Abstract
Introduction: Neuropathic pain is one of the main problems that succeeds a lesion or disease of the somatosensory system. In this study, the effect of exercise on oxidative stress after neuropathic pain due to sciatic nerve injury in male and female rats was evaluated.
Methods: For this study, 70 adult wistar rats (35 males and 35 females) weighing 180 ? 220 grams were divided into single-sex intact, sham, exercised sham, neuropathy, and exercised neuropathy groups, with 7 rats in each group. To induce neuropathy, chronic constriction injury (CCI) of the sciatic nerve was used. The exercise program included 4 weeks of swimming and medium-intensity. Von-Frey filament and plantar test devices were used to evaluate neuropathic pain. Malondialdehyde (MDA) and the ferric-reducing ability of plasma (FRAP) were determined using a spectrophotometer.
Results: Our results showed that nerve damage significantly reduced the response threshold to mechanical and thermal stimulation in both sexes, and continuous exercise significantly improved neuropathic pain in both sexes. In addition, nerve injury did not significantly generate oxidative stress in male or female rats. Meanwhile, exercise significantly reduced MDA levels and increased FRAP levels in neuropathic male rats but it did not affect oxidative stress parameters in female neuropathic rats.
Conclusions: Long-term exercise reduces neuropathic pain. Swimming exercise significantly modified MDA and FRAP levels in neuropathic male rats but not in female rats. Sex hormones appear to play different roles in the oxidative stress response.
Introduction
Neuropathic pain is a chronic condition that develops after a lesion or disease of the somatosensory system1. Based on whether the lesion is in the peripheral or central nervous system, neuropathic pain is categorized as peripheral or central. Neuropathic pain is characterized by sensory disturbances, including allodynia and hyperalgesia, which can be spontaneous or evoked2. Various mechanisms driving neuropathic pain have been suggested; however, oxidative stress is also prominently involved in the pathogenesis of neuropathic pain3. Despite various pharmacological treatments, neuropathic pain remains a major problem in medicine. The extent of the involved mechanisms and change over time present challenges in the treatment of neuropathic pain4. Given these difficulties, using non-pharmacological methods as adjunct therapies could be useful.
Of the non-pharmacological approaches to managing neuropathic pain, exercise is of particular importance. The beneficial effects of exercise to treat disease and support health have been emphasized. Among the mechanisms that have been suggested to explain the positive effects of exercise, oxidative stress suppression is prominent5. Oxidative stress describes the inability of the antioxidant defense system to scavenge reactive oxygen species (ROS)6.
Submaximal exercise reportedly reduces ROS production and improves antioxidant capacity7. Regular physical activity prevents oxidative-stress-induced injuries by stimulating endogenous antioxidant capacity5. The oxidative stress response is affected by sex, age, and lifestyle8.
The antioxidant system’s activity seems to be higher in females than in males9. Women suffer from oxidative stress injuries less than men10.
As oxidative stress is a potential contributor to various diseases, studying the effect of oxidative stress on any problem in either sex is helpful to clarify the available treatment pathways.
Some studies have shown that the response to oxidative stress differs by sex in some disorders; for example, female organisms are more resistant than males against the ischemic heart and ischemic brain disease that are associated with oxidative stress11.
Previously, we showed that swimming exercise significantly improved glutathione peroxidase levels in female rats with trigeminal neuropathic pain, but not in male rats12.
Little information about sex differences affecting the effect of exercise on the response to oxidative stress under illness is available. A review of past studies showed no reports on the difference in the oxidative stress response in male and female rats with sciatic neuropathy. As most research is performed on male animals of different ages and sex differences are less thoroughly studied, the potential effect of sex is important in designing research and treatment programs. Therefore, this study sought to investigate whether oxidative stress is involved in neuropathic pain, if the effect of aerobic exercise on neuropathic pain was mediated through the suppression of oxidative stress, and whether the response in both sexes was the same.
Materials and Methods
Animals
In this study, 70 adult wistar rats (35 male rats and 35 female rats) weighing 180 - 220 grams were used. The rats had free access to food and water and were housed at standard temperature (22 ± 2°C) and humidity (40 — 50%), and a 12 h light–dark cycle. The male and female rats were each divided into five groups: intact, sham, exercised sham, neuropathy, and exercised neuropathy, with seven rats in each group. Because of their inability to perform the tests, three male rats and four female rats were excluded from the study.
All experimental protocols of this study were approved by the research committees of the Semnan University of Medical Sciences (IR.SEMUMS.REC.1399.298) and were conducted according to the national health institute’s guidelines for the use and care of laboratory animals. All of the behavioral experiments were performed between 9 and 12 a.m to minimize diurnal variations.
Surgery to induce neuropathic pain
Neuropathic pain was induced via chronic constriction injury (CCI) as described by Bennett and Xie13. Each rat was anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg); then, the right thigh was shaved and a 2-cm incision was made at the sciatic nerve. The exposed sciatic nerve was ligated with four moveable 4/0 catgut chromic sutures. The ligations were placed 1 mm apart. Then, the incision was closed with 4/0 silk suture. Rats in the sham group underwent the same surgery without nerve ligation. The rats were placed in individual cages until they reached full consciousness and recovered.
Exercise protocol
Moderate-intensity swimming exercise was performed as described by Jose14. Animals were exercised for 4 weeks (5 days a week for 20 minutes daily). During swimming, a weight equal to 3% of the animal’s body weight was hung on its tail. A plastic cylinder (60 cm tall and 30 cm in diameter) filled with tap water (36 ± 1° C) was used. To accommodate the exercise program, the animals swam for 5, 10, and 20 minutes a day during the week before the experimental treatment began, and the animals that were unable to complete the program were excluded from the study (three male rats and four female rats).
Evaluation of pain-like behavior
Mechanical allodynia and thermal hyperalgesia (paw withdrawal threshold in response to mechanical and thermal stimulation), were evaluated via Von-Frey filament and plantar test devices, respectively, on the 30th day after surgery. To adapt to the experimental conditions, the animals were transferred to the lab 30 minutes before the experiments were performed.
Mechanical allodynia
Mechanical allodynia was determined with Von-Frey filaments according to the method described by Ren15. A Von-Frey filament is a polyethylene hair that, according to its diameter, exerts a certain amount of force on the surface to which it is applied. Von-Frey filaments are calibrated by diameter; a small-diameter filament is usually used initially. Stimulation was applied to the dorsal surface of the injured paw at the junction between the second and third toes. Each filament was applied five times at intervals of 10 seconds. If the paw withdrawal response was observed at three consecutive stimulations, that force was considered the response threshold; otherwise, the stimulation would be repeated with a larger-diameter filament. The cutoff force was 60 grams.
Thermal hyperalgesia
Thermal hyperalgesia (paw withdrawal latency to thermal stimulation) was detected by the method described by Bennett and Xie13 using the plantar test device. After the animal was placed in the device, the source of the infrared beam was focused on the plantar surface of the injured paw, irradiation began, and the paw withdrawal latency to irradiation was recorded automatically. The infrared beam was irradiated three times at 5-minute intervals. The average of three latency times was considered the paw withdrawal response. The cutoff time of response was 40 seconds.
Biochemical experiments
Biochemical tests included malondialdehyde (MDA) and ferric-reducing ability of plasma (FRAP) assays in blood serum. A spectrophotometer was used to read the wavelength results.
Blood sampling
To prepare the serum, blood samples were taken from rats’ hearts and centrifuged for 10 minutes at 2,000 RPM. The prepared serum was kept at -80° C until the biochemical tests were performed.
MDA measurement
Malondialdehyde was quantified via the method described by Mihara16 using thiobarbituric acid. MDA is one of the end products of lipid peroxidation and is a marker for oxidative stress. The reaction of MDA with thiobarbituric acid was evaluated spectrophotometrically, with the maximum absorption at 535 nm.
FRAP measurement
FRAP was measured via the method described by Benzei17. The test was used to determine the total antioxidant capacity of the plasma. This method is based on the ability of the sample to convert Fe3+ ions to Fe2+ ions. The output is a blue solution, which was measured spectrophotometrically, with the maximum light absorption at 593 nm.
Statistical analyses
One-way analyses of variance and two-way analyses of variance were used to analyze the data. Tukey’s and Bonferroni posthoc tests were used on these results, respectively. The data were analyzed with GraphPad Prism version 8.0 software (GraphPad, San Diego, CA, USA). All data are presented as mean ± SEM and p < 0.05 was considered statistically significant. The sample size in behavioral tests was 6–7 rats per group and 4–5 rats per group for biochemical experiments. Experiments were performed according to the following timeline.
Results
In this study, we evaluated the effect of swimming exercise on oxidative stress following neuropathic pain induced via CCI in male and female rats. The results are presented as behavioral and biochemical sections.
Behavioral results
Effect of swimming exercise on mechanical allodynia induced via CCI in male and female rats
The paw withdrawal response to mechanical stimulation significantly increased (p < 0.01) in male neuropathic rats compared to the sham group (Figure 2A). Further, CCI increased (p < 0.05) the paw withdrawal response to mechanical stimulation in female neuropathic rats compared to the sham group (Figure 2B). Four weeks of swimming exercise significantly (p < 0.05) increased the paw withdrawal threshold (decreased withdrawal response) in male and female neuropathic rats (Figure 2A, B). Furthermore, our results do not show a significant difference between male and female rats’ paw withdrawal threshold in response to mechanical stimulation (Figure 2C).
Effect of swimming exercise on thermal hyperalgesia induced via CCI in male and female rats
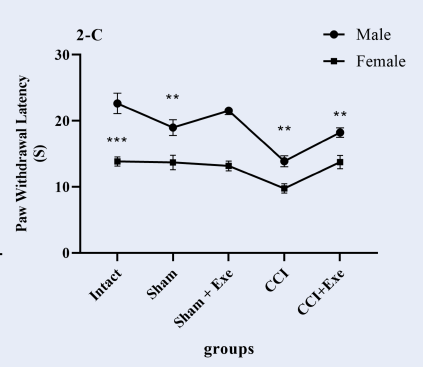
Paw withdrawal latency to thermal stimulation significantly decreased (p < 0.01) in male neuropathic rats compared to the sham group (Figure 3A). CCI also decreased (p < 0.05) paw withdrawal latency to thermal stimulation compared to the sham group in female neuropathic rats (Figure 3B). Four weeks of swimming exercise significantly (p < 0.05) increased paw withdrawal latency (decreased withdrawal response) in exercised neuropathic pain male and female rats (Figure 3A, B). However, we found that female rats’ paw withdrawal latency in response to thermal stimulation was significantly lower than that of male rats (Figure 3C), indicating that thermal hyperalgesia in female neuropathic rats is more intense than in male neuropathic rats.
Biochemical Results
MDA assay
In this study, MDA level was assayed in blood serum. Our data showed that the MDA levels in male and female neuropathic rats were not significantly different from those of the sham groups (Figure 4A, B), although they did increase in male neuropathic rats. On the other hand, exercise significantly (p < 0.01) decreased the MDA level in male neuropathic rats compared to the neuropathy group (Figure 4A). A comparison of male and female rats’ MDA levels showed that exercise led to a significant difference between them; the MDA level was significantly (p < 0.05) lower in exercised male neuropathic rats than in female ones (Figure 4 C).
FRAP assay
FRAP as an index of the total antioxidant capacity of blood plasma was measured in the serum. We observed that the FRAP level was not significantly different in male neuropathic rats compared to sham rats (Figure 5A), and exercise significantly (p < 0.05) increased FRAP toward the level of the sham rats. However, the FRAP levels in female rats were not significantly different between groups (Figure 5B). A comparison of FRAP levels between male and female rats showed no significant difference (Figure 5C).
Discussion
In this study, we showed that swimming exercise improves neuropathic pain in both sexes and operates as an antioxidant in male rats.
We showed that CCI decreased the paw withdrawal threshold and paw withdrawal latency in both sexes compared to the respective sham groups. Consistent with our results, Cardenas et al. reported in 2021 that cuff compression injury to the sciatic nerve led to mechanical allodynia and thermal hyperalgesia in male and female mice18. Further, Dominguez et al. reported that sciatic injury led to mechanical allodynia and thermal hyperalgesia in rats of both sexes19.
Most of the available information is the result of research on male animals, and sex and gender differences are less frequently considered20. Various physiological differences have been identified between male and female animals, including nervous responses, cardiovascular responses, respiratory responses, and hormones21. In addition, sex and gender are known to play a role in the pathology of chronic pain22. In this study, we observed that thermal hyperalgesia following CCI was significantly greater in female rats than in males. Similarly, LaCroix-Fralish et al. showed that when mechanical allodynia and thermal hyperalgesia are measured, female rats are more sensitive than ovariectomized rats and male rats23. Further, Meyer et al. reported that females are more sensitive than males to thermal stimulation after polyneuropathy24. Boullon et al. showed that the response threshold to skin irritation caused by acetone in female neuropathic rats is significantly lower than that of male neuropathic rats25. Biological factors play an important role in the different responses of the two sexes to painful stimuli. Estrogen receptors occur in different central and peripheral regions associated with pain26. Estrogen also increases pain sensitivity by stimulating the expression of NMDAR1 (N-methyl-D-aspartate acid receptor in the spinal dorsal horn ganglion, reducing the response threshold to noxious stimuli in females27. Moreover, brain imaging studies have shown that the activity of pain-inhibitory regions of the brain (the rostral ventrolateral medulla) is reduced in women who take contraceptives28.
Although various medicinal methods have been developed to stop neuropathic pain, not only have none of them been completely effective but they exert many side effects on patients. Given the many adverse effects of pharmacological medication, adjuvant non-pharmacologic therapies play a prominent role in patients’ pain management and reduced drug consumption.
In this study, we used swimming exercise as a non-pharmacological approach to improve CCI-induced neuropathic pain. Our results showed that exercise reduces mechanical allodynia and thermal hyperalgesia in both sexes. Several studies have investigated the hypoalgesic effect of exercise and reported that exercise improves sensory and motor performance after nerve injury29. Furthermore, we have previously shown that swimming exercise reduced neuropathic pain after infraorbital nerve injury in both sexes12. Similarly, Sumizono et al. reported the hypoalgesic effect of treadmill exercise in rats with injured sciatic nerves30.
Evidence has suggested the role of oxidative stress in neuropathic pain30, 31. ROS production after nerve injury reportedly leads to oxidative stress and endoneurial lipid peroxidation32, 33. In this study, CCI did not increase the MDA level in male or female neuropathic rats compared to the equivalent sham groups. Tang et al. reported similar results, indicating that ischemic sciatic nerve lesions cause no change in MDA levels in male and female mice34. Conversely, Yuceli et al. reported that MDA levels noticeably increased in male rats after sciatic nerve ischemia via femoral artery clamping35. Furthermore, contrary to our results, Etienne et al. reported increased MDA levels in male and female diabetic neuropathy patients36 but found no significant difference between the sexes. Oxidative stress parameters change in response to physical activity in a time-dependent manner37, so the time of measurement will affect the determined value. The inconsistency between our results and other mentioned studies may be due to either a difference in the evaluated sample (e.g., serum in our study, but sciatic tissue in others), a difference in the time of measuring malonaldehyde (e.g., in our study, one month after the intervention, but in Yuceli et al., one day after the intervention), a difference in the type of intervention (e.g., in our study, pressure injury on the nerve, but in other studies, ischemia reperfusion), or a difference in the study subject (rats in our experiment, but humans in Etienne et al.).
According to our results, exercise significantly (p < 0.01) reduced MDA levels in the male neuropathic rats but not in the female neuropathic rats. Jiankang38 and Shirvani39 separately showed that physical training decreases MDA levels compared to a control group.
Physical training reportedly increases parasympathetic nervous system activity40. Increased parasympathetic tone has an anti-inflammatory effect by suppressing cytokine release41. In this study, financial limitations prevented us from evaluating inflammatory mediators such as cytokines, but in a previous study42, we showed the anti-inflammatory effect of exercise in CCI-treated male rats. As pain is one of the signs of inflammation, the observed hypoalgesia in this study may be due to increased parasympathetic activity and the resulting inflammation suppression. However, a close and direct relationship between inflammation and oxidative stress also exists43; therefore, the reduction of inflammatory factors through the attenuation of oxidative stress may reduce neuropathic pain.
We observed that the FRAP levels of neuropathic rats of both sexes were not significantly different from the sham groups. In addition, the FRAP levels of male neuropathic rats were not significantly different from those of female neuropathic rats. Heidari et al. observed that FRAP levels were significantly lower in female diabetic neuropathic patients compared to female diabetic patients without neuropathic pain44. Furthermore, Etienne et al. reported that FRAP level was significantly lower in male and female patients compared to their control groups, but there was no significant sex difference36. The difference between our results and those of the researchers mentioned above may reflect aspects such as the time of FRAP measurement or the examined subjects. In this study, we examined the serum level of FRAP at 4 weeks post-surgery, when the level of this parameter may have returned to control levels, as previously we showed that the FRAP level was significantly reduced 3 weeks after sciatic nerve CCI in male rats42. In addition, the difference between our results and Etienne et al.’s and Heidari et al.’s may be due to the difference in the examined species. Importantly, as in malondialdehyde, the total antioxidant capacity may also change over time.
Our result showed that exercise led to a significant increase in the FRAP levels of male neuropathic rats but not female neuropathic rats. These results align with Rytz et al., who showed that aerobic exercise prominently increased FRAP levels in men with metabolic syndrome but not in women with the same problem45. Jolien Hendrix also reported that repeated exercise increases total antioxidant capacity and exerts hypoalgesia in male rats46. Previously, we showed that 3 weeks of treadmill exercise increased FRAP levels in CCI-treated male rats42. Human and animal studies show that sex hormones, especially estrogen, exert antioxidant and neuroprotective effects, and estrogen’s role is more pronounced than testosterone’s47.
In addition to reproduction, estrogen plays a role in immune system performance through receptors on immune cells, such as lymphocytes, monocytes, and macrophages48. Estrogen prevents cytokine release by inhibiting microglia and astrocytes49. Meanwhile, as mentioned earlier, there is a close and direct relationship between inflammation and oxidative stress50, so cytokines can stimulate oxidative stress and vice versa51. Therefore, estrogen may prevent oxidative stress by inhibiting the release of inflammatory agents. The levels of vitamin E and glutathione peroxidase enzyme activity are high in female rats9, and these levels may have prevented oxidative stress and, therefore, limited changes in malondialdehyde and FRAP levels. Confirming this possibility, other studies have shown that the mitochondrial DNA damage caused by oxidative stress products is significantly reduced in female rats compared to male rats52. Several studies have indicated the direct protective effect of estrogen against oxidative stress damage in the heart and liver53, 54. In vitro reports have shown that the protective effect of estrogen and progesterone may be mediated through antioxidant properties or genomic effects55. According to the mentioned studies, which have shown that the amount and activity of antioxidant enzymes are higher in female than in male organisms, the stronger antioxidant potential in females may prevent oxidative stress or neutralize it in the initial stages. Therefore, the protective and antioxidant properties of estrogen may have prevented the changes in malondialdehyde and FRAP levels that were observed in male rats during this study.
Reportedly, in castrated male rats, MDA and the antioxidant power of plasma significantly increased and decreased, respectively, and either continuous exercise or testosterone therapy significantly reversed these trends toward control levels56. Moderate-intensity exercise has been shown to not only increase testosterone levels57 but also potentiate the effects of testosterone58. According to the literature, it is possible that in our study, exercise increased the level of testosterone in male neuropathic rats, thereby decreasing the level of malondialdehyde and increasing the FRAP level. This mechanism should be studied further to increase the reliability of the conclusions. Although financial constraints prevented us from using sex hormone antagonists or measuring testosterone levels in the rat sample, doing so would permit us to ascertain whether the reducing oxidative stress response effects of exercise could be attributed to sex hormones. This is one of the limitations of this study.
Conclusion
In this study, we did not detect oxidative stress in neuropathic rats of either sex but affirmed that exercise improves oxidative stress parameters in male rats. There may be an association between male sex hormones and oxidative stress suppression by exercise that does not manifest in females. The potential antioxidative properties of female sex hormones do not appear to be affected by exercise.
Abbreviations
CCI: Chronic Constriction Injury, FRAP: Ferric-reducing ability of plasma, MDA: Malondialdehyde, NMDAR: N-methyl-D-aspartate acid receptor, ROS: Reactive Oxygen Species, RPM: Revolutions Per Minute
Acknowledgments
The authors of this article express their gratitude to Mr. Hossein Ali Safakhah for technical assistances. The authors wish to thank the Deputy of Research and Technology of Semnan University of Medical Sciences, Semnan, Iran for offering the grants (grant number: IR.SEMUMS.REC.1398.137) for present study.
Author’s contributions
Ali Ghanbari: Conceptualization, Methodology, Supervision, Reviewing and Editing, Sahar Ghasemi: Methodology, Writing- Original draft preparation, Data curation, Ahmad Reza Bandegi: Software, Visualization, Investigation, Reviewing, Ali Rashidy-pour: Software, Validation, Reviewing. All authors have accepted responsibility for the entire content of this manuscript and approved its submission. All authors read and approved the final manuscript.
Funding
This study was supoorted by Deputy of Research and Technology of Semnan University of Medical Sciences, Semnan, Iran (permit number: IR.SEMUMS.REC.1398.137).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Ethics approval
Study involving animals complied with all relevant national regulations and institutional policies (permit number: IR.SEMUMS.REC.1398.137) for the care and use of animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Haroutounian
S.,
Nikolajsen
L.,
Bendtsen
T.F.,
Finnerup
N.B.,
Kristensen
A.D.,
Hasselstr∅m
J.B.,
Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain.
2014;
155
(7)
:
1272-9
.
View Article PubMed Google Scholar -
Nazemi
S.,
Manaheji
H.,
Zaringhalam
J.,
Sadeghi
M.,
Haghparast
A.,
Post-injury repeated administrations of minocycline improve the antinociceptive effect of morphine in chronic constriction injury model of neuropathic pain in rat. Pharmacology, Biochemistry, and Behavior.
2012;
102
(4)
:
520-5
.
View Article PubMed Google Scholar -
Mallet
M.L.,
Hadjivassiliou
M.,
Sarrigiannis
P.G.,
Zis
P.,
The role of oxidative stress in peripheral neuropathy. Journal of Molecular Neuroscience.
2020;
70
(7)
:
1009-17
.
View Article PubMed Google Scholar -
Woolf
C.J.,
Mannion
R.J.,
Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet.
1999;
353
(9168)
:
1959-64
.
View Article PubMed Google Scholar -
Gökbel
H. ,
Acute exercise induced oxidative stress and antioxidant changes. European Journal of General Medicine.
2006;
3
(3)
:
126-31
.
-
Marotti
T.,
Sobočanec
S.,
Mačak-Šafranko
Z.,
Šarić
A.,
Kušić
B.,
Balog
T.,
Sensitivity to oxidative stress: sex matters. Medicinske znanosti.
2010;
2010
(35)
:
59-68
.
View Article Google Scholar -
Fatouros
I.G.,
Jamurtas
A.Z.,
Villiotou
V.,
Pouliopoulou
S.,
Fotinakis
P.,
Taxildaris
K.,
Oxidative stress responses in older men during endurance training and detraining. Medicine and Science in Sports and Exercise.
2004;
36
(12)
:
2065-72
.
View Article PubMed Google Scholar -
Vezzoli
A.,
Pugliese
L.,
Marzorati
M.,
Serpiello
F.R.,
La Torre
A.,
Porcelli
S.,
Time-course changes of oxidative stress response to high-intensity discontinuous training versus moderate-intensity continuous training in masters runners. PLoS One.
2014;
9
(1)
:
e87506
.
View Article PubMed Google Scholar -
Yamamoto
T.,
Ohkuwa
T.,
Itoh
H.,
Sato
Y.,
Naoi
M.,
Effect of gender differences and voluntary exercise on antioxidant capacity in rats. Comparative Biochemistry and Physiology. Toxicology & Pharmacology : CBP.
2002;
132
(4)
:
437-44
.
View Article PubMed Google Scholar -
Català-Niell
A.,
Estrany
M.E.,
Proenza
A.M.,
Gianotti
M.,
Lladó
I.,
Skeletal muscle and liver oxidative metabolism in response to a voluntary isocaloric intake of a high fat diet in male and female rats. Cellular Physiology and Biochemistry.
2008;
22
(1-4)
:
327-36
.
View Article PubMed Google Scholar -
Crea
F.,
Battipaglia
I.,
Andreotti
F.,
Sex differences in mechanisms, presentation and management of ischaemic heart disease. Atherosclerosis.
2015;
241
(1)
:
157-68
.
View Article PubMed Google Scholar -
Rostami
Z.,
Ghasemi
S.,
Farzadmanesh
H.,
Safari
M.,
Ghanbari
A.,
Sex difference in trigeminal neuropathic pain response to exercise: role of oxidative stress. Pain Research and Management.
2020;
2020
:
3939757
.
View Article Google Scholar -
Bennett
G.J.,
Xie
Y.K.,
A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain.
1988;
33
(1)
:
87-107
.
View Article PubMed Google Scholar -
Cechella
J.L.,
Leite
M.R.,
Dobrachinski
F.,
da Rocha
J.T.,
Carvalho
N.R.,
Duarte
M.M.,
Moderate swimming exercise and caffeine supplementation reduce the levels of inflammatory cytokines without causing oxidative stress in tissues of middle-aged rats. Amino Acids.
2014;
46
(5)
:
1187-95
.
View Article PubMed Google Scholar -
Ren
K.,
An improved method for assessing mechanical allodynia in the rat. Physiology {&}amp; Behavior.
1999;
67
(5)
:
711-6
.
View Article PubMed Google Scholar -
Mihara
M.,
Uchiyama
M.,
Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry.
1978;
86
(1)
:
271-8
.
View Article PubMed Google Scholar -
Benzie
I.F.,
Strain
J.J.,
The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Analytical Biochemistry.
1996;
239
(1)
:
70-6
.
View Article PubMed Google Scholar -
Cardenas
A.,
Caniglia
J.,
Keljalic
D.,
Dimitrov
E.,
Sex differences in the development of anxiodepressive-like behavior of mice subjected to sciatic nerve cuffing. Pain.
2020;
161
(8)
:
1861-71
.
View Article PubMed Google Scholar -
Dominguez
C.A.,
Kouya
P.F.,
Wu
W.P.,
Hao
J.X.,
Xu
X.J.,
Wiesenfeld-Hallin
Z.,
Sex differences in the development of localized and spread mechanical hypersensitivity in rats after injury to the infraorbital or sciatic nerves to create a model for neuropathic pain. Gender Medicine.
2009;
6
:
225-34
.
View Article PubMed Google Scholar -
Pang
L.,
Lian
X.,
Liu
H.,
Zhang
Y.,
Li
Q.,
Cai
Y.,
Understanding diabetic neuropathy: focus on oxidative stress. Oxidative Medicine and Cellular Longevity.
2020;
2020
:
1-3
.
View Article Google Scholar -
Ansdell
P.,
Thomas
K.,
Hicks
K.M.,
Hunter
S.K.,
Howatson
G.,
Goodall
S.,
Physiological sex differences affect the integrative response to exercise: acute and chronic implications. Experimental Physiology.
2020;
105
(12)
:
2007-21
.
View Article PubMed Google Scholar -
Abu-Arefeh
I.,
Russell
G.,
Prevalence of headache and migraine in schoolchildren. BMJ (Clinical Research Ed.).
1994;
309
(6957)
:
765-9
.
View Article PubMed Google Scholar -
LaCroix-Fralish
M.L.,
Tawfik
V.L.,
DeLeo
J.A.,
The organizational and activational effects of sex hormones on tactile and thermal hypersensitivity following lumbar nerve root injury in male and female rats. Pain.
2005;
114
(1-2)
:
71-80
.
View Article PubMed Google Scholar -
Meyer-Frie\ssem
C.H.,
Attal
N.,
Baron
R.,
Bouhassira
D.,
Finnerup
N.B.,
Freynhagen
R.,
Pain thresholds and intensities of CRPS type I and neuropathic pain in respect to sex. European Journal of Pain (London, England).
2020;
24
(6)
:
1058-71
.
View Article PubMed Google Scholar -
Boullon
L.,
Finn
D.P.,
Llorente-Berzal
Á.,
Sex differences in a rat model of peripheral neuropathic pain and associated levels of endogenous cannabinoid ligands. Frontiers in Pain Research (Lausanne, Switzerland).
2021;
2
:
673638
.
View Article PubMed Google Scholar -
Ray
S.K.,
Samntaray
S.,
Banik
N.L.,
Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regeneration Research.
2016;
11
(9)
:
1418-9
.
View Article PubMed Google Scholar -
Deng
C.,
Gu
Y.J.,
Zhang
H.,
Zhang
J.,
Estrogen affects neuropathic pain through upregulating N-methyl-D-aspartate acid receptor 1 expression in the dorsal root ganglion of rats. Neural Regeneration Research.
2017;
12
(3)
:
464-9
.
View Article PubMed Google Scholar -
Craft
R.M.,
Modulation of pain by estrogens. Pain.
2007;
132
:
3-12
.
View Article PubMed Google Scholar -
Pieh
C.,
Altmeppen
J.,
Neumeier
S.,
Loew
T.,
Angerer
M.,
Lahmann
C.,
Gender differences in outcomes of a multimodal pain management program. Pain.
2012;
153
(1)
:
197-202
.
View Article PubMed Google Scholar -
Sumizono
M.,
Sakakima
H.,
Otsuka
S.,
Terashi
T.,
Nakanishi
K.,
Ueda
K.,
The effect of exercise frequency on neuropathic pain and pain-related cellular reactions in the spinal cord and midbrain in a rat sciatic nerve injury model. Journal of Pain Research.
2018;
11
:
281-91
.
View Article PubMed Google Scholar -
Kim
H.K.,
Park
S.K.,
Zhou
J.L.,
Taglialatela
G.,
Chung
K.,
Coggeshall
R.E.,
Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain.
2004;
111
(1-2)
:
116-24
.
View Article PubMed Google Scholar -
Khalil
Z.,
Khodr
B.,
A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radical Biology & Medicine.
2001;
31
(4)
:
430-9
.
View Article PubMed Google Scholar -
Sayan
H.,
Ugurlu
B.,
Babül
A.,
Take
G.,
Erdogan
D.,
Effects of L-arginine and NG-nitro L-arginine methyl ester on lipid peroxide, superoxide dismutase and nitrate levels after experimental sciatic nerve ischemia-reperfusion in rats. The International Journal of Neuroscience.
2004;
114
(3)
:
349-64
.
View Article PubMed Google Scholar -
Dai
C.,
Tang
S.,
Biao
X.,
Xiao
X.,
Chen
C.,
Li
J.,
Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice. Molecular Biology Reports.
2019;
46
(2)
:
1963-72
.
View Article PubMed Google Scholar -
Yuceli
S.,
Yazici
G.N.,
Mammadov
R.,
Suleyman
H.,
Ozdogan
S.,
The Effect of Lutein on Ischemia-reperfusion-induced Vasculitic Neuropathic Pain and Neuropathy in Rats. In vivo.
2021;
35
(3)
:
1537-43
.
View Article Google Scholar -
Etienne
I.,
Magalhães
L.V.,
Cardoso
S.A.,
de Freitas
R.B.,
de Oliveira
G.P.,
Palotás
A.,
Oxidative stress markers in cognitively intact patients with diabetic neuropathy. Brain Research Bulletin.
2019;
150
:
196-200
.
View Article PubMed Google Scholar -
Michailidis
Y.,
Jamurtas
A.Z.,
Nikolaidis
M.G.,
Fatouros
I.G.,
Koutedakis
Y.,
Papassotiriou
I.,
Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Medicine and Science in Sports and Exercise.
2007;
39
(7)
:
1107-13
.
View Article PubMed Google Scholar -
Jiang
H.K.,
Miao
Y.,
Wang
Y.H.,
Zhao
M.,
Feng
Z.H.,
Yu
X.J.,
Aerobic interval training protects against myocardial infarction-induced oxidative injury by enhancing antioxidase system and mitochondrial biosynthesis. Clinical and Experimental Pharmacology & Physiology.
2014;
41
(3)
:
192-201
.
View Article PubMed Google Scholar -
Shirvani
H.,
Aslani
J.,
Fallah Mohammadi
Z.,
Arabzadeh
E.,
Short-term effect of low-, moderate-, and high-intensity exercise training on cerebral dopamine neurotrophic factor (CDNF) and oxidative stress biomarkers in brain male Wistar rats. Comparative Clinical Pathology.
2019;
28
(2)
:
369-76
.
View Article Google Scholar -
Hautala
A.J.,
Kiviniemi
A.M.,
Tulppo
M.P.,
Individual responses to aerobic exercise: the role of the autonomic nervous system. Neuroscience and Biobehavioral Reviews.
2009;
33
(2)
:
107-15
.
View Article PubMed Google Scholar -
Bonaz
B.,
Sinniger
V.,
Pellissier
S.,
Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. The Journal of Physiology.
2016;
594
(20)
:
5781-90
.
View Article PubMed Google Scholar -
Safakhah
H.A.,
Moradi Kor
N.,
Bazargani
A.,
Bandegi
A.R.,
Gholami Pourbadie
H.,
Khoshkholgh-Sima
B.,
Forced exercise attenuates neuropathic pain in chronic constriction injury of male rat: an investigation of oxidative stress and inflammation. Journal of Pain Research.
2017;
10
:
1457-66
.
View Article PubMed Google Scholar -
Jha
J.C.,
Ho
F.,
Dan
C.,
Jandeleit-Dahm
K.,
A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clinical Science (London, England).
2018;
132
(16)
:
1811-36
.
View Article PubMed Google Scholar -
Heidari
N.,
Sajedi
F.,
Mohammadi
Y.,
Mirjalili
M.,
Mehrpooya
M.,
Ameliorative effects of N-acetylcysteine as adjunct therapy on symptoms of painful diabetic neuropathy. Journal of Pain Research.
2019;
12
:
3147-59
.
View Article PubMed Google Scholar -
Rytz
C.L.,
Pialoux
V.,
Mura
M.,
Martin
A.,
Hogan
D.B.,
Hill
M.D.,
Impact of aerobic exercise, sex, and metabolic syndrome on markers of oxidative stress: results from the Brain in Motion study. Journal of Applied Physiology (Bethesda, Md.).
2020;
128
(4)
:
748-56
.
View Article PubMed Google Scholar -
Hendrix
J.,
Nijs
J.,
Ickmans
K.,
Godderis
L.,
Ghosh
M.,
Polli
A.,
The interplay between oxidative stress, exercise, and pain in health and disease: potential role of autonomic regulation and epigenetic mechanisms. Antioxidants.
2020;
9
(11)
:
1166
.
View Article PubMed Google Scholar -
Gatto
N.M.,
Deapen
D.,
Stoyanoff
S.,
Pinder
R.,
Narayan
S.,
Bordelon
Y.,
Lifetime exposure to estrogens and Parkinson's disease in California teachers. Parkinsonism & Related Disorders.
2014;
20
(11)
:
1149-56
.
View Article PubMed Google Scholar -
Danel
L.,
Souweine
G.,
Monier
J.C.,
Saez
S.,
Specific estrogen binding sites in human lymphoid cells and thymic cells. Journal of Steroid Biochemistry.
1983;
18
(5)
:
559-63
.
View Article PubMed Google Scholar -
Rosen
S.,
Ham
B.,
Mogil
J.S.,
Sex differences in neuroimmunity and pain. Journal of Neuroscience Research.
2017;
95
(1-2)
:
500-8
.
View Article PubMed Google Scholar -
McGarry
T.,
Biniecka
M.,
Veale
D.J.,
Fearon
U.,
Hypoxia, oxidative stress and inflammation. Free Radical Biology & Medicine.
2018;
125
:
15-24
.
View Article PubMed Google Scholar -
Pan
L.,
Yu
L.,
Wang
L.,
He
J.,
Sun
J.,
Wang
X.,
Inflammatory stimuli promote oxidative stress in pancreatic acinar cells via Toll-like receptor 4/nuclear factor-κB pathway. International Journal of Molecular Medicine.
2018;
42
(6)
:
3582-90
.
View Article PubMed Google Scholar -
Borrás
C.,
Sastre
J.,
García-Sala
D.,
Lloret
A.,
Pallardó
F.V.,
Viña
J.,
Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radical Biology & Medicine.
2003;
34
(5)
:
546-52
.
View Article PubMed Google Scholar -
Busserolles
J.,
Mazur
A.,
Gueux
E.,
Rock
E.,
Rayssiguier
Y.,
Metabolic syndrome in the rat: females are protected against the pro-oxidant effect of a high sucrose diet. Experimental Biology and Medicine (Maywood, N.J.).
2002;
227
(9)
:
837-42
.
View Article PubMed Google Scholar -
Persky
A.M.,
Green
P.S.,
Stubley
L.,
Howell
C.O.,
Zaulyanov
L.,
Brazeau
G.A.,
Protective effect of estrogens against oxidative damage to heart and skeletal muscle in vivo and in vitro. Proceedings of the Society for Experimental Biology and Medicine.
2000;
223
(1)
:
59-66
.
View Article PubMed Google Scholar -
Roof
R.L.,
Hall
E.D.,
Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. Journal of Neurotrauma.
2000;
17
(5)
:
367-88
.
View Article PubMed Google Scholar -
Chodari
L.,
Smailnejad
S.,
Fallahi
M.,
Khalaji
N.,
Ghorbanzadeh
V.,
Oxidative stress is markedly reduced by combined voluntary exercise and testosterone in the heart of diabetic rats. Acta Endocrinologica.
2019;
15
(2)
:
173-81
.
View Article PubMed Google Scholar -
Vaamonde
D.,
Da Silva-Grigoletto
M.E.,
García-Manso
J.M.,
Barrera
N.,
Vaamonde-Lemos
R.,
Vaamonde-Lemos
R.,
Physically active men show better semen parameters and hormone values than sedentary men. European Journal of Applied Physiology.
2012;
112
(9)
:
3267-73
.
View Article PubMed Google Scholar -
Fry
A.C.,
Lohnes
C.A.,
Acute testosterone and cortisol responses to high power resistance exercise. Fiziologiia Cheloveka.
2010;
36
(4)
:
102-6
.
PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 6 (2023)
Page No.: 5726-5734
Published on: 2023-06-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3672 times
- PDF downloaded - 1329 times
- XML downloaded - 132 times
 Biomedpress
Biomedpress







