Scholar -
Martínez
R.,
Reyes
S.,
Menéndez
R.,
Pulmonary nocardiosis: risk factors, clinical features, diagnosis and prognosis. Current Opinion in Pulmonary Medicine.
2008;
14
(3)
:
219-27
.
View Article Google Scholar -
Blackmon
K.N.,
Ravenel
J.G.,
Gomez
J.M.,
Ciolino
J.,
Wray
D.W.,
Pulmonary nocardiosis: computed tomography features at diagnosis. Journal of Thoracic Imaging.
2011;
26
(3)
:
224-9
.
View Article Google Scholar -
Liu
B.,
Zhang
Y.,
Gong
J.,
Jiang
S.,
Huang
Y.,
Wang
L.,
CT findings of pulmonary nocardiosis: a report of 9 cases. Journal of Thoracic Disease.
2017;
9
(11)
:
4785-90
.
View Article Google Scholar -
Ramirez
J.A.,
Musher
D.M.,
Evans
S.E.,
Cruz
C. Dela,
Crothers
K.A.,
Hage
C.A.,
Treatment of Community-Acquired Pneumonia in Immunocompromised Adults: A Consensus Statement Regarding Initial Strategies. Chest.
2020;
158
(5)
:
1896-911
.
View Article Google Scholar -
Woodworth
M.H.,
Saullo
J.L.,
Lantos
P.M.,
Cox
G.M.,
Stout
J.E.,
Increasing Nocardia Incidence Associated with Bronchiectasis at a Tertiary Care Center. Annals of the American Thoracic Society.
2017;
14
(3)
:
347-54
.
View Article Google Scholar -
Rouzaud
C.,
Rodriguez-Nava
V.,
Catherinot
E.,
Mécha\"\i
F.,
Bergeron
E.,
Farfour
E.,
Clinical Assessment of a Nocardia PCR-Based Assay for Diagnosis of Nocardiosis. Journal of Clinical Microbiology.
2018;
56
(6)
:
e00002-18
.
View Article Google Scholar -
Minero
M.V.,
Marín
M.,
Cercenado
E.,
Rabadán
P.M.,
Bouza
E.,
Muñoz
P.,
Nocardiosis at the turn of the century. Medicine.
2009;
88
(4)
:
250-61
.
View Article Google Scholar -
Kancherla
R.,
Ramanathan
R.P.,
Appalaraju
B.,
Rajagopala
S.,
Pulmonary Nocardiosis Presenting as Exacerbation of Chronic Pulmonary Disease. Indian journal of critical care medicine.
2019;
23
(10)
:
467-74
.
View Article PubMed Google Scholar -
Castellana
G.,
Grimaldi
A.,
Castellana
M.,
Farina
C.,
Castellana
G.,
Pulmonary nocardiosis in Chronic Obstructive Pulmonary Disease: A new clinical challenge. Respiratory Medicine Case Reports.
2016;
11
(18)
:
14-21
.
View Article PubMed Google Scholar -
Lafont
E.,
Conan
P.L.,
Rodriguez-Nava
V.,
Lebeaux
D.,
Invasive Nocardiosis: Disease Presentation, Diagnosis and Treatment - Old Questions, New Answers? . Infection and drug resistance.
2020;
13
:
4601-13
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 7 (2023)
Page No.: 5763-5770
Published on: 2023-07-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4709 times
- PDF downloaded - 1200 times
- XML downloaded - 157 times
 Biomedpress
Biomedpress







