Abstract
Introduction: Ulcerative colitis (UC) and other inflammatory bowel diseases (IBDs) are common chronic, inflammatory gastrointestinal diseases. Due to their antioxidant, anti-inflammatory, and antibacterial properties, polyphenols are beneficial in the treatment of IBD. Caffeine acid phenethyl ester (CAPE) has been shown to have cytotoxic, antibacterial, antioxidant, and anti-inflammatory effects. This study focuses on the biochemical and molecular levels of the mode of action of CAPE in DSS-induced UC in rats.
Methods: Thirty male Wistar rats were distributed into five groups, with six rats in each group: group I was administered 3 mL of distilled water orally, group II was administered CAPE (10 mg/kg.b.w.) orally, group III was administered 5% DSS orally, group IV was administered 5% DSS and CAPE (10 mg/kg.b.w.) orally; and group V was administered 5% DSS and sulfasalazine (100 mg/kg b.w.) orally.
Results: Individually, oral treatment with CAPE or sulfasalazine significantly ameliorated body weight, DAI score, and colon length in DSS-induced colitis and raised blood PLT count, NO, NF-kß, and vitamin C levels. In addition, animals given CAPE had a considerable increase in colon GSH, GPx, CAT, and SOD levels compared with rats given DSS. Compared with the DSS control group, colon TBAR, IL-6, and INF-ɣ were lower in the CAPE-treated rats. Histopathological examination revealed that CAPE treatment caused tissue injury and improved vanin-1, AKT, and miRNA-203 genes in the distal colon and triggered apoptosis.
Conclusions: The gastroprotective impact of CAPE was more noticeable than sulfasalazine. CAPE treatment caused biochemical and histopathological improvements, indicating that CAPE may have antioxidant and anti-inflammatory effects in colitis; therefore, CAPE may be a potential therapeutic agent for the amelioration of IBD. This finding is promising for future therapies and research goals.
Introduction
Several factors, including genetics, microbiome, and environmental stressors, are the causes of ulcerative colitis (UC)1, 2, 3. In UC, the epithelial cell lining of the colon becomes inflamed4, 5, 6, 7. Dextran sulphate sodium (DSS) is a polysaccharide with varying molecular weight ranging from 5 to 1400 kDa8. Due to its toxicity to colonic epithelial cells, DSS promotes human UC-like diseases, resulting in depressed mucosal barrier function9. Weight loss, diarrhea, and occult blood in the stool are common observations in the DSS-treated rat model10. Many documents have demonstrated the pharmaceutical importance of phytochemicals in reducing UC symptoms, enhancing immune activity, and providing antioxidants that reduce inflammation in animal models11, 12, 13. However, the data indicate a need for further studies to elucidate the benefits and mechanisms of these compounds.
Caffeic acid phenethyl ester (CAPE) is a major active phenolic compound of some types of propolis14, 15. It has been shown to be protective against oxidative stress-mediated tissue damage16, 17, 18, 19. CAPE has been associated with a variety of in vitro and in vivo pharmacological effects20, 21, 22, 23, and there have been reports of its gastroprotective activity in animal models. In addition, its anticancer properties were observed in the skin of mice treated with bee propolis and exposed to 12-O-tetradecanoylphorbol-13-acetate24, 25.
Our findings indicate that the presence of vanin-1 in tissues of the epithelium influences the perception of stress26 by innate immune cells as an inhibitor of inflammatory processes and the treatment of colitis27. In addition, research indicates the upregulation of miRNA 203 and AKT gene expression in certain inflammatory tissues and organs28, 29, 30. As a measure of our interest research program in the treatment of inflammatory diseases31, 32, 33, 34, 35, 36. In the present study, we aimed to evaluate the therapeutic potential of CAPE in DSS-induced UC in rats.
Methods
Chemicals
All chemicals were purchased from Sigma Aldrich, Germany.
- Caffeic acid phenethyl ester (97%) powder: Formula [C17H16O4], molecular weight (284.31), CAS. No. (104594-70-9).
- Dextran sulfate sodium (98%) powder: Formula [(C6H7Na3O14S3)n], molecular weight (>500,000), CAS. No. (9011-18-1).
- Sulfasalazine (99%) powder: Formula (C18H14N4O5S), molecular weight (398.394), CAS. No. (599-79-1).
Animals
Male albino rats weighing 150 ± 10 g each were donated by the National Cancer Institute Animal House at Cairo University in Giza, Egypt. They were kept in plastic cages with stainless steel covers at a humidity level of 55 — 60% and a temperature of 22 °C in a light-controlled environment. The animals were maintained for 2 weeks to adapt and were provided regular feed and water at will.
Design of experiment
This experiment was designed to evaluate the gastroprotective effect of CAPE in DSS-induced UC. According to the guidelines of the Faculty of Applied Health Sciences Committee, the present study was design. The treatment grouping is described in Table 1.
| Group NO. | Groups | Treatment description |
|---|---|---|
| I | Normal control | Received 3 mL of distilled water, orally for 15 days. |
| II | CAPE (10 mg/kg.b.w.) | Received 10 mg / kg bw. CAPE, orally, daily for 15 days 36 . |
| III | Positive control DSS, 5% in distilled water | Received DSS, 5% orally, for 15 days 37 . |
| IV | DSS + CAPE | Received DSS + CAPE, orally, for 15 days |
| V | DSS + Sulfasalazine (100 mg/kg b.w.) | Received DSS + sulfasalazine (100 mg/kg b.w.), orally, for 15 days 37 . |
Calculation of disease activity index
In experimental colitis, the disease activity index (DAI) was calculated according to the method outlined by Bang and Lichtenberger38.
Sample collection
On day 16, 1 day after the last dose, blood samples were collected in tubes containing heparin from the retroorbital venous plexus of each animal. Heparinized blood samples were divided into two parts: the first part was used to estimate PLT count using a Sysmex KX-21N automated hematology analyzer (Sysmex Corp., Kobe, Japan)39, and the second part was centrifuged at 1000 g for 20 min. Separated plasma was used to estimate plasma levels of NF-κB using an ELISA kit (MyBioSource Inc., San Diego, CA, USA, 92195-3308), NO using the calorimetrically calibrated diagnostic kit, interleukin 6 (IL-6) using an ELISA kit (Abcam plc, USA), and vitamin C (Vit. C) using an ELISA kit (Novus kits, Novus Biologicals, LLC, California, USA).
At the end of the experiment, the colon of each animal was excised, washed with phosphate-buffer saline (PBS), gently stretched, and the distance between the colocecal junction and the distal end of the rectum was measured40. The distal sections of the colons were then separated; one piece was used for histopathological analysis, and the other piece was kept frozen at 80 °C until biochemical analysis of TBARS, GSH, SOD, and CAT41, 42, 43, 44 using colorimetric methods in the diagnostic kit. In addition, the IFN-γ content was determined using an ELISA kit (Abcam plc, USA).
Real-time PCR
Using the RNA-spinTM (QiaGen GmbH, Hilden, Germany), total RNA from colon tissues was extracted45. As instructed by the manufacturer, cDNA was used for qPCR using the SYBR Green PCR master mix (iNtRON Biotechnology, Korea). The reverse transcription kit was used to produce cDNA from 1 – 5 g total RNA (Applied Biosystems, Foster City, CA). The sequences of the genes evaluated (vanin-1, AKT, and miRNA-203) and the housekeeping primer used in RT-PCR, β-actin (Primer Design Ltd, USA), are shown in Table 2.
| Gene | Sequences | |
|---|---|---|
| Vanin-1 | forward | 5'-AACTGGATACCCTGTGATAACCC-'3 |
| reverse | 5'- GTCTCCCATGTTCGCCACAA-'3 | |
| AKT | forward | 5'-CCCTGCTCCTAGTCCACCA–'3 |
| reverse | 5'-TGTCTCTGTTTCAGTGGGCTC-'3 | |
| miRNA-203 | forward | 5'- GGGGTGAAATGTTTAGGAC-'3 |
| reverse | 5'- CAGTGCGTGTCGTGGAGT-'3 | |
| β-actin (housekeeping) | forward | 5'-TGACTGACTACCTCATGAAGATCC-'3 |
| reverse | 5'-TCTCCTTAATGTCACGCACGATT-'3 | |
Histological examination
A sample of colon tissues was collected and fixed in 10% neutral buffered formalin. It was then dehydrated in ascending graders of ethyl alcohol (50–100%), cleared in xylene and embedded in melted paraffin wax (MP 59), embedded in paraffin as blocks, and 5–6-micron-thickness sections were cut using a rotary microtome46. The paraffin sections were stained and exanimated using a light microscope (Olympus, Münster, Germany). A photomicrograph of the colon tissue was taken at 400 x magnification.
Statistical analysis
Data are presented as mean ± standard deviation (SD) for six measurements for both spectrophotometric measurements and ELISA. However, there are three separate determinations for PCR analysis of gene expression. All data were analyzed using SPSS version 20 software47. The one-way analysis of variance (ANOVA) test was used to assess the hypotheses. Statistical significance was defined as P < 0.05.
| No. | Groups | Number of days/ Body weight of rats(g) | |||
|---|---|---|---|---|---|
| 1 | 5 | 10 | 15 | ||
| (I) | Normal control 3 mL of distilled water, orally | 160.42 ± 3.72 bA | 163.72 ± 5.08 aA | 169.79 ± 7.69 | 174.63 ± 3.24 cC |
| (II) | CAPE (10 mg/kg.b.w.) | 162.29 ± 8.53 A | 166.63 ± 8.23 AB | 170.58 ± 10.54 C | 175.69 ± 8.06 cD |
| (III) | Positive control DSS, 5% in distilled water | 158.15 ± 10.78 aA | 160.50 ± 9.29 aA | 161.57 ± 10.73 a | 161.99 ± 4.95 aB |
| (IV) | DSS + CAPE | 160.43 ± 10.45 A | 163.01 ± 7.72 aA | 168.33 ± 5.28 ab | 172.37 ± 9.62 C |
| (V) | DSS + Sulfasalazine (100 mg/kg b.w.) | 159.73 ± 4.47 aA | 162.07 ± 6.14 aAB | 165.70 ± 8.00 aBC | 167.73 ± 6.03 aC |
| Groups | Treatment Description | PLT (10 3 /mL) | NO (ng/mL) | NF-kβ (ng/mL) | Vit. C (µmol/L) |
|---|---|---|---|---|---|
| I | Normal control 3 mL of distilled water, orally | 261.27 ± 23.18 c | 10.79 ± 1.87 a | 0.81 ± 0.10 a | 83.13 ± 7.96 d |
| II | CAPE (10 mg/kg.b.w.) | 265.33 ± 18.62 c | 9.67 ± 0.54 a | 0.93 ± 0.16 a | 82.04 ± 5.46 d |
| III | Positive control DSS, 5% in distilled water | 209.28 ± 12.08 a | 36.13 ± 4.39 d | 3.51 ± 0.37 d | 59.76 ± 6.46 a |
| IV | DSS + CAPE | 265.00 ± 26.38 c | 14.52 ± 3.30 b | 1.36 ± 0.22 b | 77.13 ± 6.14 c |
| V | DSS + Sulfasalazine (100 mg/kg b.w.) | 217.58 ± 14.00 B | 21.94 ± 2.56 c | 2.40 ± 0.31 c | 65.98 ± 5.25 b |
| Groups | Treatment Description | GSH (nmole/mg protein) | GPx (U/mg protein) | CAT (U/mg protein) | SOD (U/mg protein) |
|---|---|---|---|---|---|
| I | Normal control 3 mL of distilled water, orally | 29.89 ± 2.79d | 25.97 ± 2.85d | 32.67 ± 2.75d | 97.44 ± 11.13 d |
| II | CAPE (10 mg/kg.b.w.) | 30.71 ± 3.21d | 22.39 ± 3.77cd | 35.48 ± 3.62d | 96.17 ± 9.49 d |
| III | Positive control DSS, 5% in distilled water | 13.64 ± 2.65a | 8.98 ± 0.84a | 13.82 ± 2.27a | 53.09 ± 6.99 a |
| IV | DSS + CAPE | 23.04 ± 3.20 c | 19.28 ± 3.21c | 27.50 ± 2.23 c | 79.25 ± 6.48 c |
| V | DSS + Sulfasalazine (100 mg/kg b.w.) | 17.45 ± 3.35b | 13.78 ± 2.65 | 21.60 ± 2.44b | 61.19 ± 5.82b |
| Groups | Treatment Description | TBARs (nmol/mg protein) | IL-6 (pg/mg protein) | INF-γ (pg/mg protein) |
|---|---|---|---|---|
| I | Normal control 3 mL of distilled water, orally | 70.34 ± 5.72 a | 25.71 ± 4.49 a | 0.41 ± 0.06 a |
| II | CAPE (10 mg/kg.b.w.) | 68.83 ± 5.81 a | 27.85 ± 2.58 a | 0.38 ± 0.05 a |
| III | Positive control DSS, 5% in distilled water | 142.71 ± 8.73 d | 46.59 ± 4.69 d | 1.46 ± 0.13 d |
| IV | DSS + CAPE | 93.06 ± 8.06 b | 33.08 ± 4.13 b | 0.64 ± 0.08 b |
| V | DSS + Sulfasalazine (100 mg/kg b.w.) | 114.65 ± 11.44 c | 40.17 ± 4.25 c | 1.14 ± 0.17 c |
Results
Effect of CAPE and sulfasalazine on the body weight of DSS-treated rats
Table 3 shows non-significant changes in body weight of CAPE (10 mg/kg.b.w.)-treated rats (group II) as compared with normal control rats (group I). Additionally, there was a significant depletion of 7.2% body weight in rats treated with DSS (5%) (group III) as compared with the control group after 15 days of oral administration (P< 0.05).
In contrast, administration of CAPE (10 mg/kg.b.w.) to DSS rats produced a significant increase in body weight by 60.40 % compared with the DSS-treated control group (P < 0.05). Administration of sulfasalazine (100 mg/kg b.w.) produced a non-significant increase in body weight in DSS-treated rats by 3.54 % when compared to the DSS-treated control group of rats.
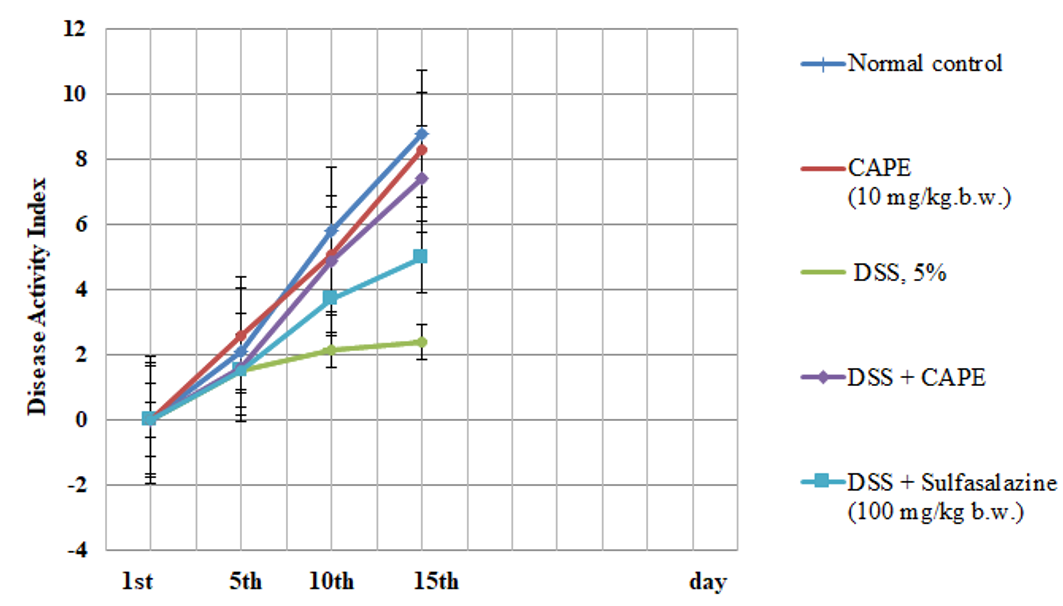
Effect of CAPE and sulfasalazine on disease activity index in rats with DSS-induced colitis
The results in Figure 1 indicate a non-significant change in the DAI of CAPE-treated rats (group II) as compared to normal control rats (group I). However, the present data show a significant change in the DAI score after DSS treatment compared with the DAI of the control group. Treatment with CAPE (10 mg/kg.b.w.) and sulfasalazine produced significantly improved DAI scores in DSS-treated rats as compared with the group treated with DSS alone (P < 0.05).
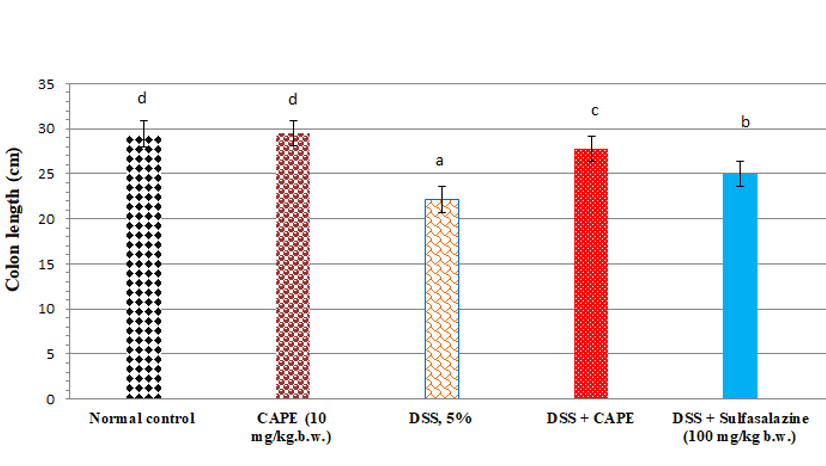
Effect of CAPE and sulfasalazine on colon length in DSS-treated rats
The colon length of CAPE-treated normal rats (group II) was non-significantly changed when compared with the normal rats (Figure 2). Compared with the normal group of rats, treatment with DSS (5%) resulted in a significant reduction in colon length by 24.7 %. Furthermore, administration of CAPE to the DSS-treated group produced a significant increase in colon length by 25.38 % when compared with the DSS-treated control group (P < 0.05). However, the administration of sulfasalazine to DSS-treated rats produced a significant increase in colon length by 12.78% compared with the DSS-treated control group (P < 0.05).
Effect of CAPE and sulfasalazine on blood PLT count and plasma NO, NF-kβ, and vitamin C in DSS-treated rats.
Administration of CAPE to normal rats produced non-significant changes in plasma PLT, NO, NF-kβ, and Vit. C when compared with normal rats (Table 4).
Compared with normal rats, DSS treatment (5%) resulted in a significant reduction in blood PLT count and plasma Vit. C levels by 19.89% and 28.11%, respectively, and a significant increase in plasma NO and NF-kβ by 234.84% and 333.30%, respectively (P < 0.05).
Additionally, compared with the DSS-treated control group, CAPE treatment resulted in significantly elevated PLT count and plasma Vit. C levels by 26.62% and 29.06%, respectively, and a significant decrease in plasma NO and NF-kβ by 59.81% and 61.25%, respectively (P < 0.05).
Sulfasalazine administration significantly increased blood PLT and plasma Vit. C levels by 3.96% and 10.4%, respectively, and produced a significant increase in plasma NO and NF-kβ by 39.27% and 31.62%, respectively, when compared with the DSS-treated control group (P < 0.05) (Table 4).
Effect of CAPE and sulfasalazine on colonic GSH, GPx, CAT, and SOD in DSS-treated rats
Administration of CAPE to normal rats produced nonsignificant changes in colon GSH, GPx, CAT, and SOD when compared with normal rats. However, compared with normal rats, DSS (5%) treatment resulted in a significant reduction in colon GSH, GPx, CAT, and SOD of 54.36%, 55.42%, 57.69%, and 45.51%, respectively. Furthermore, administration of CAPE to rats treated with DSS produced a significant increase in colon GSH, GPx, CAT, and SOD by 68.91%, 114.64%, 98.9%, and 49.27%, respectively, when compared with rats treated with DSS alone (P < 0.05) (Table 5).
Treatment of rats with sulfasalazine (100 mg/kg b.w.) produced a significant increase in colon GSH, GPx, CAT, and SOD by 27.93%, 53.45%, 56.29%, and 15.25%, respectively, compared with the DSS-treated control group (P < 0.05).
Effect of CAPE and sulfasalazine on colonic thiobarbituric acid-reactive substances (TBARs), interleukin 6 (IL-6), and interferon γ (INF-γ) in DSS-treated rats
Table 6 shows the effects of CAPE and sulfasalazine individually on colon TBARs, IL-6, and INF-γ in normal and DSS-treated rats. Oral administration of normal rats with CAPE showed nonsignificant changes in colonic TBAR, IL-6, and INF-γ when compared to normal rats. In contrast, oral administration of DSS (5%) resulted in a significant increase in colon levels of TBAR, IL-6, and INF-γ by 102.88%, 81.21%, and 256.09%, respectively (P < 0.05).
Compared with the DSS-treated control group, CAPE administration in the X group resulted in a significant reduction in colonic TBAR, IL-6, and INF-γ by 35.22%, 28.99%, and 56.10%, respectively. Furthermore, administration of sulfasalazine (100 mg/kg b.w.) significantly decreased colonic TBARs, IL-6, and INF-γ by 19.66%, 13.77% and 21.91%, respectively, when compared with the DSS-treated control group (P < 0.05).
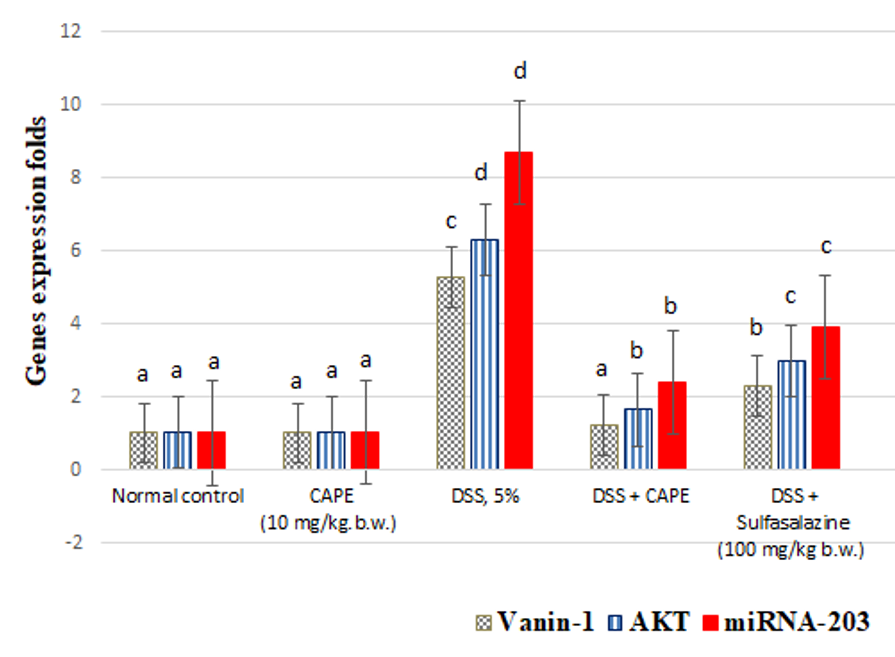
Effect of CAPE and sulfasalazine on colonic vanin-1, AKT, and miRNA-203 gene expression in DSS-treated rats
Figure 3 shows the individual effects of CAPE and sulfasalazine on the expression of the vnin-1, AKT and miRNA-203 genes in normal rats treated with DSS. Treatment of normal rats with CAPE (10 mg/kg.b.w.) produced a nonsignificant change in colon vanin-1, AKT, and miRNA-203 gene expression.
When compared with the normal control group, oral administration of DSS (5%) resulted in a significant increase in colon levels of vanin-1, AKT, and miRNA-203 gene expression by 416.66%, 511.65%, and 761.4%, respectively (P < 0.05).
However, compared with the DSS-treated control group, CAPE treatment resulted in a significant reduction in colonic expression of vanin-1, AKT, and miRNA-203 gene by 55.35%, 52.53%, and 72.41%, respectively. Furthermore, the administration of sulfasalazine to rats treated with DSS significantly decreased the expression of vanin-1, AKT, and miRNA-203 genes in the colon by 74.95%, 73.65%, and 55.17%, respectively, compared with the control group (P < 0.05).
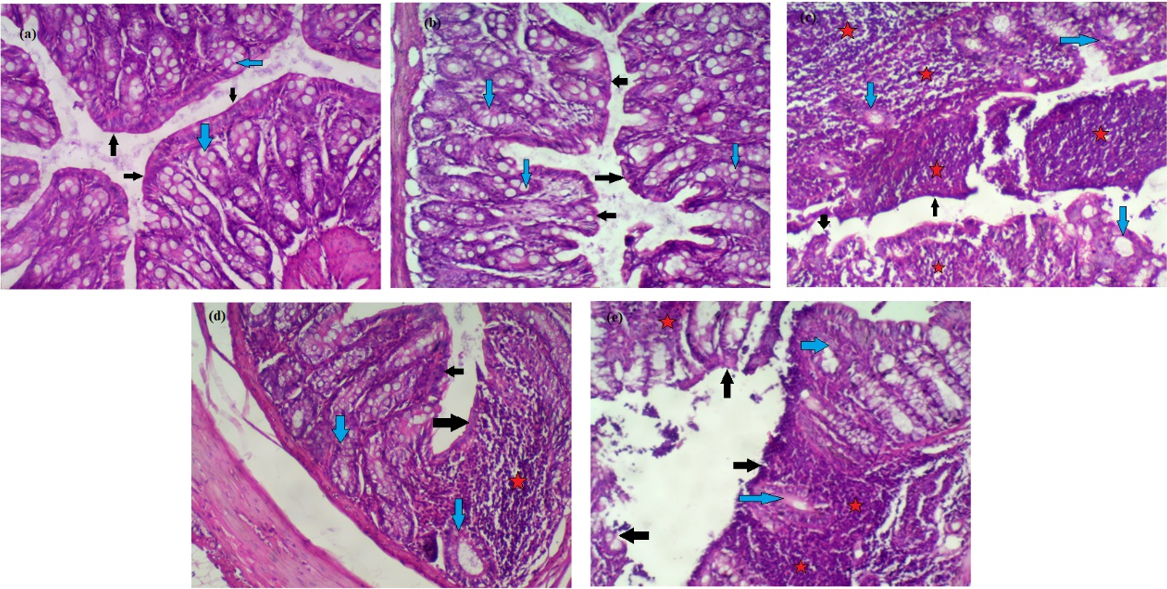
Effect of CAPE and sulfasalazine on histological alterations in colon tissues
Figure 4 (a-e) shows the individual effects of CAPE and sulfasalazine on colon histopathology of normal and DSS-treated rats.
In Figure 4 a&b, histopathological examination of normal and CAPE-treated groups (I&II) revealed intact surface epithelium (black arrows) and regular glands with adequate mucin production (blue arrows). The lamina propria did not show inflammatory cell aggregates.
In Figure 4c, colon histopathological examination of DSS-treated rats revealed surface erosions and ulcerations (black arrows). Many glands were replaced by inflammation, with mucin depletion (blue arrows). Many lymphoid aggregates (stars) are observed.
Furthermore, oral administration of CAPE to rats treated with DSS produced an intact surface epithelium (black arrows); mucin depletion was partially corrected (blue arrows), and few focal lymphoid aggregates were still observed (stars) compared with rats treated with DSS.
However, histological examination of rats treated with sulfasalazine/DSS showed a partly eroded surface epithelium (black arrows) with glandular mucin depletion in many glands (blue arrows); large lymphoid aggregates were still seen (stars).
Discussion
UC is believed to be a TH2-mediated inflammatory disease, while Crohn's disease is thought to be a TH1-mediated inflammatory disease48. In animal models, the inflammatory imitate UC mediated by TH1 and TH 2 was elevated49.
Numerous studies have shown that polyphenols and flavonoids can be used therapeutically to prevent invasion and metastasis of colorectal cancer cells50, 51.
In that study, we suggested that CAPE inhibited the expression of inflammatory mediators and biomarkers of oxidative stress. A recent study reported a previously unknown mechanism in which CAPE could inhibit invasion and migration by modulating the MMP-2 and MMP-9 signaling pathways52.
Effect of CAPE and sulfasalazine on the body weight of DSS-treated rats
In UC animal models, the degree of inflammation is measured by determining the daily change in body weight of the animals during each experiment and measuring the length of the resected colon53, 54. Although weight loss alone is a poor predictor of well-being55, it is still accepted that weight loss >20% is a criterion for euthanasia and an indication that the experimental design may be too aggressive55. The three primary symptoms of IBD in DSS-induced rats with colitis were considerable weight loss, bloody diarrhea, and shortening of the colon56, 57. In addition, in rats treated with DSS, colon shortening may be related to thickening due to edema and infiltration of inflammatory cells into the lamina propria and submucosa58.
Effect of CAPE and sulfasalazine on colon DAI score and length in DSS treated rats
In our investigation, treatment of rats with DSS for 15 days caused acute colitis with an elevated DAI score and a decreased colon weight/length ratio. A substantial correlation between the DAI score and inflammation in DSS-induced acute and chronic colitis has been reported by Bullich et al.57 Following treatment with CAPE, colon length, DAI score, and body weight improved. Many researchers have reported the antioxidant, anti-inflammatory, anticancer, prebiotic, immunomodulatory, and gastroprotective properties of polyphenols and their metabolites59, 60. CAPE treatment led to a marked reduction in the inflammatory infiltrate in both the lamina propria and the submucosa and protected against changes in colon length in a dose-dependent manner. Espíndola et al.52 showed that CAPE inhibits the release of inflammatory cytokines from human hepatocellular carcinoma cells.
Effect of CAPE and sulfasalazine on blood PLT count and plasma NO, NF-kβ, and Vit. C in DSS-treated rats
In the present study, we observed a depletion of blood PLT levels in DSS-treated rats. Our results were in line with those reported by Zamora et al.61 and Honjo et al.,62 who showed that UC patients had the lowest levels of PLT and Vita. C due to the production of inflammatory mediators NO and NF-kβ63 and the depletion of endogenous antioxidant biomarkers (i.e., Vit. C), which reduced the binding of PLTs to monocytes through the membrane, favoring an inflammatory response in UC patients with onset flare. The PLT changes in DSS-treated rats were restored upon treatment with CAPE and sulfasalazine. CAPE exhibited potent antioxidant activity by restoring the levels of PLT, NO, NF-kβ, and Vit. C. Our results are in line with the results of Gupta et al.64 and Nakashima et al.65, who noticed that phenolics significantly reduced levels of inflammatory mediators, thereby suppressing their inflammatory response in UC. Our results suggest that CAPE treatment decreases the expression of NO and NF-kβ, increases the production of Vit. C, and ameliorates intestinal mucosal barrier dysfunction in UC.
Effect of CAPE and sulfasalazine on colon GSH, GPx, CAT, and SOD in DSS-treated rats
In the present study, we noticed depletion of colon GSH, GPx, CAT, and SOD in DSS-treated rats. Our results were in line with the results reported by Zieliska et al.66, who observed a significant decrease in GSH, GPx, CAT, and SOD levels in patients with IBD when compared with controls. Our results indicate that the administration of CAPE significantly improves macroscopic damage, colon length, increases the activity of GPx, CAT, and SOD, depresses TBARs and NO levels, and increases GSH levels in the colon tissues of experimental colitis. Many articles have shown that the administration of flavonoids and polyphenols modulates the levels of antioxidant biomarkers and inflammatory mediators67, 68.
Effect of CAPE and sulfasalazine on colonic TBARs, IL-6, and INF-γ in DSS-treated rats
Our study indicated the elevation of TBAR, IL-6, and INF-γ levels in the colon tissues of experimental colitis. Yan et al.69 reported elevation of TBAR, IL-6, and INF-γ levels in colon tissue. These markers increase the inflammatory response of IL-6, INF-γ, NF-κB, destructive enzymes, and TBARs that cause damage to colon tissue70. In our study, CAPE significantly ameliorated tissue damage, IL-6, INF-γ, NF-κB, and TBARs in rats treated with DSS. Furthermore, our results indicate that DSS upregulates colon vanin-1 gene expression in DSS-treated rats. Our results were confirmed by the results of Gensollen et al.71, who reported vanin-1 upregulation in the intestinal tract in DSS-induced colitis. Our study suggests that elevation of vanin-1 gene expression in DSS-treated rats is a direct target for NF-κB in the colon, and this could be related to susceptibility to UC.
Effect of CAPE and sulfasalazine on colon vanin-1, AKT, and miRNA-203 gene expression in DSS-treated rats
Colon Akt gene expression was significantly upregulated in DSS-treated in rats. Our results were in line with a study by Li et al.72, who reported upregulation of Akt in DSS-treated rats. Furthermore, the Akt signaling pathway was significantly inhibited by CAPE administration, leading to the recovery of intestinal microbiota diversity. Our results indicated the downregulation of colon Akt gene expression by CAPE administration due to its inhibitory activity against colon NF-κB production in DSS-treated rats. Additionally, our study revealed upregulation of miRNA-203 gene expression in DSS-treated rats. Tian et al.73 studied the effect of inflammation on miRNAs and found that the mucosa of UC patients that was infiltrated with inflammatory cells had elevated miRNA levels, while the levels were reduced to non-inflammatory levels in patients under remission. Further investigation revealed the role of miRNA in suppressing inflammatory mediator genes within colonic epithelial cells, all implicated in IBD74. However, miR-203 downregulation was observed in DSS-treated rats after oral CAPE administration. An association between vanin-1 and AKT suggests that overexpressed vanin-1 decreases the extent of AKT74 phosphorylation. Furthermore, miR-203 can activate the Akt signaling pathway through IL-8 in the regulation of radioresistance in nasopharyngeal carcinoma cells75. Moreover, the AKT signaling pathway has been shown to be effective in preventing ventilator-induced lung injury76. Our findings suggest regulation of miR-203 gene expression due to inhibition of IL-6, INF-γ, and NF-κB production and vanin-1 and Akt gene expression by CAPE administration.
Our results support and indicate downregulation of colon vanin-1 gene expression in DSS-treated rats after CAPE administration for 15 days due to inhibition of colon NF-κB levels.
Effect of CAPE and sulfasalazine on histological changes in colon tissues
In rats treated with DSS, histological analysis of colon tissue indicated altered mucosal architecture and inflammation. Our observations of microscopic changes were consistent with a large body of research on DSS-induced UC models in rats77. CAPE inhibits changes in colon architecture and length and significantly reduces inflammatory infiltration in both the lamina propria and submucosa.
CAPE treatment significantly decreased colon injury and contributed to the anti‑inflammatory and anti‑apoptotic effects. Furthermore, the anti‑inflammatory and anti‑apoptotic activities of CAPE and its effects on vanin-1, AKT, and miRNA-203 gene expression in IBD rats has not been previously documented, and this study may be the first of its kind.
Conclusions
The current study used biochemical and molecular analysis to show that IBD is associated with increased levels of oxidative stress and apoptosis. The data show that IL-6, INF-α, and NF-kβ are actively involved in gut mucosal inflammation in DSS-treated rats. Furthermore, we found significant improvement in miRNA-203, vanin-1, and Akt gene expression in DSS-treated rats. The potential benefits of CAPE on colon lipid peroxidation, inflammatory mediators, and antioxidant systems led researchers to hypothesize that it would be a viable choice for treating IBD.
Abbreviations
AKT: Protein kinase B, CAPE: caffeic acid phenethyl ester, CAT: catalase, DAI: disease activity index, DSS: dextran sulfate sodium, GPx: glutathione peroxidase, GSH: reduced glutathione, IL-6: interleukine-6, INF-γ: interferon-γ, IBD: inflammatory bowel disease, NO: nitric oxide, NF-kβ: nuclear factor kappa, PLT: platelet count, SOD: superoxide dismutase, TBARs: thiobarbituric acid reactive substances, Vit. C: vitamin C.
Acknowledgments
None.
Author’s contributions
All authors contributed significantly to this work, read, and approved the final manuscript.
Funding
None.
Availability of data and materials
Supporting data, including analytical (colorimetric ELISA-PCR) data, will be available to demonstrate the hepatoprotective activity of morin against paracetamol-induced liver toxicity.
Ethics approval and consent to participate
Data collection was ethically approved by the Research Ethics Committee of the Faculty of Applied Medical Sciences, October 6 University, Egypt (No. 20210614). in vivo lab studies formed the basis of this research, no human volunteers were used.
Consent for publication
The authors granted their permission for their personal information to be used in the article.
Competing interests
The authors declare that they have no competing interests.
References
-
Salah
A.,
Hussein
A.,
Hassan
S.,
Hussein
M.A.,
Bassiouny
K.,
Green Synthesis of RES-CMCS: A Promising Modulator of the GLUT-4/Leptin Signaling Pathway in HFD-induced Insulin Resistance. Biomedical Research and Therapy.
2022;
9
(7)
:
5166-78
.
View Article Google Scholar -
Pan
T.,
Guo
H.Y.,
Zhang
H.,
Liu
A.P.,
Wang
X.X.,
Ren
F.Z.,
Oral administration of Lactobacillus paracasei alleviates clinical symptoms of colitis induced by dextran sulphate sodium salt in BALB/c mice. Beneficial Microbes.
2014;
5
(3)
:
315-22
.
View Article PubMed Google Scholar -
Li
G.,
Liu
Z.,
Ren
F.,
Shi
H.,
Zhao
Q.,
Song
Y.,
Alterations of gut microbiome and fecal fatty acids in patients with polycystic ovary syndrome in central China. Frontiers in Microbiology.
2022;
13
:
911992
.
View Article PubMed Google Scholar -
Zhou
L.,
Ni
Z.,
Yu
J.,
Cheng
W.,
Cai
Z.,
Yu
C.,
Correlation between fecal metabolomics and gut microbiota in obesity and polycystic ovary syndrome. Frontiers in Endocrinology (Lausanne).
2020;
11
:
628
.
View Article PubMed Google Scholar -
Cheng
L.,
Jin
H.,
Qiang
Y.,
Wu
S.,
Yan
C.,
Han
M.,
High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. International Immunopharmacology.
2016;
40
:
1-10
.
View Article PubMed Google Scholar -
Wang
K.,
Han
G.,
Dou
Y.,
Wang
Y.,
Liu
G.,
Wang
R.,
Opposite role of tumor necrosis factor receptors in dextran sulfate sodium-induced colitis in mice. PLoS One.
2012;
7
(12)
:
e52924
.
View Article PubMed Google Scholar -
Zhang
H.,
Sun
Y.,
Li
K.,
Zhang
J.,
Chen
X.,
Multiple lesions at different stages of pyoderma gangrenosum in a crohn's disease patient. Clinical, Cosmetic and Investigational Dermatology.
2022;
15
:
1593-6
.
View Article PubMed Google Scholar -
Laroui
H.,
Ingersoll
S.A.,
Liu
H.C.,
Baker
M.T.,
Ayyadurai
S.,
Charania
M.A.,
Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One.
2012;
7
(3)
:
e32084
.
View Article PubMed Google Scholar -
Piccinin
E.,
Cariello
M.,
De Santis
S.,
Ducheix
S.,
Sabbà
C.,
Ntambi
J.M.,
Role of oleic acid in the gut-liver axis: from diet to the regulation of its synthesis via stearoyl-CoA desaturase 1 (SCD1). Nutrients.
2019;
11
(10)
:
2283
.
View Article PubMed Google Scholar -
Mar-Solís
L.M.,
Soto-Domínguez
A.,
Rodríguez-Tovar
L.E.,
Rodríguez-Rocha
H.,
García-García
A.,
Aguirre-Arzola
V.E.,
Analysis of the Anti-Inflammatory Capacity of Bone Broth in a Murine Model of Ulcerative Colitis. Medicina (Kaunas, Lithuania).
2021;
57
(11)
:
1138
.
View Article PubMed Google Scholar -
Zhu
L.,
Gu
P.,
Shen
H.,
Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. International Immunopharmacology.
2019;
67
:
129-37
.
View Article PubMed Google Scholar -
Fang
W.,
Zhu
S.,
Niu
Z.,
Yin
Y.,
The protective effect of syringic acid on dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Development Research.
2019;
80
(6)
:
731-40
.
View Article PubMed Google Scholar -
Muthusami
S.,
Ramachandran
I.K.,
Babu
K.N.,
Krishnamoorthy
S.,
Guruswamy
A.,
Queimado
L.,
Role of inflammation in the development of colorectal cancer. Endocrine, Metabolic & Immune Disorders Drug Targets.
2021;
21
(1)
:
77-90
.
View Article PubMed Google Scholar -
Zhang
P.,
Tang
Y.,
Li
N.G.,
Zhu
Y.,
Duan
J.A.,
Bioactivity and chemical synthesis of caffeic acid phenethyl ester and its derivatives. Molecules (Basel, Switzerland).
2014;
19
(10)
:
16458-76
.
View Article PubMed Google Scholar -
Tolba
M.F.,
Omar
H.A.,
Azab
S.S.,
Khalifa
A.E.,
Abdel-Naim
A.B.,
Abdel-Rahman
S.Z.,
Caffeic Acid Phenethyl Ester: A Review of Its Antioxidant Activity, Protective Effects against Ischemia-reperfusion Injury and Drug Adverse Reactions. Critical Reviews in Food Science and Nutrition.
2016;
56
(13)
:
2183-90
.
View Article PubMed Google Scholar -
Zabaiou
N.,
Fouache
A.,
Trousson
A.,
Baron
S.,
Zellagui
A.,
Lahouel
M.,
Biological properties of propolis extracts: something new from an ancient product. Chemistry and Physics of Lipids.
2017;
207
:
214-22
.
View Article PubMed Google Scholar -
Turan
I.,
Demir
S.,
Misir
S.,
Kilinc
K.,
Mentese
A.,
Aliyazicioglu
Y.,
Cytotoxic Effect of Turkish Propolis on Liver, Colon, Breast, Cervix and Prostate Cancer Cell Lines. Tropical Journal of Pharmaceutical Research.
2015;
14
(5)
:
777-82
.
View Article Google Scholar -
Taslidere
E.,
Gul
M.,
Elbe
H.,
Cetin
A.,
Vardi
N.,
Ozyalin
F.,
The effects of caffeic acid phenethyl ester on streptozotocin-induced diabetic liver injury. Bratisl Lek Listy.
2016;
117
(5)
:
276-82
.
View Article PubMed Google Scholar -
Akyol
S.,
Ozturk
G.,
Ginis
Z.,
Armutcu
F.,
Yigitoglu
M.R.,
Akyol
O.,
In vivo and in vitro ant\ineoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutrition and Cancer.
2013;
65
(4)
:
515-26
.
View Article PubMed Google Scholar -
Armutcu
F.,
Akyol
S.,
Ustunsoy
S.,
Turan
F.F.,
Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review). Experimental and Therapeutic Medicine.
2015;
9
(5)
:
1582-8
.
View Article PubMed Google Scholar -
Ghorab
M.M.,
Ismail
Z.H.,
Abdalla
M.,
Synthesis and biological activities of some novel triazoloquinazolines and triazinoquinazolines containing benzenesulfonamide moieties. Arzneimittel-Forschung.
2010;
60
(2)
:
87-95
.
View Article PubMed Google Scholar -
Abdel-Gawad
S.M.,
Ghorab
M.M.,
El-Sharief
A.M.,
El-Telbany
F.A.,
Abdel-Alla
M.,
Design, synthesis, and antimicrobial activity of some new pyrazolo[3,4-d] pyrimidines. Heteroatom Chemistry.
2003;
14
(6)
:
530-4
.
View Article Google Scholar -
Abou-Taleb
N.I.,
Elblasy
O.A.,
Elbesoumy
E.A.,
Basuny
H.I.,
Elhamadi
E.A.,
Nasr eldin
M.S.,
Nasr eldin MS, Emara AA, Ali AA, Salem MA, Ahmed FM, Hussein MA. Mechanism of antiangiogenic and antioxidant activity of newly synthesized CAMBA in ehrlich ascites carcinoma-bearing mice. Asian Journal of Chemistry.
2021;
33
(10)
:
2465-71
.
View Article Google Scholar -
Borik
R.M.,
Hussein
M.A.,
A Novel Quinazoline-4-one Derivatives as a Promising Cytokine Inhibitors: Synthesis, Molecular Docking, and Structure-activity Relationship. Current Pharmaceutical Biotechnology.
2022;
23
(9)
:
1179-203
.
View Article PubMed Google Scholar -
Murtaza
G.,
Karim
S.,
Akram
M.R.,
Khan
S.A.,
Azhar
S.,
Mumtaz
A.,
Caffeic acid phenethyl ester and therapeutic potentials. BioMed Research International.
2014;
2014
:
145342
.
View Article PubMed Google Scholar -
Wang
G.,
Wang
J.,
Du
L.,
Li
M.,
Visualization-Based Discovery of Vanin-1 Inhibitors for Colitis. Frontiers in Chemistry.
2022;
9
:
809495
.
View Article PubMed Google Scholar -
Lu
P.,
Zhang
C.,
Fu
L.,
Wei
Y.,
Huang
Y.,
Wang
X.,
Near-Infrared Fluorescent Probe for Imaging and Evaluating the Role of Vanin-1 in Chemotherapy. Analytical Chemistry.
2021;
93
(29)
:
10378-87
.
View Article PubMed Google Scholar -
Ling
L.,
Lu
H.T.,
Wang
H.F.,
Shen
M.J.,
Zhang
H.B.,
MicroRNA-203 Acts as a Potent Suppressor in Septic Shock by Alleviating Lung Injury via Inhibition of VNN1. Kidney & Blood Pressure Research.
2019;
44
(4)
:
565-82
.
View Article PubMed Google Scholar -
Gobba
N.A.,
Ali
A. Hussein,
Sharawy
D.E. El,
Hussein
M.A.,
The potential hazardous effect of exposure to iron dust in Egyptian smoking and nonsmoking welders. Archives of Environmental & Occupational Health.
2018;
73
(3)
:
189-202
.
View Article PubMed Google Scholar -
Boshra
S.A.,
Hussein
M.A.,
Cranberry extract as a supplemented food in treatment of oxidative stress and breast cancer induced by N-Methyl-N-Nitrosourea in female virgin rats. International Journal of Phytomedicine.
2016;
8
(2)
:
217-27
.
-
Elgizawy
H.A.,
Ali
A.A.,
Hussein
M.A.,
Resveratrol: Isolation, and Its Nanostructured Lipid Carriers, Inhibits Cell Proliferation, Induces Cell Apoptosis in Certain Human Cell Lines Carcinoma and Exerts Protective Effect Against Paraquat-Induced Hepatotoxicity. Journal of Medicinal Food.
2021;
24
(1)
:
89-100
.
View Article PubMed Google Scholar -
Hussein
M.A.,
Synthesis of some novel triazoloquinazolines and triazinoquinazolines and their evaluation for anti-inflammatory activity. Medicinal Chemistry Research.
2012;
21
(8)
:
1876-86
.
View Article Google Scholar -
Abdel Maksoud
H.A.,
Elharrif
M.G.,
Mahfouz
M.K.,
Omnia
M.A.,
Abdullah
M.H.,
Eltabey
M.E.,
Biochemical study on occupational inhalation of benzene vapours in petrol station. Respiratory Medicine Case Reports.
2019;
27
:
100836
.
View Article PubMed Google Scholar -
Gizawy
Heba A El,
Abo-Salem
Heba M,
Ali
Ali A,
Hussein
Mohammed A,
Phenolic Profiling and Therapeutic Potential of Certain Isolated Compounds from Parkia roxburghii against AChE Activity as well as GABAA α5, GSK-3β, and p38α MAP-Kinase Genes. ACS omega.
2021;
6
(31)
:
20492-20511
.
-
Borik
R.M.,
Hussein
M.A.,
Synthesis, molecular docking, biological potentials, and structure activity relationship of new quinazoline and quinazoline-4-one derivatives. Asian Journal of Chemistry.
2021;
33
(2)
:
423-38
.
View Article Google Scholar -
Li
M.,
Wang
X.F.,
Shi
J.J.,
Li
Y.P.,
Yang
N.,
Zhai
S.,
Caffeic acid phenethyl ester inhibits liver fibrosis in rats. World Journal of Gastroenterology.
2015;
21
(13)
:
3893-903
.
View Article PubMed Google Scholar -
Hussein
M.A.,
Ismail
N.E.,
Mohamed
A.H.,
Borik
R.M.,
Ali
A.A.,
Mosaad
Y.O.,
Plasma Phospholipids: A Promising Simple Biochemical Parameter to Evaluate COVID-19 Infection Severity. Bioinformatics and Biology Insights.
2021;
15
:
11779322211055891
.
View Article PubMed Google Scholar -
Bang
B.,
Lichtenberger
L.M.,
Methods of Inducing Inflammatory Bowel Disease in Mice. Curr Protoc Pharmacol.
2016;
72
:
5.58.1-5.58.42
.
View Article Google Scholar -
Kitamura
Y.,
Suzuki
M.,
Tsukioka
T.,
Isobe
K.,
Tsujino
T.,
Watanabe
T.,
Spectrophotometric determination of platelet counts in platelet-rich plasma. International Journal of Implant Dentistry.
2018;
4
(1)
:
29
.
View Article PubMed Google Scholar -
Metgud
R.,
Anandani
C.,
Singh
K.,
Estimation of salivary nitric oxide in oral precancer patients. Biotechnic & Histochemistry.
2015;
90
(4)
:
302-8
.
View Article PubMed Google Scholar -
Nagib
M.M.,
Tadros
M.G.,
ElSayed
M.I.,
Khalifa
A.E.,
Anti-inflammatory and anti-oxidant activities of olmesartan medoxomil ameliorate experimental colitis in rats. Toxicology and Applied Pharmacology.
2013;
271
(1)
:
106-13
.
View Article PubMed Google Scholar -
Gao
B.,
Saralamba
S.,
Lubell
Y.,
White
L.,
Dondorp
A.M.,
Aguas
R.,
Determinants of MDA impact and designing MDAs towards malaria elimination. Elife.
2020;
15
(9)
:
e51773
.
View Article Google Scholar -
Owen
J.B.,
Butterfield
D.A.,
Measurement of oxidized/reduced glutathione ratio. Methods in Molecular Biology (Clifton, N.J.).
2010;
648
:
269-77
.
View Article PubMed Google Scholar -
Bresciani
G.,
da Cruz
I.B.,
González-Gallego
J.,
Manganese superoxide dismutase and oxidative stress modulation. Advances in Clinical Chemistry.
2015;
68
:
87-130
.
View Article PubMed Google Scholar -
Glorieux
C.,
Zamocky
M.,
Sandoval
J.M.,
Verrax
J.,
Calderon
P.B.,
Regulation of 303 catalase expression in healthy and cancerous cells. Free Radical Biology and 304 Medicine.
2015;
87
:
84-97
.
View Article Google Scholar -
Alturkistani
H.A.,
Tashkandi
F.M.,
Mohammedsaleh
Z.M.,
Histological Stains: A Literature Review and Case Study. Global Journal of Health Science.
2015;
8
(3)
:
72-9
.
View Article PubMed Google Scholar -
SPSS. 2018. (SPSS 20), Inc., Chicago, IL, USA. 2018
.
-
Chapuy
L.,
Bsat
M.,
Rubio
M.,
Harvey
F.,
Motta
V.,
Schwenter
F.,
Transcriptomic analysis and high-dimensional phenotypic mapping of mononuclear phagocytes in mesenteric lymph nodes reveal differences between ulcerative colitis and crohn's disease. Journal of Crohn's and Colitis.
2020;
14
(3)
:
393-405
.
View Article PubMed Google Scholar -
Duran
N.E.,
Hommes
D.W.,
Stem cell-based therapies in inflammatory bowel disease: promises and pitfalls. Therapeutic Advances in Gastroenterology.
2016;
9
(4)
:
533-47
.
View Article PubMed Google Scholar -
Geng
J.,
Fan
J.,
Ouyang
Q.,
Zhang
X.,
Zhang
X.,
Yu
J.,
Loss of PPM1A expression enhances invasion and the epithelial-to-mesenchymal transition in bladder cancer by activating the TGF-β/Smad signaling pathway. Oncotarget.
2014;
5
(14)
:
5700-11
.
View Article PubMed Google Scholar -
Ji
Q.,
Liu
X.,
Fu
X.,
Zhang
L.,
Sui
H.,
Zhou
L.,
Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One.
2013;
8
(11)
:
e78700
.
View Article PubMed Google Scholar -
Espíndola
K.M.,
Ferreira
R.G.,
Narvaez
L.E.,
Silva Rosario
A.C.,
da Silva
A.H.,
Silva
A.G.,
Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Frontiers in Oncology.
2019;
9
:
541
.
View Article PubMed Google Scholar -
Jeoung
B.,
lee Kd, na cS, Kim Ye, Kim B, Kim Yr. Ganghwaljetongyeum, an anti-arthritic remedy, attenuates synoviocyte proliferation and reduces the production of proinflmmatory mediators in macrophages: the therapeutic effect of GHJTY on rheumatoid arthritis. BMC Complementary and Alternative Medicine.
2013;
13
:
47
.
View Article PubMed Google Scholar -
O'Connor
J.W.,
Gomez
E.W.,
Biomechanics of TGFβ-induced epithelial-mesenchymal transition: implications for fibrosis and cancer. Clinical and Translational Medicine.
2014;
3
(65)
:
23-34
.
View Article PubMed Google Scholar -
Lee
J.,
Fecal calprotectin in inflammatory bowel disease. Korean J Gastroenterol.
2016;
67
(5)
:
233-237
.
View Article Google Scholar -
Uko
V.,
Thangada
S.,
Radhakrishnan
K.,
Liver disorders in inflammatory bowel disease. Gastroenterology Research and Practice.
2012;
2012
(2)
:
642923
.
View Article PubMed Google Scholar -
Bullich
C.,
Keshavarzian
A.,
Garssen
J.,
Kraneveld
A.,
Perez-Pardo
P.,
Gut vibes in parkinson's disease: the microbiota-gut-brain axis. Movement Disorders Clinical Practice (Hoboken, N.J.).
2019;
6
(8)
:
639-51
.
View Article PubMed Google Scholar -
da Silva
L.M.,
de Souza
P.,
Jaouni
S.K.,
Harakeh
S.,
Golbabapour
S.,
de Andrade
S.F.,
Propolis and its potential to treat gastrointestinal disorders. Evidence-Based Complementary and Alternative Medicine.
2018;
2018
:
2035820
.
View Article PubMed Google Scholar -
Chiu
H.F.,
Venkatakrishnan
K.,
Golovinskaia
O.,
Wang
C.K.,
Gastroprotective effects of polyphenols against various gastro-intestinal disorders: A mini-review with special focus on clinical evidence. Molecules (Basel, Switzerland).
2021;
26
(7)
:
2090
.
View Article PubMed Google Scholar -
Santino
A.,
Scarano
A.,
De Santis
S.,
De Benedictis
M.,
Giovinazzo
G.,
Chieppa
M.,
Gut microbiota modulation and anti-inflammatory properties of dietary polyphenols in IBD: New and Consolidated Perspectives. Current Pharmaceutical Design.
2017;
23
(16)
:
2344-51
.
View Article PubMed Google Scholar -
Zamora
C.,
Canto
E.,
Nieto
J.C.,
Garcia-Planella
E.,
Gordillo
J.,
Ortiz
M.A.,
Inverse association between circulating monocyte-platelet complexes and inflammation in ulcerative colitis patients. Inflammatory Bowel Diseases.
2018;
24
(4)
:
818-28
.
View Article PubMed Google Scholar -
Honjo
T.,
Toyota
K.,
Kanada
M.,
Itoh
T.,
Vitamin C enema advances induction of remission in the dextran sodium sulfate-induced colitis model in rats. Journal of Nutritional Science and Vitaminology.
2021;
67
(2)
:
91-8
.
View Article PubMed Google Scholar -
Kurtovic
J.,
Segal
I.,
Recent advances in biological therapy for inflammatory bowel disease. Tropical Gastroenterology.
2004;
25
(1)
:
9-14
.
PubMed Google Scholar -
Gupta
R.A.,
Motiwala
M.N.,
Mahajan
U.N.,
Sabre
S.G.,
Protective effect of Sesbania grandiflora on acetic acid induced ulcerative colitis in mice by inhibition of TNF-α and IL-6. Journal of Ethnopharmacology.
2018;
219
:
222-32
.
View Article PubMed Google Scholar -
Nakashima
T.,
Maeda
T.,
Nagamoto
H.,
Kumakura
T.,
Takai
M.,
Mori
T.,
Rebamipide enema is effective for treatment of experimental dextran sulfate sodium induced colitis in rats. Digestive Diseases and Sciences.
2005;
50
(S1)
:
124-31
.
View Article PubMed Google Scholar -
Zielińska
A.K.,
Sa\laga
M.,
Siwiński
P.,
W\lodarczyk
M.,
Dziki
A.,
Fichna
J.,
Oxidative stress does Not influence subjective pain sensation in inflammatory bowel disease patients. Antioxidants.
2021;
10
(8)
:
1237
.
View Article PubMed Google Scholar -
Xing
J.F.,
Sun
J.N.,
Sun
J.Y.,
You
C.Y.,
Dong
K.,
Lv
J.,
Protective effects of 3,4-oxo-isopropylidene-shikimic acid on experimental colitis induced by trinitrobenzenesulfonic acid in rats. Digestive Diseases and Sciences.
2012;
57
(8)
:
2045-54
.
View Article PubMed Google Scholar -
Vrdoljak
J.,
Kumric
M.,
Ticinovic Kurir
T.,
Males
I.,
Martinovic
D.,
Vilovic
M.,
Effects of wine components in inflammatory bowel diseases. Molecules (Basel, Switzerland).
2021;
26
(19)
:
5891
.
View Article PubMed Google Scholar -
Yan
Y.,
Wang
P.,
Sun
Y.,
Dong
Y.,
Xing
J.,
Potential mechanisms of 3, 4-Oxo-isopropylidene-shikimic acid in ameliorating 2, 4, 6-trinitrobenzenesulfonic acid-induced colitis in rats. Journal of Interferon & Cytokine Research.
2019;
39
(9)
:
554-63
.
View Article PubMed Google Scholar -
Vrdoljak
J.,
Kumric
M.,
Vilovic
M.,
Martinovic
D.,
Tomic
I.J.,
Krnic
M.,
Effects of olive oil and its components on intestinal inflammation and inflammatory bowel disease. Nutrients.
2022;
14
(4)
:
757
.
View Article PubMed Google Scholar -
Gensollen
T.,
Bourges
C.,
Rihet
P.,
Rostan
A.,
Millet
V.,
Noguchi
T.,
Functional polymorphisms in the regulatory regions of the VNN1 gene are associated with susceptibility to inflammatory bowel diseases. Inflammatory Bowel Diseases.
2013;
19
(11)
:
2315-25
.
View Article PubMed Google Scholar -
Li
M.Y.,
Luo
H.J.,
Wu
X.,
Liu
Y.H.,
Gan
Y.X.,
Xu
N.,
Anti-inflammatory effects of huangqin decoction on dextran sulfate sodium-induced ulcerative colitis in mice through regulation of the gut microbiota and suppression of the ras-PI3K-Akt-HIF-1α and NF-κB pathways. Frontiers in Pharmacology.
2020;
10
:
1552
.
View Article PubMed Google Scholar -
Tian
Y.,
Xu
J.,
Li
Y.,
Zhao
R.,
Du
S.,
Lv
C.,
MicroRNA-31 Reduces inflammatory signaling and promotes regeneration in colon epithelium, and delivery of mimics in microspheres reduces colitis in mice. Gastroenterology.
2019;
156
(8)
.
View Article PubMed Google Scholar -
Chen
S.,
Zhang
W.,
Tang
C.,
Tang
X.,
Liu
L.,
Liu
C.,
Vanin-1 is a key activator for hepatic gluconeogenesis. Diabetes.
2014;
63
(6)
:
2073-85
.
View Article PubMed Google Scholar -
Qu
J.Q.,
Yi
H.M.,
Ye
X.,
Zhu
J.F.,
Yi
H.,
Li
L.N.,
MiRNA-203 Reduces nasopharyngeal carcinoma radioresistance by targeting IL8/AKT signaling. Molecular Cancer Therapeutics.
2015;
14
(11)
:
2653-64
.
View Article PubMed Google Scholar -
Yan
X.,
Li
W.,
Yang
L.,
Dong
W.,
Chen
W.,
Mao
Y.,
MiR-135a Protects vascular endothelial cells against ventilator-induced lung Injury by inhibiting PHLPP2 to activate PI3K/Akt pathway. Cellular Physiology and Biochemistry.
2018;
48
(3)
:
1245-58
.
View Article PubMed Google Scholar -
Huang
T.C.,
Tsai
S.S.,
Liu
L.F.,
Liu
Y.L.,
Liu
H.J.,
Chuang
K.P.,
Effect of Arctium lappa L. in the dextran sulfate sodium colitis mouse model. World Journal of Gastroenterology.
2010;
16
(33)
:
4193-9
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 9 (2022)
Page No.: 5313-5325
Published on: 2022-09-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5738 times
- PDF downloaded - 1530 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress