Abstract
Objectives: To evaluate the effectiveness and safety of the first sirolimus-eluting stent made in Vietnam (Xplosion stent) on Vietnamese patients via the following outcomes: restenosis rate in the stent and at the two heads of stent, stent occlusion rate, and mortality rate due to myocardial infarction at 6 months and 12 months following stent placement.
Methods and Results: The prospective, open-label, non-randomized, longitudinal study enrolled 43 patients diagnosed with stable angina and de novo lesions with a 2.5 – 3.5 mm reference diameter and 24 mm in length. The patients received one stent per branch, and some received stents in multiple branches. All patients were scheduled for angiographic follow-up at 6 months, and if subjects consented, at 12 months. The subjects' mean age was 60.05 +/- 11.07 years, and many had cardiovascular risk factors, such as hypertension (83.72%), dyslipidemia (53.49%), and diabetes mellitus (25.58%). Stenting was performed on 50 lesions with a sirolimus drug-eluting stent (1.16 stent/patient on average). Of the patients, 92% had single-vessel coronary artery disease, and type B lesions (AHA) accounted for the majority (56%), with distribution on the LAD (42%), LCx (24%), and RCA (34%). The technical and procedural success rate of stent placement was 97.67%, with a very low complication rate (0%). The restenosis rate at 6 months was 0%. All patients were followed up to 12 months. Only two patients had recurrent chest pain and underwent coronary angiography; however, there was no in-stent restenosis and no need for revascularization. Therefore, the rate of chest pain recurrence after 12 months was 4.65% (2/43), and the rate of target vessel revascularization at 12 months was 0%. At 6- and 12-month follow-up, we observed no death due to unknown causes, no target-vessel myocardial infarction, and two clinically-driven re-angiographies with no need for revascularization. No additional events were reported beyond the 6-month follow-up. During the entire 12-month follow-up period, none of the patients experienced a definite or probable stent thrombosis.
Conclusion: The new sirolimus-eluting stents manufactured in Vietnam use a new technology transferred from the United States. They were placed successfully and showed a sustained favorable safety profile for up to 12 months. These findings suggest that these new stents could be used in many catheterization laboratories in Viet Nam.
Introduction
Percutaneous coronary intervention (PCI) performed with a balloon or stent is used to open a narrowed or occluded coronary artery. Following the advent of cardiac catheterization in the late 1920s and the development of coronary contrast technology in the late 1950s, balloon angioplasty (BA) was first introduced in the mid-1960s. Although this was a significant advancement in interventional cardiology, BA has major limitations, including the risk of uncontrolled atherosclerotic plaque disruption and vascular constriction, which can lead to coronary artery occlusion and myocardial infarction (MI)1, 2.A stent is a metal mesh that was developed to prevent restenosis after BA. The clinical efficacy of stenting compared with BA was studied in two landmark clinical trials. The STRESS study (North American Stent Restenosis Study) comparing a stenting group and BA group and reported a lower rate of restenosis on coronary angiography (31.6% vs 42.1%) and a lower rate of revascularization (10.2% vs 15.4%) in the stenting group3. Acute stent closure and late in-stent restenosis are the two primary adverse events initially encountered with the widespread use of bare metal stents (BMS)4, 5. Many clinical trials on drug-eluting stents (DES) were conducted in the late 1990s to find a solution to this problem5, and they demonstrated a reduction in the restenosis rate.The advantages of DES have completely altered the complications associated with PCI in recent years. First-generation DES reduce endothelial proliferation and thereby decrease the need for revascularization of the target coronary artery compared with BMS despite some residual issues in terms of the long-term safety of this generation of DES, especially with late stent thrombosis2. Recent scientific advances, such as fabrication of stent materials, improvement of stent frame design, and enhancement of the biocompatibility of the stent-coated polymer layer, have helped address these problems6. Since being granted approval by the U.S. Food and Drug Administration in April 2003, clinical trials using sirolimus-eluting stents have demonstrated an antiproliferative effect on vascular smooth muscle and a decrease in restenosis rate and major adverse events on follow-up7.
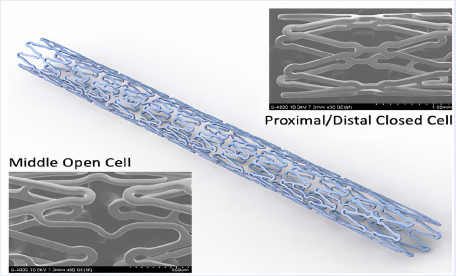
The new sirolimus stent’s platform, with low strut thickness (65 µm) and optimal cell size and shape, open cells in the middle, and closed cells at the two heads, showed high flexibility and low crossing profile, suiting complex anatomies in vitro. The new stent is expected to minimize edge dissection with good vessel wall coverage, no plaque protrusion, and uniform drug distribution. We aimed to evaluate the efficacy, safety, and technical success of sirolimus-eluting stents manufactured in Viet Nam at 6-month and 1-year follow-up.
Methods
Study design and population
The study was conducted from September 2016 to March 2018 at Cho Ray Hospital. Eligible patients had stable angina or documented silent ischemia, a maximum of three single de novo lesions in three separate coronary arteries, with a reference vessel diameter between 2.5 mm and 3.5 mm, a lesion length ≤24 mm, and a stenosis diameter between 70% and 99%. Exclusion criteria included evidence of left ventricular systolic dysfunction (i.e., left ventricular ejection fraction [LVEF] ≤ 30%), evidence of MI within 72 hours prior to the procedure, history of allergy to acetylsalicylic acid (ASA), clopidogrel, ticlopidine, heparin, contrast media, cobalt-chromium (CoCr), or polylactide-co-glycolide (PLGA), platelets < 100,000 cells/mm3 or > 700,000 cells/mm3, white blood cells < 3,000 cells/mm3, acute or chronic renal failure (i.e., estimated glomerular filtration rate according to Cockroft < 30 ml/min), stenosis of more than 50% of the lumen in the pre- or post-injury segment, severe calcification damage, bifurcation lesion requiring two-stent technique, or LM disease. Clinical follow-up was planned at 6 and 12 months. Angiographic follow-up was scheduled at 6 months for all patients, and an additional, pre-specified imaging follow-up was scheduled at 12 months if subjects consented.
Study device
The new sirolimus stent’s platform is constructed of cobalt-chromium L605, which is characterized by a unique edge design with low strut thickness (65 µm) and optimal cell size and shape. The polymer/drug coating of the Xplosion stent is approximately 3 – 5 μm thick, and the drug load has a nominal density of 1.33 μg/mm2. This new design provides high flexibility and lower crossing profile to suit complex anatomies in vitro. The coating is composed of biodegradable polymers and sirolimus (anti-proliferative agent). The new technology has achieved optimal cell size and shape, with open cells in the middle and closed cells at the two heads (Figure 1).
Endpoints and definitions
The primary endpoint was death from all causes, major adverse cardiovascular events (MACE) defined as composite endpoint of cardiac death, MI, target lesion revascularization (TLR) at 6 – 12 months, or in-segment late lumen loss (LLL) at 6-month follow-up. Secondary endpoints were technical success, defined as successful stent placement at the correct target lesion site with residual narrowing of the stent < 10% (measured on QCA), and procedural success was defined as patients not having serious complications or death during the procedure.
Procedure
Hospitalized patients who met the inclusion criteria for the study and met no exclusion criteria were examined clinically, underwent laboratory tests, and received drug treatment according to the protocol (Figure 2).
Patients were fully informed about the indications, benefits, and risks of PCI, signed a consent form to perform the procedure, and consented to participating in the study.
Percutaneous coronary angiography was performed for patients using a DSA angiography machine. When the coronary angiogram of patients with coronary artery stenosis met the criteria for inclusion in the study, PCI was performed with the studied stent.
The patient continued to be monitored and treated after PCI until discharge. The patients were admitted for evaluation by coronary angiography after 6 months and at 12 months if they consented.
Data processing & statistical analyses
SPSS 20.0 software was used to process the data. Discrete identified variables are presented as n (%); quantitative variables with a normal distribution are presented as mean ± standard error (SE) and as median (1st quartile — 2nd quartile) if not normally distributed.
| Baseline clinical and lesion characteristics (n = 43) | Frequency (Percentage %) Mean ± SD Median (IQR) |
|---|---|
| Age (years) | 60.05 ± 11.07 |
| Male (%) | 33 (76.74%) |
| BMI (kg/m 2 ) | 22.52 ± 2.94 |
| Risk factors | |
| Obesity (BMI ≥ 23 kg/m 2 ) | 19 (44.18%) |
| Smoking (%) | 16 (37.21%) |
| Hypertension (%) | 36 (83.72%) |
| Diabetes (%) | 11 (25.58%) |
| High cholesterol level (%) | 23 (53.49%) |
| History of coronary diseases (%) | 23 (53.49%) |
| Myocardial infarction (%) | 5 (11.62%) |
| Prior PCI (%) | 3 (6.97%) |
| Prior CABG (%) | 0 (0%) |
| NYHA dyspnea grades | |
| I | 9 (20.93%) |
| II | 8 (18.6%) |
| CCS angina grades | |
| I | 1 (2.33%) |
| II | 12 (27.91%) |
| III | 30 (69.76%) |
| Ejection fraction (EF) | 51.93 ± 11.62 |
| Blood creatinin (mg/dl) | 1.30 (1.20 – 1.40) |
| Troponin I (ng/mL) | 2.7 (0.6 – 79.4) |
| CK-MB (U/L) | 25 (17 – 40) |
| Position | Number of lesions (%) | Mean ± SD (n = 50) | ||
|---|---|---|---|---|
| Length (mm) | Diameter (mm) | Stenosis (%) | ||
| LAD I | 3 (6.97%) | 19.1 ± 4.91 | 3.33 ± 0.29 | 75.67 ± 7.37 |
| LAD II | 13 (30.23%) | 18.58 ± 3.98 | 2.99 ± 0.26 | 79 ± 4.05 |
| LAD III | 1 (2.32%) | 13 | 2.5 | 70 |
| LAD –other | 4 (9.3%) | 16 ± 4.97 | 2.69 ± 0.24 | 83.67 ± 5.51 |
| LCX I | 3 (6.97%) | 21.1 ± 5.02 | 2.73 ± 0.25 | 75 ± 5 |
| LCX II | 4 (9.30%) | 17 ± 3.56 | 2.49 ± 0.23 | 79.04 ± 7.64 |
| LCX - other | 5 (11.62%) | 19.2 ± 3.95 | 2.76 ± 0.45 | 81.67 ± 2.08 |
| RCA I | 1 (2.32%) | 24 | 3.4 | 76 |
| RCA II | 8 (16.27%) | 18.26 ± 5.28 | 2.86 ± 0.43 | 78.14 ± 8.8 |
| RCA III | 2 (4.65%) | 16 ± 8.48 | 3 ± 0.71 | 70 |
| RCA-other | 6 (13.95%) | 20.32 ± 3.18 | 2.75 ± 0.42 | 82.17 ± 5.04 |
| Characteristics | Frequency (Percentage %) Median (IQR) (n = 50) |
|---|---|
| Vascular Access (n = 43) | |
| Radial artery | 42 (97.67 %) |
| Femoral artery | 1 (2.33 %) |
| AHA coronary lesion classification | |
| A | 21 (42 %) |
| B | 28 (56 %) |
| B1 | 15 (30 %) |
| B2 | 13 (26 %) |
| C | 1 (2 %) |
| Target vessels | |
| LAD | 21 (42 %) |
| LCx | 12 (24%) |
| RCA | 17 (34 %) |
| Distribution of coronary lesions | |
| Single branch | 46 (92%) |
| Double branches | 3 (6%) |
| Triple branches | 1 (2%) |
| Predilated by balloon | 26 (52%) |
| Ratio Stent/patients | 1.16 |
| Length of stent (mm) | 23 (18 - 23) |
| Diameter of stent (mm) | 3 (2.50 – 3.25) |
| Characteristics | Frequency (Percentage %) Mean ± SD n = 50 |
|---|---|
| Technical success | 43 (100%) |
| Procedural success | 42 (97.67%) |
| TIMI flow after tent implantation | |
| II | 1 (2%) |
| III | 49 (98%) |
| Residual stenosis (%) | 2 (1.2%) |
| Late lumen loss (mm) | 0.1 ± 0.05 |
Results
Between September 2016 and March 2018, 43 patients were enrolled in the study. The baseline parameters are listed in Table 1. Most patients had only one coronary artery (92%), the coronary branches were damaged in the anterior interventricular artery (LAD) in most patients (42%), stenosis rate was >70%, type B lesions accounted for the majority (56%), while type C lesions accounted for only 2% (Table 2 and Table 3). The average number of stents placed per patient was 1.16 stents. Most of the patients in the study (52%) underwent Bloomsable BA before stenting, and all the balloons were fully expanded according to procedural protocol. The implanted stents had a length of 23 mm (18 – 23 mm) and were 3.0 mm in diameter (Table 3).
In the immediate phase after insertion, 43 cases were stented with a technical success rate of 100% and procedural success rate of 97.67%. The angiographic results were TIMI III flow (98%). Only one case (2%) had TIMI II flow, which improved to TIMI III flow and no restenosis was observed at 6 months. One procedure involved a hematoma in the right arm, but the patient recovered well with no need for blood transfusion and no recorded arteriovenous catheterization during follow-up. At 6 months, nine cases with residual stenosis after placement were observed; however, the restenosis was insignificant and did not require target vessel revascularization, and there was no acute occlusion. LLL was 0.1 ± 0.05 mm. No deaths or MACEs were recorded. At 1 year, two cases (4.65%) were hospitalized because of CCS III chest pain; however, no restenosis was observed on angiography (Table 4).
Discussion
The DES Xplosion is first manufactured by USM Healthcare Medical Devices Factory in Viet Nam. The stent’s platform is cobalt-chromium L605, which is characterized by a unique edge design, low strut thickness (65 µm), and optimal cell size and shape. The stent has a special design with proximal and distal closed cells and an open middle cell. This unique design provides high flexibility and low crossing profile to suit complex anatomies and decrease risk of edge dissection and acute closure during PCI. The coating is composed of a biodegradable polymers and sirolimus (anti-proliferative agent). These design features result in several important advantages in comparison with first-generation DES. The proven biocompatibility of a polymer-based sirolimus-eluting coated stent surface when it comes in contact with blood may reduce the early and late thrombosis risk and may play an important role in early endothelialization of the stent while maintaining its antirestenotic properties. Therefore, the Xplosion stent is indicated for use in patients with symptomatic ischemic coronary heart disease due to de novo lesions or restenosed lesions of the coronary arteries with a reference vessel diameter of 2.25 – 4.00 mm. Compared with BMS, the use of sirolimus-coated stents should lead to better results in the patients after 6 months, specifically significant reduction of in-stent restenosis and LLL. In this trial using Xplosion stents, at 6 months, 40 (93%) patients completed the follow-up period and only 3 patients (7%) did not return for follow-up because they lived far from the hospital and had difficulty obtaining transportation from their provinces to Chơ Rẫy hospital. However, their follow-up health status was assessed by telephone, and they reported neither serious adverse events nor symptoms. Angiography showed no significant restenosis needing revascularization in the stent or at the two ends. Compared with a study by Lemos et al.8, where the Supraflex sirolimus-eluting stent was used, all-cause mortality at 6 months and 1 year were 1% and 2.2%, respectively. At 6 and 12 months, MACEs were 2.2% and 3.7%, and revascularization rates were 0.5% and 0.7%, respectively. In a study by Urban et al.9 using sirolimus-coated Cypher stents, all-cause mortality at 6 and 12 months was 1.44% and 2.18%, MACEs were 3.38% and 5.8%, and revascularization rates were 1.49% and 3.07%, respectively. In our trial, we measured LLL of 0.1 ± 0.05 mm at 6 months.At 1-year follow-up, two cases had recurrent chest pain (CCS III). Both were hospitalized at Cho Rẫy hospital and underwent repeated coronary angiography; however, no binary restenosis was observed in the stent and/or two proximal stents that would have required revascularization.
The results of this trial have proven that effectiveness and safety of the first sirolimus-eluting stent manufactured in Vietnam (Xplosion stent) used on Vietnamese patients with minimum cost. Our study included a limited population with simple lesions and short-term data (6 months to 1 year). The favorable outcomes of stent placement need to be confirmed in more complex patient and lesion scenarios.
Conclusions
The new sirolimus-eluting stents manufactured in Vietnam with a new technology transferred from the United States were placed successfully and showed a sustained and favorable safety profile up to 12 months. Clinical outcomes at 6-month follow-up consisted of no death due to unknown causes, no target-vessel MI, and low LLL. During the follow-up of 12 months, there were two cases that required clinically-driven re-angiography, but neither required TLR. None of the patients experienced a definite or probable stent thrombosis. These findings suggest that this stent can be considered as an alternative to current stents.
Abbreviations
AHA: American heart association, ASA: acetylsalicylic acid, BA: balloon angioplasty, BMS: bare metal stents, CoCr: cobalt-chromium, DES: drug-eluting stents, LVEF: left ventricular ejection fraction, LLL: late lumen loss, LM: left main, LAD: left anterior descending artery, LCx: left circumflex artery, MI: myocardial infarction, MACE: major adverse cardiovascular events, PCI: Percutaneous coronary intervention, PLGA: polylactide- co-glycolide, RCA: right coronary artery, TLR: target lesion revascularization
Acknowledgments
None.
Author’s contributions
All authors equally contributed to this work, read and approved the final manuscript.
Funding
Sponsored by USM Healthcare Company.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Cho Ray Hospital. This study was conducted in accordance with the amended Declaration of Helsinki. All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
McKavanagh
P.,
Zawadowski
G.,
Ahmed
N.,
Kutryk
M.,
The evolution of coronary stents. Expert Review of Cardiovascular Therapy.
2018;
16
(3)
:
219-28
.
View Article PubMed Google Scholar -
Pfeiffer
D.,
Monsuez
J.J.,
Grüntzig
J.W.,
Laufs
U.,
Coronary Balloon Angioplasty is due to two physicians born in Saxony, Germany. European Heart Journal.
2020;
41
(15)
:
1462-3
.
View Article PubMed Google Scholar -
Serruys
P.W.,
de Jaegere
P.,
Kiemeneij
F.,
Macaya
C.,
Rutsch
W.,
Heyndrickx
G.,
Benestent Study Group
A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. The New England Journal of Medicine.
1994;
331
(8)
:
489-95
.
View Article PubMed Google Scholar -
Reejhsinghani
R.,
Lotfi
A.S.,
Prevention of stent thrombosis: challenges and solutions. Vascular Health and Risk Management.
2015;
11
:
93-106
.
PubMed Google Scholar -
Torrado
J.,
Buckley
L.,
Durán
A.,
Trujillo
P.,
Toldo
S.,
Valle Raleigh
J.,
Restenosis, stent thrombosis, and bleeding complications: navigating between Scylla and Charybdis. Journal of the American College of Cardiology.
2018;
71
(15)
:
1676-95
.
View Article PubMed Google Scholar -
Kandzari
D.E.,
Mauri
L.,
Koolen
J.J.,
Massaro
J.M.,
Doros
G.,
Garcia-Garcia
H.M.,
Investigators
BIOFLOW V,
Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet.
2017;
390
(10105)
:
1843-52
.
View Article PubMed Google Scholar -
Vale
N.,
Madeira
S.,
Almeida
M.,
Raposo
L.,
Freitas
P.,
Castro
M.,
Ten-year survival of patients undergoing coronary angioplasty with first-generation sirolimus-eluting stents and bare-metal stents. Revista Portuguesa de Cardiologia (English Ed.).
2020;
39
(11)
:
639-47
.
View Article PubMed Google Scholar -
Lemos
P.A.,
Chandwani
P.,
Saxena
S.,
Ramachandran
P.K.,
Abhyankar
A.,
Campos
C.M.,
Clinical outcomes in 995 unselected real-world patients treated with an ultrathin biodegradable polymer-coated sirolimus-eluting stent: 12-month results from the FLEX Registry. BMJ Open.
2016;
6
(2)
:
e010028
.
View Article PubMed Google Scholar -
Urban
P.,
Gershlick
A.H.,
Guagliumi
G.,
Guyon
P.,
Lotan
C.,
Schofer
J.,
e-Cypher Investigators
Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation.
2006;
113
(11)
:
1434-41
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 7 (2022)
Page No.: 5154-5160
Published on: 2022-07-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 4918 times
- PDF downloaded - 1589 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress




