
Treatment of arteriovenous malformations associated with ruptured intracranial aneurysm
- Digital subtraction angiography Unit, Can Tho S.I.S General Hospital, Can Tho, Viet Nam
- Department of Surgery, Can Tho S.I.S General Hospital, Can Tho, Viet Nam
- Department of Radiology, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Viet Nam
Abstract
Spontaneous subarachnoid hemorrhage (SAH) is a specific characteristic of aneurysmal rupture. SAH coexisting with arteriovenous malformations is challenging to treat because the patient status at admission is severe and the therapeutic approach is multidisciplinary: endovascular treatment, cerebral fluid drainage, and decompressive craniectomy. We report a clinical case successfully treated for cerebellar hemorrhage due to a ruptured aneurysm with arteriovenous malformations. A 65-year-old woman was admitted to Can Tho SIS General Hospital within six hours of stroke onset. She experienced loss of consciousness, aphasia, and high blood pressure. Non-contrast computed tomography showed a diffused SAH in both cerebellum hemispheres, Sylvian fissure, intraventricular hemorrhage, and non-communicating hydrocephalus. Her medical history included uncontrolled hypertension. The patient underwent multidisciplinary treatment for hydrocephalus, cerebellar edema, and embolization to control recurrent ruptured arteriovenous malformations. She recovered well and was discharged on day 30 with a Modified Rankin Scale score of 1.
Introduction
The two common treatment options for spontaneous subarachnoid hemorrhage (SAH) due to aneurysmal rupture are surgical clipping and endovascular treatment1, 2. In addition, external ventricular drains are placed early to control hydrocephalus, and elevated intracranial pressure is present after SAH. While magnetic resonance (MRA) or computed tomography angiography (CTA) can identify the ruptured aneurysm, digital subtraction angiography (DSA) is the gold standard for exactly detecting these aneurysms and other specific lesions3. During craniectomy, intracranial arteriovenous malformations (AVMs) related to these ruptured aneurysms sometimes appear in the same operative field3. The order of therapeutic options based on the clinical condition at admission is thus essential for deciding successful treatment strategies. AVMs and feeder artery aneurysms have a stronger relationship in infra-tentorial than supra-tentorial regions4. The incidence of AVMs with intracranial aneurysms is more frequent than each lesion alone. In these cases, endovascular embolization is the optimal treatment option, with or without neurosurgery1, 3. We report a clinical case with an AVM-associated ruptured intracranial aneurysm successfully treated with surgery at the acute stage and endovascular embolization at the stable stage.
CASE REPORT
Patient information
A 65-year-old woman was admitted to Can Tho SIS General Hospital after a sudden onset of headache, vomiting, and impaired consciousness six hours before. Uncontrolled hypertension was noted within the last five years.
Clinical findings
On examination, the patient’s blood pressure was 140/70 mmHg, with a heart rate of 94 bpm, oxygen saturation of 93% on room air, respiratory rate of 20 times/min, and blood glucose of 190 mg/dL. Her Glasgow Coma Scale (GCS) score was 10 (E2V3M5) with a stiff neck.
Diagnostic assessment
Non-contrast computed tomography (NCCT) showed a diffused SAH in both cerebellum hemispheres, Sylvian fissure, intraventricular hemorrhage, and non-communicating hydrocephalus due to cerebellar edema. This uncommon SAH type might not have a typical hypertensive cause. The CTA suggested AVMs where the feeder artery arose from the right posterior inferior cerebellar artery (PICA) and drained into the sinuses’ confluence (Figure 1).
Treatment
The loss of consciousness was mostly due to hydrocephalus. A ventriculoperitoneal (VP) shunt was first placed to drain cerebrospinal fluid and relieve intracranial hypertension.
Two days after shunting, the patient could open her eyes and respond to simple commands. Three days later, she had a decreased GCS and developed respiratory distress. An immediate NCCT showed that progressive cerebellar edema compressed the brainstem. Therefore, a decompressive craniectomy was performed (Figure 2). Using the midline suboccipital approach, we followed the PICA to the superior part of the cerebellum, where we found a bleeding PICA aneurysm. The aneurysm was clipped with a 7-mm strong curved clip (Aesculap) to stop the bleeding before the surgery finished.
The postoperative NCCT showed no new hemorrhage and reduced compression to the brainstem. Twelve days after surgery, the patient had completely recovered. A DSA then showed small cerebellar AVMs, including branches of the right PICA draining directly into the sinuses’ confluence (Spetzler–Martin Grade III). The right vertebral artery was accessed via a co-axial system with a Chaperon 6F guiding catheter (Microvention) and 0.035” wire (Terumo), making a roadmap. A Magic 1.2F microcatheter (Balt) and Hybrid 0.007” microwire (Balt) then approached selectively into the right PICA, followed by one embolization with a solution comprising 1 mL glue Histoacryl (B.Braun) diluted in 1.5 mL Lipiodol Ultra-Fluid (Guerbet) at a 2:3 ratio to provide a moderate concentration (40%). Angiography showed that the AVMs were almost completely occluded (Figure 3). After 30 days of treatment, the patient’s Modified Rankin Scale score was 1.
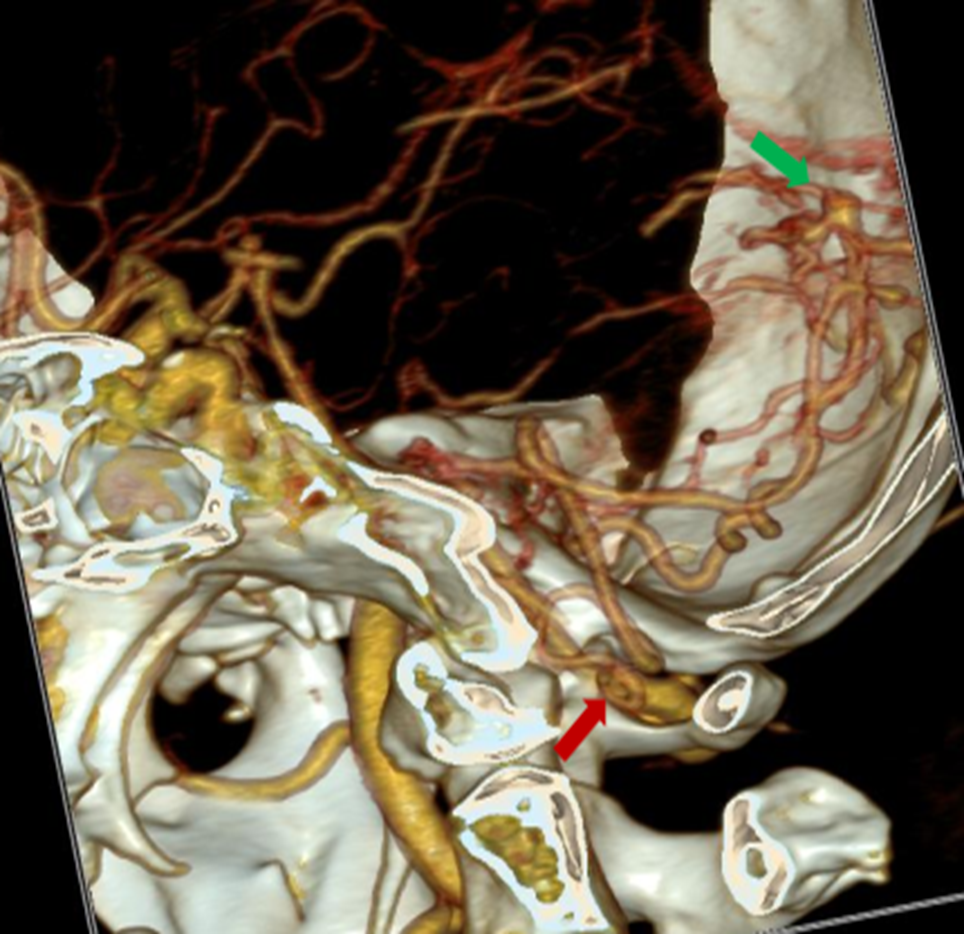
Lesions in CTA: origin of the PICA from the right vertebral artery (red arrow), with an aneurysm in the PICA (green arrow).

The order of neurosurgery approaches to improve patient outcome in the acute stage. (A) Intracranial hemorrhage in both cerebellum hemispheres (red arrow). (B) SAH in the Sylvian fissure and non-communicated hydrocephalus (green arrow). (C, D) The VP shunt approached from the right side of Keen’s point (blue arrow).
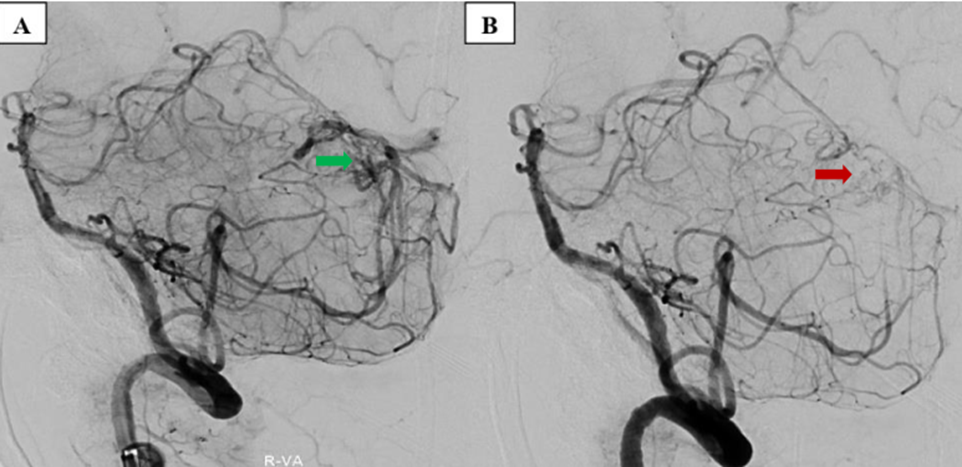
Embolization procedure in the stable stage. (A) AVM before embolization (green arrow). (B) AVM occluded after embolization (red arrow).
DISCUSSION
Among intracranial aneurysms, 10–20% are associated with AVMs, and even up to 58%3, 5, 6. In addition, the intracranial hemorrhage risk in patients with coexisting unruptured AVMs and aneurysms was 7% per year compared with 3% in those with isolated AVMs7. The size and location of intracranial aneurysms can predict their annual rupture risk and five-year cumulative rupture rate. Yu used a simplified four-type classification for AVMs associated with aneurysms2. Infratentorial AVMs are more often associated with intracranial aneurysms, hemorrhagic presentation, and unfavorable outcomes than are supratentorial AVMs1, 8.
The PICA has six segments and two loops: the basilar artery to vertebral artery to PICA junction, the anterior medullary segment, the lateral medullary segment, the tonsillomedullary segment, the telovelotonsillar segment, and the cortical segment. One study reported no aneurysms in the cortical segment among 20 patients with PICA aneurysms. In our case, the ruptured saccular aneurysm originating from the feeding (PICA) artery of the AVM (type II) was in the cortical segment. A midline suboccipital craniotomy was a suitable approach to this aneurysm9. Treatment decisions must carefully consider the clinical presentation and anatomic relationship of the intracranial aneurysm to the AVM nidus10. The intraventricular hemorrhage, SAH, and non-communicating hydrocephalus caused this patient’s impaired consciousness, severe thunderclap headache, and neck stiffness at admission. The decompressive craniectomy, clipping, and VP shunt were prioritized over endovascular therapy. After controlling the source of hemorrhage and patient recovery, the AVM was treated by embolization to prevent recurrent hemorrhage.
In low-grade (Spetzler–Martin grades I and II) AVMs treated with a Gamma Knife, the presence of an associated aneurysm was not related to bleeding after radiosurgery. This Spetzler–Martin grade III AVM was considered a high-grade AVM at high risk of recurrent hemorrhage or death and recommended for multimodality treatment11. However, the AVM was occluded completely after emergency surgery and embolization during hospitalization. After one year, the patient will be rechecked for AVMs by magnetic resonance imaging and MRA.
CONCLUSIONS
We described a case with a ruptured PICA aneurysm associated with AVM that was totally treated, despite a coma. Before the interventional treatment of AVMs, recognition of the associated anatomic abnormalities is essential to develop the treatment strategy, especially with respect to resolving both the consequences of and reasons for this complicated disease. After the recovery stage, embolization played an essential role in preventing the growth and recurrent rupture of AVMs. The combined multidisciplinary treatment is considered effective to improve patient outcomes.
Abbreviations
AVMs: Arteriovenous malformations, CTA: Computed tomography angiography, DAS: Digital subtraction angiography, SAH: Subarachnoid hemorrhage
Acknowledgments
None.
Author’s contributions
Le Minh Thang and Tran Chi Cuong contributed equally to this article as co-first authors. Le Minh Thang, Nguyen Hai Dang, and Nguyen Minh Duc contributed to write original draft. Tran Chi Cuong, Nguyen Dao Nhat Huy, and Le Minh Thang contributed to undergo interventional procedure, collect, and interpret the imaging. Nguyen Quang Hung and Nguyen Hai Dang contributed to perform VP shunt and aneurysm clipping before embolization. Le Minh Thang, Nguyen Hai Dang and Nguyen Minh Duc made substantial contributions to collect patient data and clinical data analysis. All authors have read, revised, and approved the final published version of the manuscript. All authors were responsible for submission of our study for publication.
Funding
None.
Availability of data and materials
All data generated or analyzed during this study are included in this article and/or its online supplementary material files. Further enquiries can be directed to the corresponding author.
Ethics approval and consent to participate
Ethical approval was not necessary for the preparation of this article. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Informed consent for patient information to be published in this article was obtained.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.

