Abstract
The transient receptor potential vanilloid 4 (TRPV4) channel is a member of the TRP vanilloid subfamily in the TRP superfamily of ion channels. This study aimed to provide an overview of the research progression on TRPV4 channels, from initial discovery to present, using bibliometric methods. TRPV4 channel-related articles published from 2000 onwards were retrieved from the Scopus database. Microsoft Excel and Harzing's Publish or Perish were used for the quantitative analysis of several characteristics of the retrieved articles. VOSviewer was used to construct networks based on the co-occurrence of author keywords. From 2000 onwards, 877 TRPV4 channel-related English language articles written were published and included in the final bibliometric analysis. The number of publications appeared to fluctuate over the years; however, the number is predicted to increase over time. The Journal of Biological Chemistry ranked top for publishing 46 papers. Our co-occurrence analysis of author keywords revealed four main clusters: ``Cardiovascular-related,'' ``Channelopathies,'' ``Tumorigenesis,'' and ``Smooth muscle regulation.'' Our study also highlighted four main research frontiers of TRPV4: glaucoma, mitochondria, inflammation, and cell signaling. This is the first study to examine and demonstrate the trends and outlook for TRPV4 channel research using bibliometrics.
Introduction
The mammalian transient receptor potential (TRP) superfamily is a diverse family of ion channels that is further subdivided into six subfamilies: TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystic), and TRPV (vanilloid)1. The TRPV channels have six members (TRPV1-6) and were named due to the activation of the first member by capsaicin, a vanilloid-like molecule1. The TRPV4 channel is a calcium-permeable nonselective cation channel ubiquitously expressed in a variety of tissues, including the brain, eyes, kidney, skin, gastrointestinal tract, and urinary bladder2. It is also polymodally activated by a wide range of stimuli, including physical (i.e., cell swelling, heat, and mechanical stimulation) and chemical stimuli (i.e., endocannabinoids, arachidonic acid, and 4alpha-phorbol esters)2, 3. Since its discovery as an osmosensitive calcium-permeable cation channel in 20004, TRPV4 has gained considerable attention in multidisciplinary research. Studies have assessed its physiological and pathophysiological significance5, pharmacological modulators6, 7, and therapeutic potential in a variety of human diseases8, 9.
Bibliometric analysis is a scientific method that enables the quantitative and qualitative analysis of massive bibliometric data to provide information on essential research constituents, including authors, countries, journals, and emerging trends10. Unlike other frequently used review methods, such as systematic reviews, which are confined to specific and limited aspects of a research question, bibliometric analysis provides an objective and comprehensive overview of the literature on a particular research area to illustrate overall research trends and reveal future directions. It can accommodate large datasets and is better suited for broad study scopes10, 11, 12. In recent years, bibliometric analysis has been used in various areas of research, including melatonin13, neurodegenerative diseases14, and ion channels15, 16. Although a recent bibliometric analysis has been conducted on TRP ion channels15, no prior bibliometric studies have been conducted specifically on TRPV4 channels, which belong to the TRPV subfamily of TRP channels. Moreover, unlike the previous bibliometric analysis on TRP channels, which utilized the Web of Science database15, we used the Scopus database and demonstrated the trends and future development in TRPV4-related research from 2000 to now.
Materials and Methods
The online literature search was performed on July 27th, 2022, using the Scopus database. The search query was determined after an exhaustive review of the literature for terms relevant to the research question. The National Center for Biotechnology Information’s Gene database was used to look for TRPV4 aliases (https://www.ncbi.nlm.nih.gov/gene/59341). Following this, [TS = trpv4 OR “transient receptor potential vanilloid 4 channel” OR “OTRPC4” OR “VROAC”] was used as the search strategy. The use of these search queries within article titles facilitated the retrieval of the maximum number of documents while minimizing the presence of extraneous outcomes. Only articles and reviews written in English and published from 2000 onwards were included in the analysis. Other document types, such as notes, errata, book chapters, editorial materials, conference papers, letters, short surveys, and non-English publications, were excluded. In total, 877 documents were included for further bibliometric analysis (Figure 1). Descriptive statistics were analyzed using Microsoft Excel, citation analysis was performed using Harzing’s Publish or Perish version 8.2.3944, and the VOSviewer version 1.6.18 software tool was used for network visualization of the author keyword co-occurrence map.
Results
The trends in global publications
A total of 877 articles met the search criteria from 2000 to the present and were retrieved from the Scopus database (Figure 1). Since the publication of the first paper on TRPV4 channels (initially referred to as TRP [transient receptor potential]-like channel protein, OTRPC4) in 20004, the number of publications has fluctuated over time, reaching a peak in 2020 (Figure 2). However, the overall publication output regarding TRPV4 research has been on an upward trend, and it is predicted that the number of pertinent articles in 2022 will continue to rise based on this trend.
| Source Title | TP | TC | Publisher | Cite Score (2021) | SJR (2021) | SNIP (2021) |
|---|---|---|---|---|---|---|
| Journal of Biological Chemistry | 46 | 5937 | Elsevier | 8.8 | 1.871 | 1.239 |
| Pflugers Archiv European Journal of Physiology | 25 | 913 | Springer Nature | 6.6 | 1.133 | 1.075 |
| PLoS One | 22 | 1024 | Public Library of Science | 5.6 | 0.852 | 1.368 |
| Proceedings of the National Academy of Sciences of the United States of America | 22 | 3281 | National Academy of Sciences | 18.1 | 4.184 | 3.063 |
| Scientific Reports | 20 | 628 | Springer Nature | 6.9 | 1.005 | 1.389 |
| British Journal of Pharmacology | 17 | 464 | Wiley-Blackwell | 13.6 | 1.993 | 1.871 |
| American Journal of Physiology - Lung Cellular and Molecular Physiology | 16 | 1076 | American Physiological Society | 8.8 | 1.639 | 1.271 |
| American Journal of Physiology - Renal Physiology | 15 | 645 | American Physiological Society | 6.6 | 1.224 | 1.026 |
| Biochemical and Biophysical Research Communications | 14 | 348 | Elsevier | 6.5 | 0.805 | 0.723 |
| International Journal of Molecular Sciences | 13 | 86 | Multidisciplinary Digital Publishing Institute (MDPI) | 6.9 | 1.176 | 1.401 |
Top 10 productive journals publishing articles on TRPV4 channels
The top 10 journals that published articles on TRPV4 channels are shown in Table 1. The Journal of Biological Chemistry (Cite Score = 8.8, 2021) had the highest number of publications, with 46 articles. The Pflugers Archiv European Journal of Physiology (Cite Score = 6.6, 2021) ranked second with 25 articles, followed by the PLoS One (Cite Score = 5.6, 2021) and the Proceedings of the National Academy of Sciences of the United States of America (Cite Score = 18.1, 2021), with 22 articles each.
Co-occurrence analysis of keywords
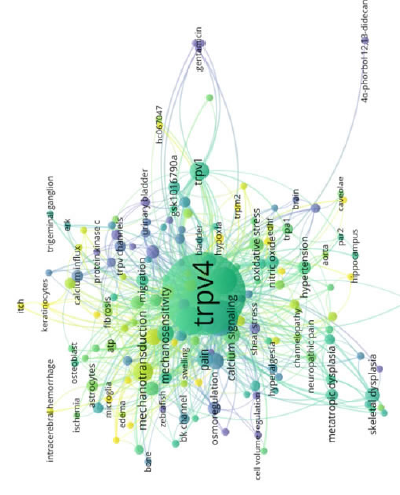
The author keywords of the 877 original articles and reviews were analyzed using VOSviewer. As shown in Fig. 3, 159 out of 1758 keywords met the threshold (defined as being used more than three times in all the articles and with a minimum cluster size of 21). Overall, 751 links were classified into four clusters: “Cardiovascular-related” (in red), “channelopathies” (in green), “tumorigenesis” (in blue), and “smooth muscle regulation” (in yellow). The “cardiovascular-related” cluster was the largest, with 59 items. Among the keywords related to this cluster were “calcium” (49 occurrences), “endothelium” (44 occurrences), “nitric oxide” (12 occurrences), “hypertension” (10 occurrences), and “vasodilation” (10 occurrences). For the “channelopathies” cluster, 46 items were identified with the primary keywords including “trpv4” (401 occurrences), “ion channels” (18 occurrences), “pain” (15 occurrences), “skeletal dysplasia” (13 occurrences), and “neuropathic pain” (6 occurrences). The “tumorigenesis” cluster comprised 31 items with primary keywords like “mechanosensitivity” (18 occurrences), “inflammation” (16 occurrences), “apoptosis” (10 occurrences), “proliferation” (9 occurrences), and “migration” (9 occurrences). For the “smooth muscle regulation” cluster, 23 items were identified, and the primary keywords included “trpv1” (20 occurrences), “vascular smooth muscle cells” (10 occurrences), “calcium influx” (8 occurrences), and “urothelium” (7 occurrences).
Research frontiers of TRPV4 channels
The research frontiers of TRPV4 channels were predicted based on the occurrences of the 159 keywords that met the threshold for the past three years (2019–2022), as analyzed by the VOSviewer software tool. Out of the 159 items, 30 were identified as the frontiers of TRPV4 research. Fifteen items were identified from Cluster 1, including “glaucoma” (10 occurrences; average publication year: 2020.40) and “angiogenesis” (9 occurrences; average publication year: 2019.33). For Cluster 2, two items were identified as research frontiers: “stroke” (3 occurrences; average publication year: 2021.33) and “mitochondria” (4 occurrences; average publication year: 2019.25). In Cluster 3, 12 items were identified: “inflammation” (16 occurrences; average publication year: 2019.25), “microglia” (4 occurrences; average publication year: 2020), “keratinocytes” (4 occurrences; average publication year: 2019.75), and “metastasis” (4 occurrences; average publication year: 2019). For Cluster 4, “cell signaling” (3 occurrences; average publication year: 2019) was the only keyword identified as a research frontier.
Discussion
This bibliometric analysis identified 877 Scopus-indexed articles related to TRPV4 channels published from 2000 to the present. Based on our data, the number of global publications on TRPV4 channels has fluctuated over this time, reaching a peak in 2020. A downward trend in global publication outputs was seen in 2021, most likely due to the COVID-19 pandemic, as this resulted in a significant realignment of priorities and research efforts, causing a decline in overall publishing rates and funding for other biomedical research areas unrelated to COVID-1918. Although there was a slight decrease in the number of publications in 2022, this was somewhat expected given that it was still within the update period when the literature search was conducted. The inclusion of the year 2022 was to allow for the analysis of the emerging trends in TRPV4 channel research. Taken together, based on the recent trends, we anticipated that the annual number of published documents on the TRPV4 channel would continue to rise in 2022, indicating that TRPV4 channel research is gaining greater attention. Similar publication trends were observed in studies of other channels15, 19, 20.
Concerning the active journals publishing TRPV4-related articles, the Journal of Biological Chemistry (a journal from the United States of America) was the leading journal, with 46 articles published and a total citation number of 5937, followed by Pflugers Archiv European Journal of Physiology, PLoS One and the Proceedings of the National Academy of Sciences of the United States of America. Interestingly, the majority of these journals have been listed among the top 10 journals for articles published on TRP channels15, indicating that these are some of the most favorable journals for publishing research on the TRP superfamily of cation channels, including TRPV4. These highly influential academic journals are expected to be among the major sources of future articles related to the TRPV4 channel.
Term clustering from our network analysis revealed four main clusters. The first and the biggest cluster, the “cardiovascular-related” cluster, had 59 items, indicating that it is currently the most popular area of research involving TRPV4 channels. The core keywords in this cluster were “calcium,” “endothelium,” “nitric oxide,” “hypertension,” and “vasodilation.” A growing number of studies have reported the physiological and pathological role of TRPV4 in the cardiovascular system21. For instance, using in vivo and in vitro study models, Zou et al. 22 demonstrated that TRPV4 activation contributes to pressure overload-induced cardiac hypertrophy and heart failure. Therefore, TRPV4 antagonism may confer a therapeutic advantage for this pathological condition.
The second cluster identified was “channelopathies”. This cluster contained 46 items, with the primary keywords being “trpv4,” “ion channels,” “pain,” “skeletal dysplasia,” and “neuropathic pain.” TRPV4 channelopathies are mutations in the TRPV4 gene that alter channel function, leading to several phenotypically distinct diseases that can be classified into two groups: skeletal dysplasias and neuropathies23. TRPV4-associated skeletal dysplasias encompass a heterogeneous group of skeletal diseases commonly characterized by a shortening of the trunk, while TRPV4-mediated neuropathies include a spectrum of hereditary neuropathies which can present with primarily motor axonal peripheral neuropathy or are associated with sensory involvement23.
The third cluster, “tumorigenesis,” had 31 items. This cluster was represented by keywords such as “mechanosensitivity,” “inflammation,” “apoptosis,” “proliferation,” and “migration.” These keywords illuminate the increasingly recognized role of TRPV4 in cancer hallmarks, including apoptosis, proliferation, migration, and metastasis24, 25, 26, 27. Therefore, TRPV4 is a viable target for cancer treatment28. The role of the mechanosensitive TRPV4 ion channel in inflammation has also been elucidated29. TRPV4 has been implicated in the inflammatory response, whereby stretch-induced TRPV4 activation causes the release of the pro-inflammatory cytokines IL-6 and IL-8 in human lung epithelial cells30. TRPV4 has also been identified as a regulator of neutrophil activation, as TRPV4 deficiency prevents neutrophil response to pro-inflammatory stimuli in acute lung injuries31.
The fourth cluster, “smooth muscle regulation,” was represented by “trpv1,” “vascular smooth muscle cells,” “calcium influx,” and “urothelium.” TRPV1, which is a close relative of TRPV4, is a receptor for capsaicin (an active component of chili peppers)32. Similar to the polymodal TRPV4 ion channel, TRPV1 is a nonselective cation channel activated by noxious heat (greater than 42°C), acidosis (pH < 6), and several endogenous agonists, including endocannabinoids, anandamide, and arachidonic acid-derived metabolites33. TRPV1 is involved in several processes, including thermoregulation, nociception, and inflammation33, 34. TRPV4 expression has been documented in vascular smooth muscle cells, such as the smooth muscle cells of rat cerebral arteries35, rat pulmonary arterial smooth muscle cells36, and human and rat smooth muscle extra-alveolar vessels37. Increasing evidence has documented the role of TRPV4 channels in vascular function regulation, including in vascular dilation and constriction, permeability, remodeling, and damage38.
The four main research frontiers of TRPV4 identified from our analysis were glaucoma, mitochondria, inflammation, and cell signaling. We have further enumerated these research frontiers below.
TRPV4 and glaucoma
The potential role of the TRPV4 channel has been investigated in several ocular diseases, including glaucoma39. Glaucoma is a group of eye diseases characterized by progressive degeneration of retinal ganglion cells that can result in subsequent loss of vision and blindness, typically due to increased intraocular pressure (IOP)40. Dysfunction of the trabecular meshwork is associated with elevated IOP in glaucoma; recent findings have provided evidence regarding the involvement of TRPV4 channels in IOP regulation41. Patel et al. 41 found that glaucomatous primary human trabecular meshwork cells showed impaired TRPV4 channel activity, reduced endothelial nitric oxide synthase (eNOS) signaling, and a subsequent reduction in nitric oxide production and elevated IOP, further implicating TRPV4-eNOS signaling in glaucoma pathogenesis. Further mechanistic studies on TRPV4’s role in glaucomatous eyes may illuminate the potential of TRPV4 as a therapeutic target for glaucoma.
TRPV4 and mitochondria
Previous studies have reported that TRPV4 is endogenously expressed in mitochondria and involved in the regulation of mitochondrial calcium homeostasis, temperature, and metabolism42. TRPV4 has also been implicated in the regulation of mitochondrial morphology, smoothness, and fusion-fission events, further highlighting the interplay between TRPV4 and mitochondria42. Zhang et al. 43 recently provided evidence on the new role of mitochondria in shaping TRPV4-mediated calcium signaling by facilitating adenosine triphosphate (ATP) release. Investigating the molecular mechanisms that link TRPV4 to mitochondria is worthy of further investigation to improve the understanding of TRPV4’s involvement in the regulation of mitochondrial function and its potential role in mitochondria-mediated diseases.
TRPV4 and inflammation
A growing body of evidence suggests TRP channels play a role in the physiology and pathophysiology of inflammation and the immune system44. Early evidence regarding the role of TRPV4 in inflammation has been demonstrated by Yin et al.31, who reported that genetic deficiency or pharmacological inhibition of TRPV4 attenuated the functional, histological, and inflammatory characteristics of acute lung injury in a murine model of acid-induced acute lung injury. Considering the importance of TRPV4 channels in various inflammatory conditions, such as chronic lung disease45 and osteoarthritis46, TRPV4 is subject to ongoing research to gain further insights into the precise molecular mechanisms underlying TRPV4-mediated inflammation. Such interest could result in the discovery of new therapies for these inflammatory conditions.
TRPV4 and cell signaling
Cell signaling refers to the fundamental, ubiquitous process that living systems utilize to respond to the environment. It provides the coordination required for multicellular organisms to function properly47. Compelling evidence has implicated some components of the calcium signaling machinery, such as TRP channels, in the development and progression of cancer. Therefore, these components may be plausible drug targets for cancer therapy48. Increasing evidence has begun to demonstrate the functional importance of TRPV4-mediated calcium signaling in several aspects of tumorigenesis, including angiogenesis, metastasis, and apoptosis, in various cancer types26, 27, 49, 50, 51, 52. Delineating the key signaling pathways involved in TRPV4-mediated oncogenesis could pave the way for the development of novel, targeted cancer therapies.
Strengths and Limitations
To the best of our knowledge, this study is the first bibliometric analysis to specifically address TRPV4 channels. Our data analysis was objective and clearly demonstrated the general global trends in research pertaining to TRPV4 channels, as well as the research frontiers, which could serve as a reference for researchers interested in conducting more in-depth studies in this field. However, since our study collected the articles from a single database only (i.e., the Scopus database) and was limited to original articles and reviews, we might have missed other relevant articles in the literature. We decided to retrieve articles from the Scopus database as it is a leading global academic database and covers a wider spectrum of journals than other databases, such as PubMed, Web of Science, and Google Scholar53. Additionally, the Scopus database is a single database with no further restrictions on content accessibility, making it an ideal data source for bibliometric applications54. Nevertheless, given that our study encompasses most articles published from 2000 to the present, the most recent publications would not have a significant impact on the results presented in this study.
Conclusions
This is the first bibliometric study demonstrating the global trends and future developments in TRPV4 channel research. Our results indicate that TRPV4 research will remain an important and emerging field of study, with an expected rise in publication output. A considerable number of papers related to TRPV4 channels have been published in highly influential journals. Four clusters, “cardiovascular-related,” “channelopathies,” “tumorigenesis,” and “smooth muscle regulation,” were identified from the author keyword co-occurrence analysis of TRPV4-related publications. Our study highlighted several research frontiers of TRPV4 channels, which will likely expand soon. TRPV4 is a subject of growing interest. Future studies could further define the pathophysiological role of TRPV4 and unveil its therapeutic potential in several human diseases.
Abbreviations
ATP: adenosine triphosphate, eNOS: endothelial nitric oxide synthase, IOP: intraocular pressure, TRP: transient receptor potential, TRPA: transient receptor potential ankyrin, TRPC: transient receptor potential canonical, TRPM: transient receptor potential melastatin, TRPML: transient receptor potential mucolipin, TRPP: transient receptor potential polycystic, TRPV: transient receptor potential vanilloid, TRPV1: transient receptor potential vanilloid 1, TRPV4: transient receptor potential vanilloid 4
Acknowledgments
None.
Author’s contributions
Conceptualization, S.Y.N.J., A.H.J. and R.Z.; methodology, R.Z.; data analysis, S.Y.N.J., A.H.J. and R.Z.; writing - original draft preparation, S.Y.N.J.; writing - review and editing, S.Y.N.J., A.H.J. and R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Samanta
A.,
Hughes
T.E.,
Moiseenkova-Bell
V.Y.,
Transient Receptor Potential (TRP) Channels. Sub-Cellular Biochemistry.
2018;
87
:
141-65
.
View Article PubMed Google Scholar -
Toft-Bertelsen
T.L.,
MacAulay
N.,
TRPing to the Point of Clarity: Understanding the Function of the Complex TRPV4 Ion Channel. Cells.
2021;
10
(1)
:
165
.
View Article PubMed Google Scholar -
Plant
T.D.,
Strotmann
R.,
TRPV4. In: Flockerzi V, Nilius B, editors. Transient Receptor Potential (TRP) Channels. Berlin, Heidelberg: Springer Berlin Heidelberg; 2007. p. 189-205.. 2007;
:
189-205
.
View Article Google Scholar -
Strotmann
R.,
Harteneck
C.,
Nunnenmacher
K.,
Schultz
G.,
Plant
T.D.,
OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nature Cell Biology.
2000;
2
(10)
:
695-702
.
View Article PubMed Google Scholar -
Rosenbaum
T.,
Benítez-Angeles
M.,
Sánchez-Hernández
R.,
Morales-Lázaro
S.L.,
Hiriart
M.,
Morales-Buenrostro
L.E.,
TRPV4: A Physio and Pathophysiologically Significant Ion Channel. International Journal of Molecular Sciences.
2020;
21
(11)
:
3837
.
View Article PubMed Google Scholar -
Vincent
F.,
Acevedo
A.,
Nguyen
M.T.,
Dourado
M.,
DeFalco
J.,
Gustafson
A.,
Identification and characterization of novel TRPV4 modulators. Biochemical and Biophysical Research Communications.
2009;
389
(3)
:
490-4
.
View Article PubMed Google Scholar -
Vincent
F.,
Duncton
M.A.,
TRPV4 agonists and antagonists. Current Topics in Medicinal Chemistry.
2011;
11
(17)
:
2216-26
.
View Article PubMed Google Scholar -
Grace
M.S.,
Bonvini
S.J.,
Belvisi
M.G.,
McIntyre
P.,
Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacology {&}amp; Therapeutics.
2017;
177
:
9-22
.
View Article PubMed Google Scholar -
White
J.P.,
Cibelli
M.,
Urban
L.,
Nilius
B.,
McGeown
J.G.,
Nagy
I.,
TRPV4: Molecular Conductor of a Diverse Orchestra. Physiological Reviews.
2016;
96
(3)
:
911-73
.
View Article PubMed Google Scholar -
Donthu
N.,
Kumar
S.,
Mukherjee
D.,
Pandey
N.,
Lim
W.M.,
How to conduct a bibliometric analysis: an overview and guidelines. Journal of Business Research.
2021;
133
:
285-96
.
View Article Google Scholar -
You
Y.,
Li
W.,
Liu
J.,
Li
X.,
Fu
Y.,
Ma
X.,
Bibliometric Review to Explore Emerging High-Intensity Interval Training in Health Promotion: A New Century Picture. Frontiers in Public Health.
2021;
9
:
697633
.
View Article PubMed Google Scholar -
You
Y.,
Wang
D.,
Liu
J.,
Chen
Y.,
Ma
X.,
Li
W.,
Physical Exercise in the Context of Air Pollution: An Emerging Research Topic. Frontiers in Physiology.
2022;
13
:
784705
.
View Article PubMed Google Scholar -
Zakaria
R.,
Ahmi
A.,
Ahmad
A.H.,
Othman
Z.,
Worldwide melatonin research: a bibliometric analysis of the published literature between 2015 and 2019. Chronobiology International.
2021;
38
(1)
:
27-37
.
View Article PubMed Google Scholar -
Liao
Z.,
Wei
W.,
Yang
M.,
Kuang
X.,
Shi
J.,
Academic Publication of Neurodegenerative Diseases From a Bibliographic Perspective: A Comparative Scientometric Analysis. Frontiers in Aging Neuroscience.
2021;
13
:
722944
.
View Article PubMed Google Scholar -
Zhu
X.,
Tian
C.,
Zhou
Y.,
Shi
J.,
Yuan
G.,
Zhang
L.,
Transient Receptor Potential channels: A Global Bibliometric analysis From 2012 to 2021. Channels (Austin, Tex.).
2021;
15
(1)
:
624-34
.
View Article PubMed Google Scholar -
Shi
J.,
Wang
H.,
Shi
S.,
Yuan
G.,
Jia
Q.,
Shi
S.,
Bibliometric analysis of calcium channel research (2010-2019). Channels (Austin, Tex.).
2020;
14
(1)
:
193-202
.
View Article PubMed Google Scholar -
Page
M.J.,
McKenzie
J.E.,
Bossuyt
P.M.,
Boutron
I.,
Hoffmann
T.C.,
Mulrow
C.D.,
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed.).
2021;
372
(71)
:
n71
.
View Article PubMed Google Scholar -
Riccaboni
M.,
Verginer
L.,
The impact of the COVID-19 pandemic on scientific research in the life sciences. PLoS One.
2022;
17
(2)
:
e0263001
.
View Article PubMed Google Scholar -
Wang
L.,
Liu
X.,
Zhang
K.,
Liu
Z.,
Yi
Q.,
Jiang
J.,
A bibliometric analysis and review of recent researches on Piezo (2010-2020). Channels (Austin, Tex.).
2021;
15
(1)
:
310-21
.
View Article PubMed Google Scholar -
Zhang
Z.,
Kang
L.,
Yan
X.,
Leng
Z.,
Fang
K.,
Chen
T.,
Global Trends and Hotspots of Transient Receptor Potential Melastatin 8 Research from 2002 to 2021: A Bibliometric Analysis. Journal of Pain Research.
2022;
15
:
3881-92
.
View Article PubMed Google Scholar -
Randhawa
P.K.,
Jaggi
A.S.,
TRPV4 channels: physiological and pathological role in cardiovascular system. Basic Research in Cardiology.
2015;
110
(6)
:
54
.
View Article PubMed Google Scholar -
Zou
Y.,
Zhang
M.,
Wu
Q.,
Zhao
N.,
Chen
M.,
Yang
C.,
Activation of transient receptor potential vanilloid 4 is involved in pressure overload-induced cardiac hypertrophy. eLife.
2022;
11
:
e74519
.
View Article PubMed Google Scholar -
Nilius
B.,
Voets
T.,
The puzzle of TRPV4 channelopathies. EMBO Reports.
2013;
14
(2)
:
152-63
.
View Article PubMed Google Scholar -
Bahari
N.N.,
Jamaludin
S.Y.,
Jahidin
A.H.,
Zahary
M.N.,
Hilmi
A.B. Mohd,
Bakar
N.H.,
The Emerging Roles of TRPV4 in Cancer. Biomedical & Pharmacology Journal.
2017;
10
(4)
:
1757-64
.
View Article Google Scholar -
Azimi
I.,
Robitaille
M.,
Armitage
K.,
So
C.L.,
Milevskiy
M.J.,
Northwood
K.,
Activation of the Ion Channel TRPV4 Induces Epithelial to Mesenchymal Transition in Breast Cancer Cells. International Journal of Molecular Sciences.
2020;
21
(24)
:
9417
.
View Article PubMed Google Scholar -
Zhang
P.,
Xu
J.,
Zhang
H.,
Liu
X.Y.,
Identification of TRPV4 as a novel target in invasiveness of colorectal cancer. BMC Cancer.
2021;
21
(1)
:
1264
.
View Article PubMed Google Scholar -
Peters
A.A.,
Jamaludin
S.Y.,
Yapa
K.T.,
Chalmers
S.,
Wiegmans
A.P.,
Lim
H.F.,
Oncosis and apoptosis induction by activation of an overexpressed ion channel in breast cancer cells. Oncogene.
2017;
36
(46)
:
6490-500
.
View Article PubMed Google Scholar -
Yu
S.,
Huang
S.,
Ding
Y.,
Wang
W.,
Wang
A.,
Lu
Y.,
Transient receptor potential ion-channel subfamily V member 4: a potential target for cancer treatment. Cell Death {&}amp; Disease.
2019;
10
(7)
:
497
.
View Article PubMed Google Scholar -
Michalick
L.,
Kuebler
W.M.,
TRPV4-A Missing Link Between Mechanosensation and Immunity. Frontiers in Immunology.
2020;
11
:
413
.
View Article PubMed Google Scholar -
Pairet
N.,
Mang
S.,
Fois
G.,
Keck
M.,
Kühnbach
M.,
Gindele
J.,
TRPV4 inhibition attenuates stretch-induced inflammatory cellular responses and lung barrier dysfunction during mechanical ventilation. PLoS One.
2018;
13
(4)
:
e0196055
.
View Article PubMed Google Scholar -
Yin
J.,
Michalick
L.,
Tang
C.,
Tabuchi
A.,
Goldenberg
N.,
Dan
Q.,
Role of Transient Receptor Potential Vanilloid 4 in Neutrophil Activation and Acute Lung Injury. American Journal of Respiratory Cell and Molecular Biology.
2016;
54
(3)
:
370-83
.
View Article PubMed Google Scholar -
Caterina
M.J.,
Schumacher
M.A.,
Tominaga
M.,
Rosen
T.A.,
Levine
J.D.,
Julius
D.,
The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature.
1997;
389
(6653)
:
816-24
.
View Article PubMed Google Scholar -
Holzer
P.,
The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. British Journal of Pharmacology.
2008;
155
(8)
:
1145-62
.
View Article PubMed Google Scholar -
Xiao
T.,
Sun
M.,
Kang
J.,
Zhao
C.,
Transient Receptor Potential Vanilloid1 (TRPV1) Channel Opens Sesame of T Cell Responses and T Cell-Mediated Inflammatory Diseases. Frontiers in Immunology.
2022;
13
:
870952
.
View Article PubMed Google Scholar -
Marrelli
S.P.,
O'neil
R.G.,
Brown
R.C.,
Bryan
R.M.,
PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. American Journal of Physiology. Heart and Circulatory Physiology.
2007;
292
(3)
:
1390-7
.
View Article PubMed Google Scholar -
Martin
E.,
Dahan
D.,
Cardouat
G.,
Gillibert-Duplantier
J.,
Marthan
R.,
Savineau
J.P.,
Involvement of TRPV1 and TRPV4 channels in migration of rat pulmonary arterial smooth muscle cells. Pfl{&}{#}x00FC;gers Archiv.
2012;
464
(3)
:
261-72
.
View Article PubMed Google Scholar -
Alvarez
D.F.,
King
J.A.,
Weber
D.,
Addison
E.,
Liedtke
W.,
Townsley
M.I.,
Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circulation Research.
2006;
99
(9)
:
988-95
.
View Article PubMed Google Scholar -
Liu
L.,
Guo
M.,
Lv
X.,
Wang
Z.,
Yang
J.,
Li
Y.,
Role of Transient Receptor Potential Vanilloid 4 in Vascular Function. Frontiers in Molecular Biosciences.
2021;
8
:
677661
.
View Article PubMed Google Scholar -
Guarino
B.D.,
Paruchuri
S.,
Thodeti
C.K.,
The role of TRPV4 channels in ocular function and pathologies. Experimental Eye Research.
2020;
201
:
108257
.
View Article PubMed Google Scholar -
Weinreb
R.N.,
Aung
T.,
Medeiros
F.A.,
The pathophysiology and treatment of glaucoma: a review. Journal of the American Medical Association.
2014;
311
(18)
:
1901-11
.
View Article PubMed Google Scholar -
Patel
P.D.,
Chen
Y.L.,
Kasetti
R.B.,
Maddineni
P.,
Mayhew
W.,
Millar
J.C.,
Impaired TRPV4-eNOS signaling in trabecular meshwork elevates intraocular pressure in glaucoma. Proceedings of the National Academy of Sciences of the United States of America.
2021;
118
(16)
:
e2022461118
.
View Article PubMed Google Scholar -
Kumar
A.,
Majhi
R.K.,
Acharya
T.K.,
Smalla
K.H.,
Gundelfinger
E.D.,
Goswami
C.,
TRPV4 interacts with mitochondrial proteins and acts as a mitochondrial structure-function regulator. bioRxiv.
2018;
2018
:
330993
.
View Article Google Scholar -
Zhang
X.,
Lee
M.D.,
Buckley
C.,
Wilson
C.,
McCarron
J.G.,
Mitochondria regulate TRPV4-mediated release of ATP. British Journal of Pharmacology.
2022;
179
(5)
:
1017-32
.
View Article PubMed Google Scholar -
Parenti
A.,
De Logu
F.,
Geppetti
P.,
Benemei
S.,
What is the evidence for the role of TRP channels in inflammatory and immune cells?. British Journal of Pharmacology.
2016;
173
(6)
:
953-69
.
View Article PubMed Google Scholar -
Belvisi
M.G.,
Birrell
M.A.,
The emerging role of transient receptor potential channels in chronic lung disease. The European Respiratory Journal.
2017;
50
(2)
:
1601357
.
View Article PubMed Google Scholar -
Zhang
K.,
Wang
L.,
Liu
Z.,
Geng
B.,
Teng
Y.,
Liu
X.,
Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: potential therapeutic targets for osteoarthritis. Channels (Austin, Tex.).
2021;
15
(1)
:
339-59
.
View Article PubMed Google Scholar -
Radhakrishnan
K.,
Halász
A.,
Vlachos
D.,
Edwards
J.S.,
Quantitative understanding of cell signaling: the importance of membrane organization. Current Opinion in Biotechnology.
2010;
21
(5)
:
677-82
.
View Article PubMed Google Scholar -
Wu
L.,
Lian
W.,
Zhao
L.,
Calcium signaling in cancer progression and therapy. The FEBS Journal.
2021;
288
(21)
:
6187-205
.
View Article PubMed Google Scholar -
Wang
H.,
Zhang
B.,
Wang
X.,
Mao
J.,
Li
W.,
Sun
Y.,
TRPV4 Overexpression Promotes Metastasis Through Epithelial-Mesenchymal Transition in Gastric Cancer and Correlates with Poor Prognosis. OncoTargets and Therapy.
2020;
13
:
8383-94
.
View Article PubMed Google Scholar -
Bahari
N.N.,
Jamaludin
S.Y.,
Jahidin
A.H.,
Zahary
M.N.,
Hilmi
A.B. Mohd,
Assessment of TRPV4 Channel and Its Role in Colorectal Cancer Cells. Biomedical & Pharmacology Journal.
2019;
12
(2)
:
629-38
.
View Article Google Scholar -
Huang
S.,
Yu
S.,
Deng
R.,
Liu
H.,
Ding
Y.,
Sun
Y.,
TRPV4 Promotes Metastasis in Melanoma by Regulating Cell Motility through Cytoskeletal Rearrangement. International Journal of Molecular Sciences.
2022;
23
(23)
:
15155
.
View Article PubMed Google Scholar -
Kanugula
A.K.,
Adapala
R.K.,
Jamaiyar
A.,
Lenkey
N.,
Guarino
B.D.,
Liedtke
W.,
Endothelial TRPV4 channels prevent tumor growth and metastasis via modulation of tumor angiogenesis and vascular integrity. Angiogenesis.
2021;
24
(3)
:
647-56
.
View Article PubMed Google Scholar -
Falagas
M.E.,
Pitsouni
E.I.,
Malietzis
G.A.,
Pappas
G.,
Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. The FASEB Journal.
2008;
22
(2)
:
338-42
.
View Article PubMed Google Scholar -
Pranckute
R.,
Web of Science (WoS) and Scopus: The Titans of Bibliographic Information in Today's Academic World. Publications / MDPI.
2021;
9
(1)
:
12
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 5 (2023)
Page No.: 5671-5679
Published on: 2023-05-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4740 times
- PDF downloaded - 1432 times
- XML downloaded - 119 times
 Biomedpress
Biomedpress





