Abstract
Introduction: In recent years, green synthesis of silver nanoparticles using natural products has been increasingly utilized in the biomedical field as a therapeutic approach for managing neurodegenerative diseases. This study aimed to determine the effects of silver nanoparticles synthesized using Tualang honey (THSN) on seizure activity and locomotor and memory functions in rats after kainic acid (KA) induction.
Methods: Male Sprague Dawley rats were randomly divided into six groups (n = 6/group), and each group was pre-treated orally with either distilled water or THSN (10 mg/kg or 50 mg/kg), according to their respective groups. Each rat was injected subcutaneously with KA (15 mg/kg) or saline after the last pre-treatment, and the onset of the first generalized seizure was recorded. After 24 hours and five days of KA induction, an open field test (OFT) and a novel object recognition test (NORT) were performed before they were sacrificed.
Results: THSN pre-treatment of KA-induced status epilepticus groups demonstrated an increment in latencies to the onset of the first generalized seizure and the number of line crossings in OFT, with a higher recognition index of NORT compared to the untreated KA-induced status epilepticus group.
Conclusion: THSN could have neuroprotective effects in ameliorating seizures, locomotor activity, and memory function after KA-induced status epilepticus in male rats.
Introduction
Seizures are a common neurological disorder associated with epilepsy, which affects more than 2% of the population worldwide1. Excitotoxic stimulation of glutamate receptors results in an excessive, hyperexcitable state of neurons, which can lead to status epilepticus2. Seizures may have an impact on cellular processes as well as synaptic plasticity, such as long–term potentiation (LTP). LTP refers to persistent changes in synaptic efficacy and plays a vital role in cellular processes necessary for learning and memory3. Repeated seizures have been shown to cause impairment of LTP-associated molecular mechanisms and saturation of synaptic responses4, potentially impacting memory function5.
Several studies have shown that an animal model of seizure induced by kainic acid (KA) administration was associated with behavioral alteration and anxiety6, 7. These abnormalities in behavior could be linked to lesions in the amygdala and hippocampus (i.e., fear expression networks)8, 9. Damage to these networks could reduce anxiety or increase impulsive, inadaptive behavior due to an incorrect interpretation of threatening circumstances8.
KA, isolated and extracted from red algae (Digenea simplex)10, is a potent analog of glutamate. KA has 30-fold neurotoxicity potential compared to glutamate11 and is widely used as a chemical neurotoxicant to investigate the mechanism involved in excitotoxicity in animal experimentation12, 13. KA receptors, which are a subtype of the ionotropic glutamate receptor family, are highly expressed in numerous parts of the brain, including the hippocampus14, which is crucially involved in learning and memory processes15.
Over the past few decades, silver nanoparticles have received significant attention due to their great stability, high bioavailability, and ability to easily cross the blood–brain barrier. In addition, they can function as antimicrobial16, anti-inflammatory17, and neuroprotective18 agents. Recently, there has been emerging research interest in the plant-mediated green synthesis of silver nanoparticles due to their cost-effectiveness, environment-friendliness, and low toxicity profile compared to their hazardous chemical-mediated counterparts19. Few strategies have been explored to enhance honey’s absorption and bioavailability, including the development of silver nanoparticles synthesized using Tualang honey (TH) formulations20, 21. In this study, we used Tualang honey–mediated silver nanoparticles (THSN) to increase the bioactivity of the substance in the rat’s brain.
Our previous work showed that nanoparticles derived from THSN possess high antioxidant activity and ferric/reducing antioxidant power, with an average size of 22 nm, which most likely improves its bioavailability in the body21. However, we have not studied the efficacy of THSN in vivo. Hence, the present research aimed to explore the possible neuroprotective effects of THSN in a KA-induced status epilepticus in vivo rat model, looking specifically at seizure and locomotor activity, as well as memory function following KA administration.
Methods
Animals
Male Sprague Dawley rats weighing between 200 and 250 g (8–10 weeks old) were acquired from the Animal Research and Service Centre (ARASC) at Universiti Sains Malaysia (USM) Health Campus. The animals were acclimatized for one week at a temperature of 25 ± 2 °C with a 12:12 hour light–dark cycle and provided with food and water ad libitum. All procedures were carried out in accordance with the guidelines approved by the Animal Ethics Committee of USM [USM/IACUC/2018/(111)(904)].
Preparation of THSN
TH was purchased from the Federal Agricultural Marketing Authority (FAMA), Kelantan, Malaysia. THSN was prepared via the green synthesis method. The synthesis and characterization of THSN are reported in our previous preliminary study21. The THSN was formulated in powder form and dissolved in 0.5 mL of distilled water before each use.
Design of experimental groups
A total of 72 male rats were randomized into two major groups (24 hours and five days), and each group contained six subgroups (n = 6). Each subgroup was pre-treated five times at 12 hours intervals:
Group 1: Control – Rats were pre-treated orally with distilled water.
Group 2: THSN 10 mg – Rats were pre-treated orally with THSN (10 mg/kg).
Group 3: THSN 50 mg – Rats were pre-treated orally with THSN (50 mg/kg).
Group 4: KA alone – Rats were pre-treated orally with distilled water.
Group 5: KA + THSN 10 mg – Rats were pre-treated orally with THSN (10 mg/kg).
Group 6: KA + THSN 50 mg – Rats were pre-treated orally with THSN (50 mg/kg).
The THSN dosages used in the present study were based on earlier reports22, 23. A recent study demonstrated that a daily dosage of 10 mg/kg of silver nanoparticles (low dose) of Azadirachta indica extract might be safer for rats24. Therefore, the current study used THSN at 10 mg/kg (low dose) and 50 mg/kg (high dose) to compare their effects on KA-induced status epilepticus in rats.
KA administration and seizure development
KA (15 mg/kg) or saline was injected subcutaneously (s.c.) into the rats 30 minutes after the last oral treatment of the respective groups. Following KA administration, each rat was placed in an individual cage, and their seizures were observed for 3–4 hours. A six-stage rating scale for seizure development in rats’ behavior was recorded as categorized by the previous study25. To minimize mortality, diazepam (10 mg/kg; Atlantic Laboratories Corp. Ltd., Thailand) was injected intraperitoneally 90 minutes after the onset of the first generalized seizure (FGS) began26, whereas animals in the control groups received an equivalent amount of saline.
Open field test (OFT)
The OFT was performed to assess the behavioral changes in rats’ locomotor activity, according to Sairazi et al. (2017). OFT is widely used in animal models of anxiety-like behavior6, 7. The animals were tested at 24 hours and five days post-KA induction. Each rat was positioned at the center of 25 equally sized squares of the OFT apparatus (40 cm height x 90 cm length x 90 cm width and surrounded by a white paper wall). Each rat was free to explore the area for five minutes, and the locomotor activity was recorded using an overhead camera (placed 100 cm above the box). After each trial, the equipment was cleaned with 30% ethanol to prevent bias from the smell of the previous animal. The locomotor activity of animals was evaluated based on the frequency of line crossings in the OFT apparatus. The animals’ behavior was analyzed by an observer blinded to the experimental groups.
Novel object recognition test (NORT)
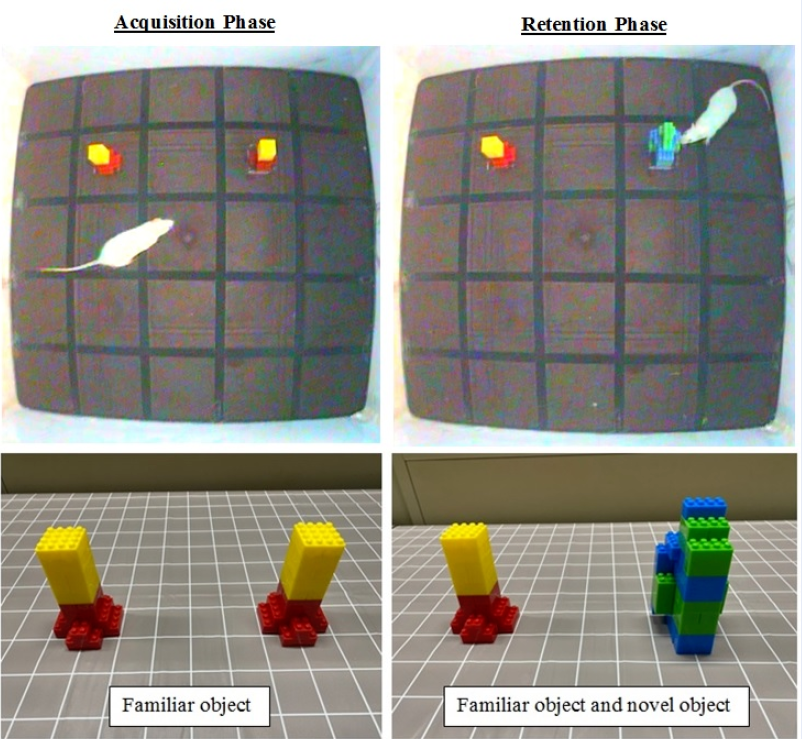
The NORT was performed to evaluate the rats’ cognitive and memory functions, according to Wang et al. (2016)27. Each rat was placed in an empty open field (40 cm height x 90 cm length x 90 cm width) with no object for 10 minutes/day for two consecutive days. The open field was used for the acquisition and retention phase. Two familiar objects (A1 and A2) were placed in the field during the acquisition phase, and each rat was permitted to explore them freely for five minutes. Their behaviors were recorded using a video camera (Sony, DCR-SX44E), and the time used to explore was documented. Exploration was described as pointing the snout toward the object, sniffing, or touching with the snout. The acquisition phase was conducted before the KA was administered.
Subsequently, retention was tested 24 hours and five days after the acquisition phase and KA administration. One of the objects used in the retention phase was substituted by a different object (novel object), and each rat was permitted to explore them for five minutes. The objects, which varied in shape and color and were made of plastic (Figure 1), were fixed on the floor. The objects were cleaned with 30% ethanol before each test to ensure the absence of olfactory cues.
A familiarity index (time spent on object A1 or A2 / total time exploring A1 and A2) was calculated during the acquisition phase. A score of 0.5 indicates that neither object was preferred. Additionally, the total exploration time of the familiar and novel objects was recorded for the retention phase, and the recognition index was calculated (time spent on the novel object / total time exploring novel and familiar objects). A recognition index of greater than 0.5 suggests a preference for the novel object.
Statistical analysis
IBM SPSS software (Version 26, Chicago, USA) was used to analyze the results. The datasets were subjected to normality and homogeneity of variance analysis using Levene’s test. A parametric test was used to analyze data with a normal distribution and equal variance. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for multiple pairwise comparisons28. All values were expressed as mean ± standard error of the mean (SEM). The differences were considered statistically significant at p < 0.05.
| Stage | Description |
|---|---|
| 1 | Staring stage – Animals crouches on all limbs, immobilized, staring, and appearing vigilant but not responding to any stimuli (5 – 15 minutes after KA administration). |
| 2 | Wet dog shakes stage – Animals develop behavioural automatisms (e.g. wet dog shakes), which later intensify. |
| 3 | Hyperactive stage – Animals display hyperactivity, including frequent forelimb movement, repeated head nodding, increasing intervals of walking and chewing. |
| 4 | Rearing stage – Onset of the FGS. Animals rear up on a hind limb, accompanied by salivation and forelimb clonic jerks. Then, animals develop more frequent and prolonged rearing with increased forelimb clonic jerks and salivation (1 – 2 hours after KA administration). |
| 5 | Rearing and falling stage – Animals lose balance while rearing and display frequent forelimb clonic jerks and salivation. |
| 6 | Jumping stage – Animals display jumping, circling, rolling, intense agitation, and wild running. These symptoms are often accompanied by death. |
| Groups | Number of line crossing | |
|---|---|---|
| 24 h | 5 days | |
| Control | 81.17 ± 11.83 | 95.17 ± 8.25 |
| THSN 10 mg | 73.33 ± 11.15 | 114.83 ± 5.17 |
| THSN 50 mg | 67.50 ± 8.14 | 110.50 ± 11.98 |
| KA alone | 6.83 ± 4.39 a,b,c | 175.50 ± 20.20 a,b |
| KA + THSN 10 mg | 36.83 ± 13.04 | 190.75 ± 19.50 a,b |
| KA + THSN 50 mg | 34.83 ± 16.58 | 123.17 ± 16.22 |
Results
Seizure activity and FGS onset
The KA administration (15 mg/kg body weight; s.c.) resulted in an epilepticus seizure in all KA-treated rats. The seizure most commonly began 1–4 hours after the KA injection. The progressive motor seizures in all KA-treated rats began with staring spells during which the animals appeared to be in motion arrest. Subsequently, the animals exhibited wet dog shakes that became progressively more frequent. The animals then displayed hyperactive behavior that included frequent head nodding, constant walking, and chewing intervals. They then began to rear up on their hind limbs, which progressed to frequent and protracted rearing, followed by forelimb clonic jerks and salivation (FGS stage). The rats then began to fall or lose their equilibrium while rearing. These rearing and falling episodes persisted until the rats were injected intraperitoneally with diazepam (10 mg/kg) around 90 minutes after the FGS started. Animals in the control group (saline, s.c.) showed no seizure activity and continued to behave normally, such as walking, sniffing, grooming, and exploring. The stages of the rating scale for seizure development were recorded (Table 1).
The KA-treated rats began to show the first generalized behavior seizure (stage 4) within 60 – 90 minutes. All animals in the pre-treatment showed significant differences in the onset of the FGS [F (2,33) = 18.905, p < 0.01; p = 0.000] in the KA + THSN 10 mg and KA + THSN 50 mg groups compared to the KA alone group. These results suggest that the pre-treatment with THSN might have anticonvulsant activity by showing longer latencies to the FGS. All KA-treated rats in pre-treatment groups showed no significant differences between each other (p > 0.05; Figure 2).
Number of line crossings in OFT
The number of line crossings during OFT was significantly different between the groups at 24 hours post-KA induction [F (5, 30) = 6.173, p < 0.01; p = 0.000; Table 2]. The post hoc test revealed that the number of line crossings was reduced significantly (p < 0.05) for the KA alone group compared to the control, THSN 10 mg, and THSN 50 mg groups. This finding indicated a decrease in animal locomotor activities 24 hours post-KA administration. At five days post-KA induction, all KA-treated rat groups, except for the KA + THSN 50 mg group, showed a significantly higher number of line crossings compared to the control group [F (5, 30) = 11.699, p < 0.01; p = 0.000; Table 2]. Contrary to the 24 hours post-KA induction results, the rats’ locomotor activities increased five days after KA administration compared to the control group.
Recognition memory performance in NORT
In the acquisition phase of the NORT, no significant differences existed (p > 0.05) in the familiarity index between all groups at both times (Figure 3). This result indicated that all rats had no preference for either left or right objects. Interestingly, in the retention phase among the 24-hours subgroups, the recognition index for the novel object in the KA alone group was significantly lower (p < 0.05) compared to the control, THSN 10 mg, and THSN 50 mg groups. The recognition index in the KA + THSN 10 mg and KA + THSN 50 mg groups was also significantly higher (p < 0.05) than that of the KA alone group (Figure 4). In the five-days subgroups, the recognition index for the novel object in the KA alone group was significantly lower (p < 0.05) compared to the control and THSN 10 mg groups, whereas the KA + THSN 10 mg and KA + THSN 50 mg groups displayed a significantly higher recognition index (p < 0.05) compared to the KA alone group. A higher recognition index represents a longer time spent with the rats’ snout directed to the novel object. The differences between control, pre-treatment, and KA-treated rats with pre-treatments were not significant (p > 0.05) at both times (Figure 4).
Discussion
In the current study, the administration of KA (15 mg/kg; s.c.) was demonstrated to induce seizures in rats, possibly via suppressing gamma-aminobutyric acid (GABA) and enhancing glutamate hyperactivity29. KA was selected because it is a potent neurotoxic analog of excitotoxic glutamate and an agonist of the kainate subtype of ionotropic glutamate receptors, which causes neuronal depolarization and seizures, specifically targeting the hippocampus12, 30. In addition, earlier studies reported that administering KA (15 mg/kg) to rats caused severe behavioral disorders and cognitive impairment, elevated glutamate levels, microglial activation, and increased neuronal loss in the brain26, 31.
The current study showed that all KA-treated rats pre-treated with THSN (10 mg/kg and 50 mg/kg) in both the 24-hours and five-days subgroups had longer latencies to the onset of the FGS compared to the KA alone group, suggesting a potential anticonvulsant property of THSN. The anticonvulsant effect of THSN could be explained due to its constituents that may be involved in this action by the binding inhibition between KA and glutamate receptors. TH, a reducing agent used to synthesize the silver nanoparticles, contains various chemical compounds such as acids, aldehydes, alcohol, ketones, terpenes, hydrocarbons, and furan derivatives32, as well as phytochemical compounds33. THSN has been reported to contain alcohols, phenols, amides, carboxylate ions, and protein and exhibit high antioxidant activity21. The presence of antioxidant components (e.g., flavonoids) may be responsible for the anticonvulsant properties exhibited by silver nanoparticles34 by acting as benzodiazepine-like molecules in the central nervous system and altering GABA-generated chloride currents in a seizure model35, 36. Previous studies have also shown that silver nanoparticles using a similar method of green synthesis demonstrated anti-epileptic properties in a rat model37, 38. Additionally, other nanoparticles of polyphenol from the tea plant (epigallocatechine-3-gallate) possess an anticonvulsant activity, evidenced by a reduction in the number of epileptic episodes and intensity of the seizure pattern39. These positive results can be attributed to its high antioxidant capacity, high bioavailability, and stability40, 41, 42.
In addition to seizures, the KA administration also induced some alterations in locomotor activity43. The OFT results demonstrated that KA administration induced anxiety in rats after 24 hours. According to Chan et al. (2017), the lower locomotor activity in the open-field arena indicates a higher level of anxiety-like behavior44. The imbalance between excitatory (glutamate) and inhibitory (GABA) synaptic activities in the brain might be the main contributor to modulating anxiety responses45, 46. The current findings demonstrated that THSN improved the locomotor activity in the OFT, suggesting potential anxiolytic action and antidepressant properties. Similarly, a previous study showed that treatment with silver nanoparticles at a dose of 10 mg/kg exhibited an anxiolytic effect in mice47. Another study also reported that animals treated with silver nanoparticles became more active and showed increased locomotor activity in the OFT48. The present findings revealed that THSN increased the rats’ locomotor activity with time. However, the pre-treatment of THSN failed to produce a significant reduction in movement in animals five days post-KA induction.
Besides OFT, NORT was performed at different intervals (24 hours and five days) after KA administration to assess several aspects of cognitive function, specifically memory and learning49. This test is a non-spatial memory task that stimulates an animal’s preference for novelty as well as their innate exploratory behavior, which confers the ability to remember50. During the retention phase, the decrease in recognition index following KA induction was improved in most of the pre-treatment groups, where the animals that recalled the familiar object spent more time examining the novel object because they have an innate preference for novelty. Additionally, a study by Ramshini et al. (2017) discovered that silver nanoparticles improved spatial learning and memory in rats by inhibiting Aβ amyloid fibril–induced neurotoxicity51. High levels of Aβ make neurons more vulnerable to excitotoxic events caused by seizures, and reducing Aβ by nanoparticles can protect neurons from Aβ-related toxicity52, 53. Additionally, a previous study reported that administering silver nanoparticles derived from green synthesis was effective in preventing and reducing deficits in recognition and spatial memory in a neurodegenerative rat model by inhibiting excess ROS formation and preserving mitochondrial activation in generating ATP54. Another finding reported that silver nanoparticle conjugate could recover spatial learning and enhance memory in other rat models55.
The disparity between the current and prior findings is most likely due to differences in experimental settings such as treatment route and type of animal used (strain and species). Further investigations examining the morphological and molecular level of the mechanism will better elucidate the protective effects of THSN against KA-induced status epilepticus and neurodegeneration in rats.
Conclusions
The present study suggests that THSN improved seizures, locomotor activity, and memory function following KA administration. This can be seen from the ability of THSN to increase the latency to seizure and the number of line crossings, as well as the higher recognition index. Further study into the cytotoxicity mechanisms of THSN is warranted to widen its nanomedical uses in diagnostics, therapeutics, and pharmaceutics.
Abbreviations
ANOVA: Analysis of variance, FGS: First generalised seizure, GABA: Gamma-aminobutyric acid, KA: Kainic acid, LTP: Long-term potentiation, NORT: Novel object recognition index, OFT: Open field test, S.C.: Subcutaneously, SEM: Standard error mean, SPSS: Statistical package for social sciences, TH: Tualang honey, THSN: Tualang honey silver nanoparticles
Acknowledgments
The authors gratefully acknowledge Universiti Malaysia Kelantan, Animal Research and Service Centre (ARASC), and Central Research Laboratory (CRL), Unversiti Sains Malaysia, Health Campus for providing the lab facilities for this research work.
Author’s contributions
SKNS developed the original idea and designed the study. PVR gave advices regarding on preparation of THSN. SM and MM assist the behavioural study. MMA and SKNS supervised the work. HH prepared the THSN, performed the experiment, analysed the results and drafted the article. All authors have read, revised and approved the article submission.
Funding
This research was financially supported by the Universiti Sains Malaysia under Research University (Individual) (RUI Grant No: 1001/PPSP/8012249).
Availability of data and materials
All data supporting the conclusions of this manuscript are provided in the text and figures. The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Paul
Y.,
Various epileptic seizure detection techniques using biomedical signals: a review. Brain Informatics.
2018;
5
(2)
:
6
.
View Article PubMed Google Scholar -
Lorigados
L.,
Orozco
S.,
Morales
L.,
Estupiñán
B.,
García
I.,
Rocha
L.,
Excitotoxicity and neuronal death in epilepsy. Biotecnologia Aplicada .
2013;
30
(1)
:
9-16
.
-
Nicoll
R.A.,
A brief history of long-term potentiation. Neuron.
2017;
93
(2)
:
281-90
.
View Article PubMed Google Scholar -
Li
W.,
Xu
X.,
Pozzo-Miller
L.,
Excitatory synapses are stronger in the hippocampus of Rett syndrome mice due to altered synaptic trafficking of AMPA-type glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America.
2016;
113
(11)
:
1575-84
.
View Article PubMed Google Scholar -
Suárez
L.M.,
Cid
E.,
Gal
B.,
Inostroza
M.,
Brotons-Mas
J.R.,
Gómez-Domínguez
D.,
Systemic injection of kainic acid differently affects LTP magnitude depending on its epileptogenic efficiency. PLoS One.
2012;
7
(10)
:
e48128
.
View Article PubMed Google Scholar -
Zhang
F.X.,
Sun
Q.J.,
Zheng
X.Y.,
Lin
Y.T.,
Shang
W.,
Wang
A.H.,
Abnormal expression of synaptophysin, SNAP-25, and synaptotagmin 1 in the hippocampus of kainic acid-exposed rats with behavioral deficits. Cellular and Molecular Neurobiology.
2014;
34
(6)
:
813-24
.
View Article PubMed Google Scholar -
Yow
H.,
Ahmad
N.,
Azmi
N.,
Bakry
M.,
The effect of curcumin on anxiety and recognition memory in kainate model of epileptic rats. Indian Journal of Pharmaceutical Sciences.
2017;
79
(2)
:
267-76
.
View Article Google Scholar -
Maia
G.H.,
Quesado
J.L.,
Soares
J.I.,
do Carmo
J.M.,
Andrade
P.A.,
Andrade
J.P.,
Loss of hippocampal neurons after kainate treatment correlates with behavioral deficits. PLoS One.
2014;
9
(1)
:
e84722
.
View Article PubMed Google Scholar -
Greening
S.G.,
Mitchell
D.G.,
A network of amygdala connections predict individual differences in trait anxiety. Human Brain Mapping.
2015;
36
(12)
:
4819-30
.
View Article PubMed Google Scholar -
Murakami
S.,
Takemoto
T.,
Shimizu
Z.,
Studies on the effective principles of Digenea-simplex aq. 1. separation of the effective fraction by liquid chromatography. Yakugaku Zasshi-Journal of the pharmaceutical society of Japan. 1953;73(9):1026-8.
.
View Article Google Scholar -
Zhang
X.M.,
Zhu
J.,
Kainic Acid-induced neurotoxicity: targeting glial responses and glia-derived cytokines. Current Neuropharmacology.
2011;
9
(2)
:
388-98
.
View Article PubMed Google Scholar -
Mohd Sairazi
N.S.,
Sirajudeen
K.N.,
Muzaimi
M.,
Mummedy
S.,
Asari
M.A.,
Sulaiman
S.A.,
Tualang Honey Reduced Neuroinflammation and Caspase-3 Activity in Rat Brain after Kainic Acid-Induced Status Epilepticus. Evidence-Based Complementary and Alternative Medicine.
2018;
2018
:
7287820
.
View Article PubMed Google Scholar -
Ullah
I.,
Park
H.Y.,
Kim
M.O.,
Anthocyanins protect against kainic acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons. CNS Neuroscience & Therapeutics.
2014;
20
(4)
:
327-38
.
View Article PubMed Google Scholar -
Lévesque
M.,
Avoli
M.,
The kainic acid model of temporal lobe epilepsy. Neuroscience and Biobehavioral Reviews.
2013;
37
(10 Pt 2)
:
2887-99
.
View Article PubMed Google Scholar -
Anand
K.S.,
Dhikav
V.,
Hippocampus in health and disease: an overview. Annals of Indian Academy of Neurology.
2012;
15
(4)
:
239-46
.
View Article PubMed Google Scholar -
Pasupuleti
V.R.,
Prasad
T.N.,
Shiekh
R.A.,
Balam
S.K.,
Narasimhulu
G.,
Reddy
C.S.,
Biogenic silver nanoparticles using Rhinacanthus nasutus leaf extract: synthesis, spectral analysis, and antimicrobial studies. International Journal of Nanomedicine.
2013;
8
:
3355-64
.
View Article PubMed Google Scholar -
Jain
A.,
Anitha
R.,
Rajeshkumar
S.,
Anti inflammatory activity of Silver nanoparticles synthesised using Cumin oil. Research Journal of Pharmacy and Technology..
2019;
12
(6)
:
2790-3
.
View Article Google Scholar -
Lawal
S.,
Olojede
S.O.,
Sulaiman
S.O.,
Aladeyelu
O.S.,
Moodley
R.,
Naidu
E.C.,
Tenofovir-silver nanoparticles conjugate ameliorates neurocognitive disorders and protects ultrastructural and cytoarchitectonic properties of the prefrontal cortex in diabetic rats. Bosnian Journal of Basic Medical Sciences.
2022;
22
(4)
:
569-79
.
View Article PubMed Google Scholar -
Roy
N.,
Gaur
A.,
Jain
A.,
Bhattacharya
S.,
Rani
V.,
Green synthesis of silver nanoparticles: an approach to overcome toxicity. Environmental Toxicology and Pharmacology.
2013;
36
(3)
:
807-12
.
View Article PubMed Google Scholar -
Haiza
H.,
Azizan
A.,
Mohidin
A.H.,
Halin
D.,
Green synthesis of silver nanoparticles using local honey. Nano Hybrids..
2013;
4
:
87-98
.
View Article Google Scholar -
Hasim
H.,
Rao
P.,
Sekhar
A.,
Muthuraju
S.,
Asari
M.,
Sirajudeen
K.,
Green synthesis and characterization of silver nanoparticles using Tualang honey and evaluation of their antioxidant activities. Advances in Natural Sciences: Nanoscience and Nanotechnology..
2020;
11
(2)
:
025010
.
View Article Google Scholar -
Sulaiman
F.A.,
Adeyemi
O.S.,
Akanji
M.A.,
Oloyede
H.O.,
Sulaiman
A.A.,
Olatunde
A.,
Biochemical and morphological alterations caused by silver nanoparticles in Wistar rats. Journal of Acute Medicine.
2015;
5
(4)
:
96-102
.
View Article Google Scholar -
Hassanen
E.I.,
Khalaf
A.A.,
Tohamy
A.F.,
Mohammed
E.R.,
Farroh
K.Y.,
Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. International Journal of Nanomedicine.
2019;
14
:
4723-39
.
View Article PubMed Google Scholar -
Nghilokwa
E.,
Sokei
J.,
Mwitari
P.,
Maina
N.,
Sub-acute and chronic toxicity of silver nanoparticles synthesized by Azadirachta indica extract. African Journal of Biotechnology.
2020;
19
(6)
:
320-31
.
View Article Google Scholar -
Zhang
X.,
Gelowitz
D.L.,
Lai
C.T.,
Boulton
A.A.,
Yu
P.H.,
Gradation of kainic acid-induced rat limbic seizures and expression of hippocampal heat shock protein-70. The European Journal of Neuroscience.
1997;
9
(4)
:
760-9
.
View Article PubMed Google Scholar -
Mohd Sairazi
N.S.,
K N S
S.,
Asari
M.A.,
Mummedy
S.,
Muzaimi
M.,
Sulaiman
S.A.,
Effect of tualang honey against KA-induced oxidative stress and neurodegeneration in the cortex of rats. BMC Complementary and Alternative Medicine.
2017;
17
(1)
:
31
.
View Article PubMed Google Scholar -
Wang
X.,
Li
P.,
Liu
J.,
Jin
X.,
Li
L.,
Zhang
D.,
Gastrodin attenuates cognitive deficits induced by 3, 3'-iminodipropionitrile. Neurochemical Research.
2016;
41
(6)
:
1401-9
.
View Article PubMed Google Scholar -
Safdar
A.,
Azman
K.F.,
Zakaria
R.,
Ab Aziz
C.B.,
Rashid
U.,
Memory-enhancing effects of goat milk in D-galactose-induced aging rat model. Biomedical Research and Therapy.
2020;
7
(1)
:
3563-71
.
View Article Google Scholar -
Furtinger
S.,
Bettler
B.,
Sperk
G.,
Altered expression of GABAB receptors in the hippocampus after kainic-acid-induced seizures in rats. Brain Research. Molecular Brain Research.
2003;
113
(1-2)
:
107-15
.
View Article PubMed Google Scholar -
Zheng
X.Y.,
Zhang
H.L.,
Luo
Q.,
Zhu
J.,
Kainic acid-induced neurodegenerative model: potentials and limitations. Journal of Biomedicine & Biotechnology.
2011;
2011
:
457079
.
View Article PubMed Google Scholar -
Lin
T.Y.,
Lu
C.W.,
Wang
S.J.,
Luteolin protects the hippocampus against neuron impairments induced by kainic acid in rats. Neurotoxicology.
2016;
55
:
48-57
.
View Article PubMed Google Scholar -
Nurul Syazana
M.S.,
Gan
S.H.,
Halim
A.S.,
Shah
N.S.,
Gan
S.H.,
Sukari
H.A.,
Analysis of volatile compounds of Malaysian Tualang (Koompassia excelsa) honey using gas chromatography mass spectrometry. African Journal of Traditional, Complementary, and Alternative Medicines.
2012;
10
(2)
:
180-8
.
View Article PubMed Google Scholar -
Chew
C.Y.,
Chua
L.S.,
Soontorngun
N.,
Lee
C.T.,
Discovering potential bioactive compounds from Tualang honey. Agriculture and Natural Resources (Bangkok).
2018;
52
(4)
:
361-5
.
View Article Google Scholar -
Cárdenas-Rodríguez
N.,
González-Trujano
M.E.,
Aguirre-Hernández
E.,
Ruíz-García
M.,
Sampieri
A.,
Coballase-Urrutia
E.,
Anticonvulsant and antioxidant effects of Tilia americana var. mexicana and flavonoids constituents in the pentylenetetrazole-induced seizures. Oxid Med Cell Longev.
2014;
2014
:
329172
.
View Article Google Scholar -
Chavan
P.J.,
Chawre
A.B.,
Redasani
V.K.,
Spectrophotometric Evaluation of Antiepileptic Activity of Ficusracemosa in Chemicals Induced Epilepsy in Mice. Asian Journal of Pharmaceutical Research and Development..
2021;
9
(5)
:
62-6
.
View Article Google Scholar -
Motevalian
M.,
Mehrzadi
S.,
Ahadi
S.,
Shojaii
A.,
Anticonvulsant activity of Dorema ammoniacum gum: evidence for the involvement of benzodiazepines and opioid receptors. Research in Pharmaceutical Sciences.
2017;
12
(1)
:
53-9
.
View Article PubMed Google Scholar -
Imad Uddin
M.,
Kalyani
D.,
Tejasri
N.,
Mounika
A.,
Sowndarya
A.,
Anitha
J.,
Green Synthesis and Characterization of Silver Nanoparticles using Glycine Max L. Seed Extract and their Antiepileptic Activity in Rats. International Journal of Pharmaceutical Sciences and Nanotechnology..
2017;
10
(6)
:
3909-14
.
View Article Google Scholar -
Joshi
N.,
Uddin
I.,
Ahmedi
Z.,
Ravali
Y.,
Reddy
V.B.,
Preparation of Silver Nano Particles From The Bark of Alstonia Scholaris and Evaluation of Its Anti-Epileptic Activity. World Journal of Pharmaceutical Research.
2018;
7
(9)
:
660-9
.
-
Cano
A.,
Ettcheto
M.,
Espina
M.,
Auladell
C.,
Calpena
A.C.,
Folch
J.,
Epigallocatechin-3-gallate loaded PEGylated-PLGA nanoparticles: A new anti-seizure strategy for temporal lobe epilepsy. Nanomedicine; Nanotechnology, Biology, and Medicine.
2018;
14
(4)
:
1073-85
.
View Article PubMed Google Scholar -
Wang
S.,
Su
R.,
Nie
S.,
Sun
M.,
Zhang
J.,
Wu
D.,
Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. The Journal of Nutritional Biochemistry.
2014;
25
(4)
:
363-76
.
View Article PubMed Google Scholar -
Khalil
I.,
Yehye
W.A.,
Etxeberria
A.E.,
Alhadi
A.A.,
Dezfooli
S.M.,
Julkapli
N.B.,
Nanoantioxidants: recent trends in antioxidant delivery applications. Antioxidants.
2019;
9
(1)
:
24
.
View Article PubMed Google Scholar -
Myint
K.Z.,
Yu
Q.,
Xia
Y.,
Qing
J.,
Zhu
S.,
Fang
Y.,
Bioavailability and antioxidant activity of nanotechnology-based botanic antioxidants. Journal of Food Science.
2021;
86
(2)
:
284-92
.
View Article PubMed Google Scholar -
Mitra
N.K.,
Goh
T.E.,
Bala Krishnan
T.,
Nadarajah
V.D.,
Vasavaraj
A.K.,
Soga
T.,
Effect of intra-cisternal application of kainic acid on the spinal cord and locomotor activity in rats. International Journal of Clinical and Experimental Pathology.
2013;
6
(8)
:
1505-15
.
PubMed Google Scholar -
Chan
J.N.,
Lee
J.C.,
Lee
S.S.,
Hui
K.K.,
Chan
A.H.,
Fung
T.K.,
Interaction Effect of Social Isolation and High Dose Corticosteroid on Neurogenesis and Emotional Behavior. Frontiers in Behavioral Neuroscience.
2017;
11
:
18
.
View Article PubMed Google Scholar -
Nuss
P.,
Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatric Disease and Treatment.
2015;
11
:
165-75
.
View Article PubMed Google Scholar -
Masneuf
S.,
Lowery-Gionta
E.,
Colacicco
G.,
Pleil
K.E.,
Li
C.,
Crowley
N.,
Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology.
2014;
85
:
190-7
.
View Article PubMed Google Scholar -
Rajadurai
U.M.,
Hariharan
A.,
Durairaj
S.,
Ameen
F.,
Dawoud
T.,
Alwakeel
S.,
Assessment of behavioral changes and antitumor effects of silver nanoparticles synthesized using diosgenin in mice model. Journal of Drug Delivery Science and Technology.
2021;
66
:
102766
.
View Article Google Scholar -
Danila
O.O.,
Berghian
A.S.,
Dionisie
V.,
Gheban
D.,
Olteanu
D.,
Tabaran
F.,
The effects of silver nanoparticles on behavior, apoptosis and nitro-oxidative stress in offspring Wistar rats. Nanomedicine (London).
2017;
12
(12)
:
1455-73
.
View Article PubMed Google Scholar -
Lueptow
L.M.,
Novel pub Recognition Test for the Investigation of Learning and Memory in Mice. Journal of Visualized Experiments.
2017;
126
(126)
:
55718
.
View Article PubMed Google Scholar -
Wong
J.H.,
Muthuraju
S.,
Reza
F.,
Senik
M.H.,
Zhang
J.,
Mohd Yusuf Yeo
N.A.,
Differential expression of entorhinal cortex and hippocampal subfields α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors enhanced learning and memory of rats following administration of Centella asiatica. Biomedicine and Pharmacotherapy.
2019;
110
:
168-80
.
View Article PubMed Google Scholar -
Ramshini
H.,
Moghaddasi
A.S.,
Aldaghi
L.S.,
Mollania
N.,
Ebrahim-Habibi
A.,
Silver nano particles ameliorate learning and spatial memory of male Wistar rats by prevention of amyloid fibril-induced neurotoxicity. Archives Italiennes de Biologie.
2017;
155
(3)
:
131-41
.
View Article PubMed Google Scholar -
Palop
J.J.,
Mucke
L.,
Epilepsy and cognitive impairments in Alzheimer disease. Archives of Neurology.
2009;
66
(4)
:
435-40
.
View Article PubMed Google Scholar -
Jamali
M.,
Mohajer
S.,
Sheikhlary
S.,
Ara
M.H.,
Z-scan optical method complements the Thioflavin T assay for investigation of anti-Alzheimer's impact of polyphenols. Photodiagnosis and Photodynamic Therapy.
2022;
39
:
102914
.
View Article PubMed Google Scholar -
Zhang
X.,
Li
Y.,
Hu
Y.,
Green synthesis of silver nanoparticles and their preventive effect in deficits in recognition and spatial memory in sporadic Alzheimer's rat model. Colloids and Surfaces. A, Physicochemical and Engineering Aspects.
2020;
605
:
125288
.
View Article Google Scholar -
Lawal
S.K.,
Olojede
S.O.,
Dare
A.,
Faborode
O.S.,
Naidu
E.C.,
Rennie
C.O.,
Silver nanoparticles conjugate attenuates highly active antiretroviral therapy-induced hippocampal nissl substance and cognitive deficits in diabetic rats. Journal of Diabetes Research.
2021;
2021
:
2118538
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 9 (2022)
Page No.: 5291-5300
Published on: 2022-09-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4753 times
- PDF downloaded - 1547 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress






