Abstract
Background: Diodia sarmentosa leaves have gained application in the local treatment of certain ailments. This study evaluated the effect of ethanol extracts of Diodia sarmentosa leaves on biochemical, antioxidant, and histopathological indices of monosodium glutamate-induced uterine leiomyoma in albino rats.
Methods: Twenty-one (21) female adult albino rats were acclimatized for 10 days and then classified into three treatment groups, each containing seven rats. Group 1 was the normal control (NC) without any induction, Group 2 was the positive control (PC) and was induced with 200 mg/kg body weight of monosodium glutamate (MSG) without treatment, and Group 3 was the treated group (TG), induced with 200 mg/kg body weight of MSG, then treated with 400 mg/kg body weight of ethanol extract of Diodia sarmentosa leaves for 30 days.
Results: The results of the study showed an impaired antioxidant system in the positive control (untreated group). Some biochemical parameters were also altered in the positive control, mostly revealing a relationship between uterine leiomyoma and renal impairment, or kidney damage. Treatment with ethanol extracts of Diodia sarmentosa leaves significantly (P < 0.05) improved the altered biochemical and antioxidant parameters. These findings were also supported by a histopathology result, which revealed the extent of tumors in the affected tissues.
Conclusion: The ethanol extract of Diodia sarmentosa (Sw) leaves mitigated oxidative stress and improved the impaired antioxidant system caused by uterine leiomyoma induction.
Introduction
According to the National Cancer Institute (NCI), the uterus is vulnerable to various diseases, which are categorized as malignant, benign, or inflammatory1. Most benign lesions are products of inflammation resulting from microbial infestations. Female hormones occasionally influence composite tissue, and this also is believed to promote genetic diseases2. Uterine leiomyoma, commonly known as a fibroid, is a benign tumor of the muscle tissue that grows in the wall of the uterus3. Uterine fibroids predominantly occur in women over the age of 304. Fibroids are a common gynecological problem as well as the most common benign genital tract tumor, which can lead to pregnancy loss and other fertility issues in women of reproductive age5, 6, 7 . Endometrial hyperplasia is the most common uterine hyperplasia8, and about 20 % of women with endometrial hyperplasia are more susceptible to developing fibroid growth during their reproductive years. Most women with uterine fibroids do not show early symptoms and therefore receive little or no medical attention5, 9. This type of tumor, known as an asymptomatic tumor, can cause pain during menstruation, frequent urination, and, in some cases, delayed pregnancy10. Most women who show signs of fibroid growth early enough for prompt diagnosis usually experience abdominal pelvic mass5, 6, 11.
Development of myomas is initiated from the transformation and growth of normal myocytes into clinically apparent tumors; in rare cases, a leiomyoma may develop into a malignant leiomyosarcoma (LMS)12, 13. Hormones may promote the development of fibroids. Other factors include, but are not limited to: menopause, diet, and hormone replacement therapy14. Estrogen and progesterone are crucial to the formation and growth of fibroid tissues15. Gonadotropin-releasing hormone analogues (GnRHa) comprise some of the chemotherapeutic drugs currently used in fibroid treatment. However, the short half-life of these drugs is a major limitation to treatment and prolonged usage can also lead to adverse effects, such as depression, reduced breast size, and vaginal dryness16, 17.
Anastrozole and Letrozole are also used in the treatment of uterine fibroids. They produce some side effects, such as vaginal dryness and musculoskeletal pain18.
Selective estrogen receptor modulators (SERMs), which are also used in the treatment of uterine fibroids, can cause some side effects, such as vasomotor symptoms and thromboembolic events18. This has spurred the search for alternative means of managing tumors using natural remedies, such as medicinal plants19. This study is part of this much-needed search for pharmacognostic solutions, with our focus being on Diodia sarmentosa (Sw) leaves.
Diodia sarmentosa has gained wide use as a medicinal plant. It is an evergreen perennial plant with a network-like stem structure and alternated leaves, and it is mostly found in dense forest zones, grassy and swampy vegetation, roadsides, and in cultivated fields. Diodia samentosa grows widely in Asia, America, Africa, and the Mascarene Islands20. Among other uses, it is used to treat injuries, eczema, oedema21 and dysentery22, 23. Several studies have reported on the anti-ulcer24, anti-diabetic25, antioxidant20, anti-inflammatory, and analgesic properties21 of Diodia sarmentosa.
As of the time of this report, there has been no prior report on the anti-tumor properties of Diodia sarmentosa leaves. This study sought to evaluate the anti-tumor activities of the ethanol extracts of Diodia sarmentosa leaves, and was carried out by assessing effects on the biochemical and histopathological indices of rat serum and tissues, respectively.
Monosodium Glutamate (MSG) is a sodium salt derived from the amino acid glutamic acid26. It is commonly used in food additives27, and has also been reported to cause oxidative stress, protein modification, DNA damage, and lysis of stromal cells28.
Methods
Collection of plant materials and extraction
Diodia sarmentosa (Sw) leaves were collected from the Federal University of Technology, Owerri, Nigeria (Latitude 5o 23.5617ˈN, Longitude 6o 59.1758ˈE). The leaves were identified by a botanist in the Department of Crop Science, Federal University of Technology, Owerri. The leaves were washed with distilled water, air-dried, and then dried in a laboratory oven (DIGITAL TT 9083, Techmel & Techmel USA) at a regulated temperature of 40°C. The dried plant material was grinded, then 800 g each of the powder was weighed and soaked separately in 4 L of ethanol for 48 h on an orbital shaker. The extracts were filtered using a Buckner funnel and Whatman No.1 filter paper and concentrated to dryness at 40oC using a rotary evaporator.
Chemicals
Synthetic monosodium glutamate (Ajinomoto Co., Inc., Tokyo, Japan) was used for this study. All other chemicals were of analytical grade.
Experimental design
Twenty-one nine-week-old, adult female wistar albino rats were acclimatized for ten days in an animal house before being classified into three groups of seven animals each. The animals were bred in the animal house of the Department of Biochemistry, Michael Okpara University of Agriculture, Umudike under standard conditions (25–27 0C, 12 h natural light-dark cycle) and fed with commercially available rat pellets and water ad libitum. The three experimental groups are as follows:
Group A (normal control (NC)): without any induction, provided with water and normal rat chew.
Group B (positive control (PC)): uterine leiomyoma induced in rats using a single daily dose of 200 mg/kg MSG, without treatment.
Group C (treatment group (TG)): uterine leiomyoma induced in rats using a single daily dose of 200 mg/kg MSG, then treated with ethanol extracts of Diodia sarmentosa (Sw) leaves.
Tumor induction
Uterine fibroids were induced with a single daily dose of 200 mg monosodium glutamate (MSG)/kg over 30 days using an oral gavage tube, with slight modifications to the protocol described by Olowofolahan et al.29. At the end of the induction period, representative samples of the uterus were harvested from the three experimental groups and processed using histological techniques and routine Hematoxylin and Eosin (H&E) staining to confirm successful induction of tumors before the commencement of treatment. Presence of densely packed, spindle-shaped fibrous tissue and multifocal tumor cells in the endometrium of the uterus confirmed successful induction of uterine fibroid.
Acute toxicity test of plant extract and administration
The LD50 of the plant extract was determined using Lorke’s method30. Nine rats (100 – 110 g) were divided into three treatment groups treated with (1600, 2900, and 5000) mg/kg of the extract, respectively. Observations were made at 24 h and symptoms of toxicity and mortality were recorded. Two mortalities were recorded in the highest (5000 mg/kg) and medium (2900 mg/kg) dose groups after 24 hours, but none were recorded in the lowest (1600 mg/kg) dose group. Based on this, a safe dose of 400 mg/kg of the plant extract was chosen for treatment.
Animals were treated with 400 mg/kg ethanol extract of Diodia sarmentosa (Sw) leaves once per day from week 5 to week 8 after tumor induction. The dried ethanol extracts of Diodia sarmentosa leaves were weighed and fresh stock solutions prepared every two days by concentrating 8 g of the dried extract in 50 ml of distilled water.
Blood collection and histopathology analysis
Rats were fasted overnight after the experiment and then sacrificed. Blood was drawn from the rats’ orbital sinus using 0.5 ml syringes. Representative samples of the uterus were harvested from the three treatment groups and preserved in Bouin’s fluid. The samples were processed using histological techniques and stained using routine Hematoxylin and Eosin stain for histopathological examinations, adhering strictly to standard protocol.
Biochemical assay
Serum electrolytes (HCO3, Cl-, K+, Na+) were determined using Sood’s spectrometric method31. Berthelot’s method was used in measuring serum urea, while serum creatinine was determined using alkaline picrate method32. Uric acid was determined with the enzymatic method described by Fôssati et al.33. Total protein was assayed using a colorimetric method based on the Biuret reaction34. 17β-oestradiol and progesterone were assayed using the NoviWell™ assay kit (HySkill Diagnostics, Bahlingen, Germany), which employs the sandwich enzyme immunoassay (SIA) microtiter method.
Antioxidant assay
Catalase was determined using the modified method by Hadwan35. Serum glutathione peroxidase was determined using Paglia and Valentine’s method36 . Lipid peroxidation was estimated using Wallin et al.'s method37. Arthur and Boyne’s method was used to determine superoxide dismutase activity38. Total antioxidant capacity was evaluated using Prieto et al.'s method39, with slight modifications. Vitamin E content was estimated using Pearson and Cox's method40. Glutathione reduction was performed using Moron et al.'s method41. Ascorbic acid (vitamin C) was assayed using the micro techniques of clinical chemistry developed by Samuel Natelson in 196142.
Statistical analysis
Data obtained from the experiment was analyzed using Statistical Package for Social Sciences (SPSS) software, version 23. The data was expressed as mean ± standard deviation. Statistical significance was determined using a one-way analysis of variance (ANOVA) followed by least significant difference (LSD) test. Statistical significance was defined as 𝑃 < 0.05.
Results
Biochemical parameters
Serum levels of urea, creatinine, chloride, sodium, and uric acid were significantly (P < 0.05) elevated in the positive control (PC) compared to the normal control (NC) (Figure 1). This suggests a possible relationship between uterine fibroids and renal impairment. Significant (P < 0.05) reductions in the serum levels of urea and creatinine were observed in the treated group (TG). No significant (P < 0.05) reduction was recorded in the serum levels of chloride, sodium, and uric acid in the treated group compared with the positive control. No significant (P < 0.05) difference was observed in the serum levels of bicarbonate (HCO3) or total protein of the positive control or treated group when compared with the normal control (Figure 1 ). This could possibly indicate non-involvement of these two parameters in the development of uterine fibroids. Significant (P < 0.05) elevated levels of serum estradiol and progesterone were observed in the positive control compared with the normal control. Estradiol and progesterone are key players in the formation of fibrotic tissues, and significant (P < 0.05) reductions in the serum levels of estradiol and progesterone were observed in the treated group compared with the positive control.
Oxidative stress and antioxidant parameters
Serum levels of malondialdehyde were significantly (P < 0.05) elevated in the positive control (PC) compared with the normal control (NC) (Figure 2). Elevated levels of malondialdehyde in the serum indicate the presence of oxidative stress. Significant (P < 0.05) reductions in the serum levels of malondialdehyde were observed in the treated group (TG) compared to the positive control. Significant (P < 0.05) reductions were also observed in the serum levels of most of the assayed antioxidants (superoxide dismutase, glutathione peroxidase, vitamin E, catalase, reduced glutathione, vitamin C, and total antioxidant capacity) in the positive control compared with the normal control (Figure 2). However, there was no significant (P < 0.05) difference in the serum levels of vitamin E and vitamin C in the treated group compared with the positive control. Significant (P < 0.05) increases were observed in the serum levels of superoxide dismutase, glutathione peroxidase, catalase, reduced glutathione, and total antioxidant capacity in the treated group compared with the normal control. Antioxidants play a key role in mitigating oxidative stress levels in tissues.
Histopathology
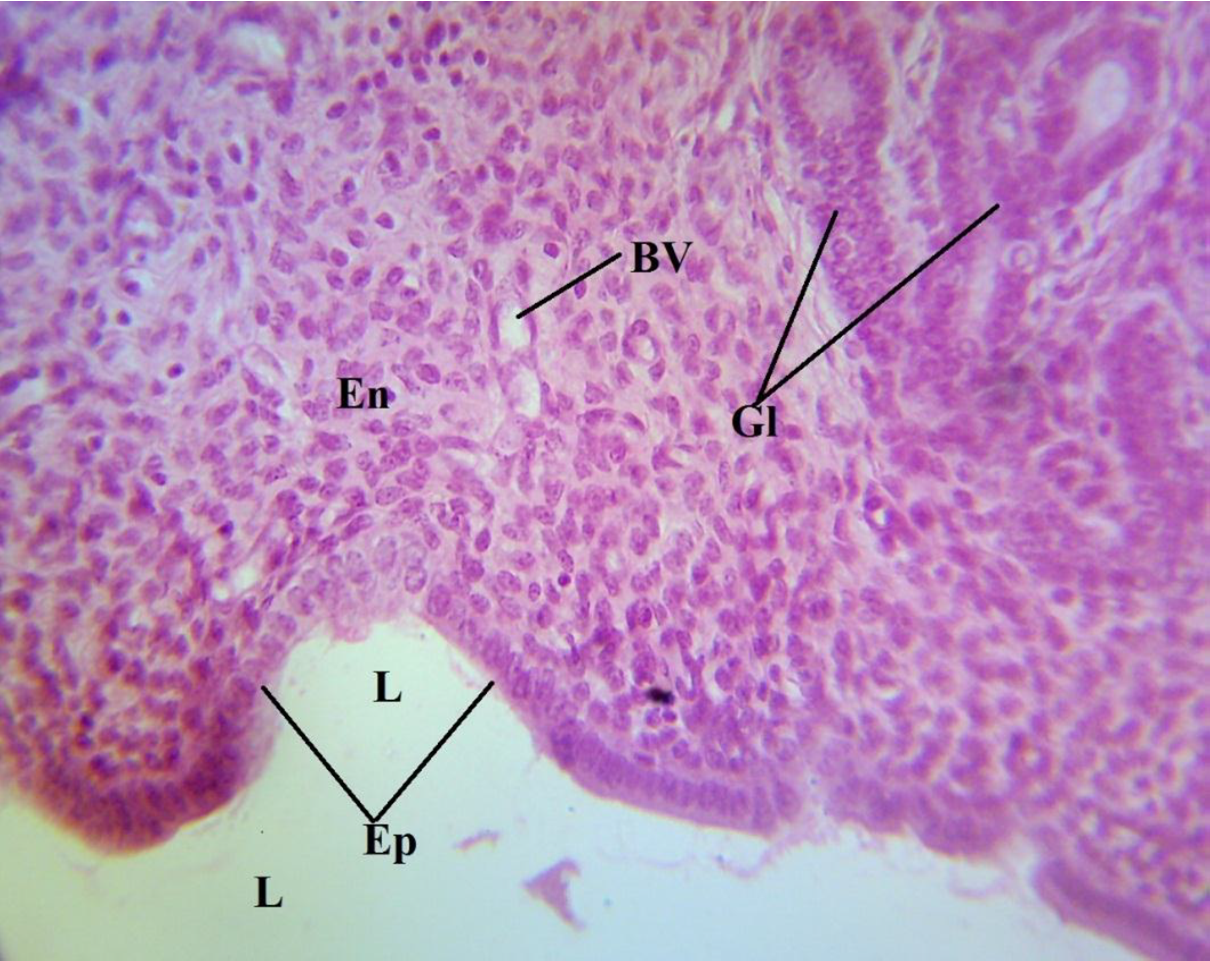
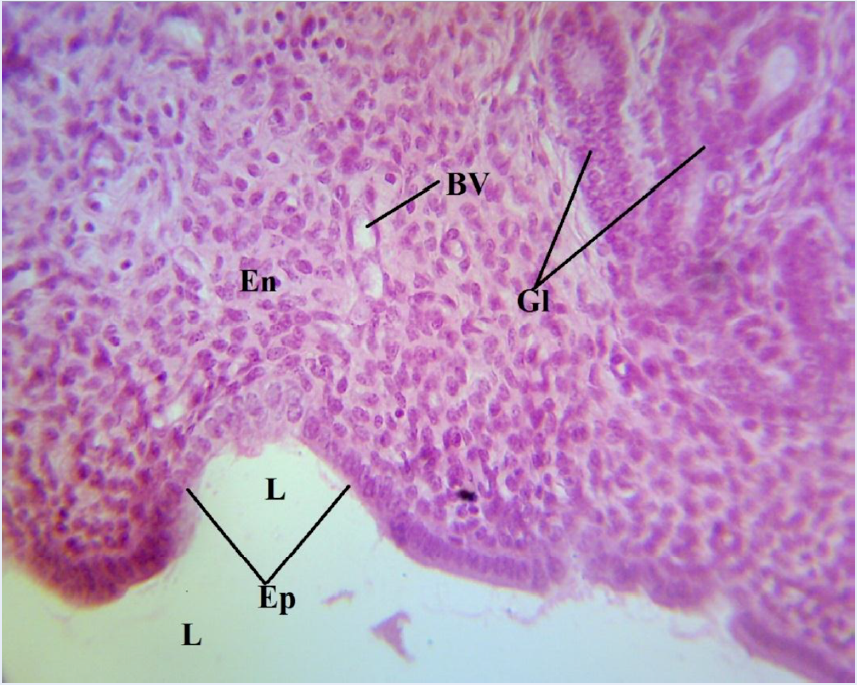
The photomicrograph of a portion of the uterus of the normal control (NC) showed a normal histologic architecture of the uterus, revealing an absence of any form of induction (Figure 3). The endometrium (En) and the epithelium (Ep) of the endometrial mucosa could be seen to be attached to a relatively straight basement membrane, with its mucosal surface facing the uterine lumen (L) and thrown into the longitudinal folds. The extensive lamina propia bears the glands (Gl) (Figure 3), and the blood vessels (BV) are also indicated. A photomicrograph of a portion of the uterus of the positive control (PC) revealed densely packed, spindle-shaped fibrous tissue (F) and multifocal tumor cells (arrow) in the endometrium (Figure 4). This is the group that was induced with uterine leiomyoma using monosodium glutamate but not given treatment. Reduced fibrous tissues and smooth muscle cells, which led to a reduced endometrial and myometrial wall thickness, could be seen in the photomicrograph of the uterus of the treated group (TG) (Figure 5).
Discussion
The significant (P < 0.05) increase in uric acid, creatinine, chloride, sodium, and urea levels and the subsequent significant (P < 0.05) decrease in serum potassium levels in the positive control (PC) compared with normal control (NC) (Figure 1) suggest a possible impairment of the kidney. This may be a result of an obstruction of the pelvic ureters by fibroid tissues. This concurs with earlier reports, which have revealed a correlation between uterine fibroids and renal impairment43. It has also been reported that serum uric acid levels can be elevated by reduced excretion via the kidneys44. No significant (P < 0.05) increase was recorded in serum levels of bicarbonate or total protein in the positive control compared with the normal control (Figure 1), suggesting that there is no relationship between serum total protein levels and fibroid growth. This disagrees with the findings of Obochi et al.45, who reported a correlation between high serum protein levels and fibroid growth. Treatment with ethanol extract of Diodia sarmentosa (Sw) leaves significantly (P < 0.05) decreased the serum levels of urea and creatinine. However, no significant difference was recorded in serum levels of bicarbonate, sodium, uric acid, or total protein in the treated group (TG) compared with the positive control (PC) (Figure 1).
There was a significant (P < 0.05) increase in serum estradiol level of the positive control (PC) compared with the normal control (NC) (Figure 1). The effects of MSG on estrogen levels can be a result of an increase in aromatase activity, which leads to an increase in estradiol synthesis46. There was also a significant (P < 0.05) increase in progesterone levels in PC compared to NC (Figure 1). This may be due to an increase in luteinizing hormone as a result of MSG treatment29. These results concur with the findings of Zia et al.46 and Olowofolahan et al.29, who reported increased progesterone levels in the plasma of MSG-treated animals. Treatment with the ethanol extract of Diodia sarmentosa (Sw) leaves reduced the elevated estrogen and progesterone levels in our study.
The significant (P < 0.05) increase in malondialdehyde (MDA) levels in PC compared with NC (Figure 2) reveals oxidative stress induced by MSG. Increased levels of MDA also indicate an upsurge in lipid peroxidation, which may lead to significant alterations in cell membrane function and DNA composition, leading to mutation. Therefore, increase in lipid peroxidation is a key factor in the progression of uterine fibroid47. Oxidative stress has also been reported as a major factor in the formation of uterine fibroid48, 49, indicating that these findings concur with previous investigations50, 51.
The significant (P < 0.05) decrease in serum levels of antioxidants in PC compared to NC (Figure 2) can be explained as follow: in trying to counter oxidative stress induced by MSG, the system used up most of its enzymatic and non-enzymatic antioxidants resulting in a decrease in serum levels of these antioxidants. These results are also in agreement with earlier reports44, 52. Additionally, uterine fibroids have been reported to be characterized by an impaired antioxidant cellular system44. The ethanol extract of Diodia sarmentosa leaves mitigated the oxidative stress induced in the rats, as seen in the significant (P < 0.05) decrease in serum malondialdehyde levels in TG compared to PC (Figure 2). There was also an improvement in antioxidant levels, as evidenced by significant (P < 0.05) increases in superoxide dismutase, glutathione, catalase, reduced glutathione, and total antioxidant capacity. However, no significant (P < 0.05) increase was recorded in vitamin E and vitamin C levels in TG compared to PC (Figure 2).
Densely packed, spindle-shaped fibrous tissue and multifocal tumor cells in the endometrium as observed in PC confirms a successful induction of uterine fibroids (Figure 4). The fibrous tissue and multifocal tumor cells seen in the positive control were significantly reduced in number in TG, resulting in reduced endometrial and myometrial wall thickness (Figure 5). This suggests diffused atrophy of the uterus, which occurs secondarily to radiotherapy and/or chemotherapy.
Conclusions
This study revealed that oxidative stress, an impaired antioxidant system, and elevated serum estrogen and progesterone levels are key players in the development of uterine fibroids. A relationship between uterine fibroids and renal impairment was also observed, as seen in the significant alterations in the serum electrolytes and uric acid. The ethanol extracts of Diodia sarmentosa leaves had a significant effect on the altered biochemical and antioxidant parameters caused by successful induction of uterine fibroid in rats.
Abbreviations
GnRHa: Gonadotropin-releasing hormone analogues, LMS: Leiomyosarcoma, MSG: Monosodium glutamate, SERMs: Selective estrogen receptor modulators
Acknowledgments
We want to specially appreciate the efforts of Dr. Amarachukwu Igwe of the Department of veterinary pathology, Michael Okpara University of Agriculture Umudike for the histopathology analysis and interpretation. Special thanks also to Dr. Egbachukwu Simon of the Shalom Laboratories for the laboratory analysis and for assisting in securing a standard well ventilated animal house used for this study. We also appreciate the Veterinary Department of Michael Okpara University of Agriculture, Umudike for providing the disease free experimental rats used in this study.
Author’s contributions
Ezejiofor T.I.N. designed the protocol and supervised the work while Okoroafor C.H. did the literature searches, carried out the experiment and wrote the manuscript. The two authors read and endorsed the final manuscript.
Funding
No external funding was received for this research.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the ethical committee on the use of animals for research, Department of Biotechnology, Federal University of Technology, Owerri, Nigeria in line with strict compliance with standard laboratory principles of animal care of the United States National Institute of Health (NIH, 1978).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
National Cancer Institute. What you need to know about the cancer of the uterus.. 2010
.
-
Ferri
S.L.,
Abel
T.,
Brodkin
E.S.,
Sex differences in autism spectrum disorder: a review. Current Psychiatry Reports.
2018;
20
(2)
:
9
.
View Article PubMed Google Scholar -
Adedokun
I.O.,
Avwloro
T.O.,
Ogharanduku
T.O.,
Enaoho
T.M.,
Onyije
F.M.,
Mokogwu
A.T.,
Age prevalence of uterine fibroid in south western Nigeria. African Journal of Cellular Pathology.
2016;
6
:
50-3
.
PubMed Google Scholar -
Viva
W.,
Juhi
D.,
Kristin
A.,
Micaela
M.,
Marcus
B.,
Ibrahim
A.,
Massive uterine fibroid: a diagnostic dilemma: a case report and review of the literature. Journal of Medical Case Reports.
2021;
15
(1)
:
344
.
View Article PubMed Google Scholar -
Whynott
R.M.,
Vaught
K.C.,
Segars
J.H.,
The Effect of Uterine Fibroids on Infertility: A Systematic Review. Seminars in Reproductive Medicine.
2017;
35
(6)
:
523-32
.
View Article PubMed Google Scholar -
Freytag
D.,
Günther
V.,
Maass
N.,
Alkatout
I.,
Uterine Fibroids and Infertility. Diagnostics (Basel).
2021;
11
(8)
:
1455
.
View Article PubMed Google Scholar -
Abam
D.S.,
Kasso
T.,
Uterine Fibroids and Pregnancy: A Review of the Challenges. In: Abduljabbar, H. S. , editor. Obstetrics [Internet]. London: IntechOpen; 2017 [cited 2022 Jul 24]. Available from: https://www.intechopen.com/chapters/57643 doi: 10.5772/intechopen.71761. 2017
.
View Article Google Scholar -
Ndubuka
G.I.,
Jervas
E.,
Ngwogu
K.O.,
Okafor
W.C.,
Nkuma-Udah
K.I.,
Iwuji
S.C.,
The Occurrence of Uterine Benign Diseases and their Histomorphologic Characters. J Foren Path..
2017;
2
(1)
:
1-2
.
-
Andrea
T.,
Uterine Fibroids Management in Asymptomatic Women. International Journal of Gynecology. Obstetrics and Neonata Care.
2019;
6
(1)
:
4-17
.
View Article Google Scholar -
Donnez
J.,
Uterine Fibroids and Progesterone treatment: Lack of evidence of its efficacy: A review. Journal of Clinical Medicine.
2020;
9
(12)
:
3948
.
View Article PubMed Google Scholar -
Dolmans
M.M.,
Cacciottola
L.,
Donnez
J.,
Conservative Management of Uterine Fibroid-Related Heavy Menstrual Bleeding and Infertility: Time for a Deeper Mechanistic Understanding and an Individualized Approach. Journal of Clinical Medicine.
2021;
10
(19)
:
4389
.
View Article PubMed Google Scholar -
Medikare
V.,
Kandukuri
L.R.,
Ananthapur
V.,
Deenadayal
M.,
Nallari
P.,
The genetic bases of uterine fibroids; a review. Journal of Reproduction & Infertility.
2011;
12
(3)
:
181-91
.
PubMed Google Scholar -
Holzmann
C.,
Saager
C.,
Mechtersheimer
G.,
Koczan
D.,
Helmke
B.M.,
Bullerdiek
J.,
Malignant transformation of uterine leiomyoma to myxoid leiomyosarcoma after morcellation associated with ALK rearrangement and loss of 14q. Oncotarget.
2018;
9
(45)
:
27595-604
.
View Article PubMed Google Scholar -
Moro
E.,
Degli Esposti
E.,
Borghese
G.,
Manzara
F.,
Zanello
M.,
Raimondo
D.,
The Impact of Hormonal Replacement Treatment in Postmenopausal Women with Uterine Fibroids: A State-of-the-Art Review of the Literature. Medicina (Kaunas, Lithuania).
2019;
55
(9)
:
549
.
View Article PubMed Google Scholar -
Afolabi-Oluyede
M.O.,
Awolola
O.O.,
Okpere
E.E.,
Ande
A.,
Okonkwo
C.A.,
Ekanem
V.J.,
Clinical significance of oestrogen and progesterone receptors in the growth and symptomatology of uterine fibroids. Tropical Journal of Obstetrics and Gynaecology.
2017;
34
(2)
:
140-4
.
View Article Google Scholar -
Sabry
M.,
Al-Hendy
A.,
Innovative oral treatments of uterine leiomyoma. Obstetrics and Gynecology International.
2012;
2012
(4)
:
943635
.
PubMed Google Scholar -
Parsanezhad
M.,
Jahromi
B.N.,
Parsa-Nezhad
M.,
Medical management of uterine fibroids. Current Obstetrics and Gynecology Reports.
2012;
1
(2)
:
81-8
.
View Article Google Scholar -
Taylor
D.K.,
Leppert
P.C.,
Treatment for uterine fibroids: searching for Effective drug therapies. Drug Discovery Today. Therapeutic Strategies.
2012;
9
(1)
:
e41-9
.
View Article PubMed Google Scholar -
Kalu
W.O.,
Okafor
P.N.,
Ijeh
I.I.,
Eleazu
C.,
Effect of kolaviron, a biflavanoid complex from Garcinia kola on some biochemical parameters in experimentally induced benign prostatic hyperplasic rats. Biomedicine and Pharmacotherapy.
2016;
83
:
1436-43
.
View Article PubMed Google Scholar -
Okoroafor
H.C.,
Awagu
F.E.,
Azeke
E.A.,
Phytochemical and antioxidant properties of Diodia sarmentosa swartz leaves. Mong J Chem..
2020;
21
(47)
:
27-32
.
View Article Google Scholar -
Umoh
U.F.,
Ajibesin
K.K.,
Ubak
N.G.,
Preliminary anti-inflammatory and analgesic effects of Diodia sarmentosa SW. leaf in rodents. World Journal of Pharmacy and Pharmaceutical Sciences.
2016;
5
(12)
:
203-12
.
-
Hemans
M.,
Akoeginou
A.,
Vander
M.J.,
Medicinal plants used to treat malaria in Southern Benin. Economic Botany.
2004;
58
:
239-52
.
View Article Google Scholar -
Soladoye
M.O.,
Osipitan
A.A.,
Sonibara
M.A.,
Chukwuma
E.C.,
From vagabond to ethno botanical relevance: weeds of the campus sites of Olabisi Onabanjo University, Awo-Iwoye, Nigeria. Ethno botanical leaflets.
2010;
2010
(4)
:
546-558
.
-
Akah
P.A.,
Orisakwe
O.E.,
Gamaniel
K.S.,
Shittu
A.,
Evaluation of Nigerian traditional medicines: II. Effects of some Nigerian folk remedies on peptic ulcer. Journal of Ethnopharmacology.
1998;
62
(2)
:
123-7
.
View Article PubMed Google Scholar -
Elechi
N.A.,
Okezie-Okoye
C.,
Abo
K.A.,
Anti-diabetic potentials of Diodia sarmentosa Sw (Rubiaceae) leaves of aloxan-induced diabetic rats. Saudi Journal of Medical and Pharmaceutical Sciences..
2020;
6
(9)
:
622-6
.
View Article Google Scholar -
Bera
T.K.,
Kar
S.K.,
Yadav
P.K.,
Mukherjee
P.,
Yadav
S.,
Joshi
B.,
Effects of monosodium glutamate on human health: A systematic review. World J Pharm Sci..
2017;
5
(5)
:
139-44
.
-
Niaz
K.,
Zaplatic
E.,
Spoor
J.,
Extensive use of monosodium glutamate: A threat to public health?. EXCLI Journal.
2018;
17
:
273-8
.
PubMed Google Scholar -
Mustafa
Z.,
Ashraf
S.,
Tauheed
S.F.,
Ali
S.,
Monosodium glutamate, commercial production, positive and negative effects on human body and remedies - a review. IJSRST.
2017;
3
:
425-35
.
-
Olowofolahan
A.O.,
Aina
O.O.,
Hassan
E.T.,
Olorunsogo
O.O.,
Ameliorative Potentials of Methanol Extract and Chloroform Fraction of Drymaria cordata on MSG induced Uterine Hyperplasia in Female Wistar Rats. European Journal of Medicinal Plants.
2017;
20
(4)
:
1-9
.
View Article Google Scholar -
Lorke
D.,
A new approach to practical acute toxicity testing. Archives of Toxicology.
1983;
54
(4)
:
275-87
.
View Article PubMed Google Scholar -
Sood
R.,
Medical Laboratory Methods and Interpretations. New Delhi, India: Jaycee Brothers Medical Publishers; 1990. 132 – 144 p. .
.
-
Tietz
N.W.,
Prude
E.,
Tietz textbook of Clinical Chemistry. In London: W.B. Saunders Company; 1994. p. 1354–74. .
.
-
Fossati
P.,
Precscipe
L.B.,
Use of 3, 5- dichloro-2-hydroxybenzene Suiphoric acid 14- aminophenazone chromagenic' sy in direct enzymatic assay of uric acid in sert andurine. Clinical Chemistry.
1980;
26
(2)
:
227-30
.
View Article PubMed Google Scholar -
Burtis
C.A.,
Ashwood
E.R.,
Bruns
D.E.,
Tietz Textbook of Clinical Chemistry and Molecular diagnosticsWB Saunders Company, Elsevier: St. Louis, USA; 2012.
Google Scholar -
Hadwan
M.H.,
New Method for Assessment of Serum Catalase Activity. Indian Journal of Science and Technology.
2016;
9
(4)
:
1-5
.
View Article Google Scholar -
Paglia
D.E.,
Valentine
W.N.,
Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine.
1967;
70
(1)
:
158-69
.
PubMed Google Scholar -
Wallin
B.,
Rosengren
B.,
Shertzer
H.G.,
Camejo
G.,
Lipoprotein oxidation and measurement of TBARS formation in single microlitre plate; it's use for evaluation of antioxidants. Analytical Biochemistry.
1993;
208
(1)
:
10-5
.
View Article PubMed Google Scholar -
Arthur
J.R.,
Boyne
R.,
Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sciences.
1985;
36
(16)
:
1569-75
.
View Article PubMed Google Scholar -
Prieto
P.,
Pineda
M.,
Aguilar
M.,
Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry.
1999;
269
(2)
:
337-41
.
View Article PubMed Google Scholar -
Pearson
D.,
Cox
E.H.,
The Chemical Analysis of foodsChurchill Livingstone: Edinburgh, New York; 1976.
Google Scholar -
Moron
M.S.,
Depierre
J.W.,
Mannervik
B.,
Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta.
1979;
582
(1)
:
67-78
.
View Article PubMed Google Scholar -
Natelson
S.,
Microtechniques of clinical chemistryCharles C Thomas: Springfield (IL); 1961.
Google Scholar -
Fletcher
H.M.,
Wharfe
G.,
Williams
N.P.,
Gordon-Strachan
G.,
Johnson
P.,
Renal impairment as a complication of uterine fibroids: a retrospective hospital-based study. Journal of Obstetrics & Gynaecology.
2013;
33
(4)
:
394-8
.
View Article PubMed Google Scholar -
Angelopoulos
T.J.,
Lowndes
J.,
Zukley
L.,
Melanson
K.J.,
Nguyen
V.,
Huffman
A.,
The effect of high-fructose corn syrup consumption on triglycerides and uric acid. The Journal of Nutrition.
2009;
139
(6)
:
1242-5
.
View Article PubMed Google Scholar -
Obochi
G.O.,
Malu
S.P.,
Obi-Abang
M.,
Alozie
Y.,
Iyam
M.A.,
Effect of Garlic Extracts on Monosodium Glutamate (MSG) Induced Fibroid in Wistar Rats. Pakistan Journal of Nutrition.
2009;
8
(7)
:
970-6
.
View Article Google Scholar -
Zia
M.S.,
Qamar
K.,
Hanif
R.,
Khalil
M.,
Effect of monosodium glutamate on the serum estrogen and progesterone levels in female rat and prevention of this effect with diltiazem. Journal of Ayub Medical College, Abbottabad.
2014;
26
(1)
:
18-20
.
PubMed Google Scholar -
Bilal
K.M.,
Measurement of some biochemical parameters in serum of uterine cancer. Raf J Sci..
2013;
24
(6)
:
37-44
.
View Article Google Scholar -
Vural
M.,
Camuzcuoglu
H.,
Toy
H.,
Camuzcuoglu
A.,
Aksoy
N.,
Oxidative stress and prolidase activity in women with uterine fibroids. Journal of Obstetrics {&}amp; Gynaecology.
2012;
32
(1)
:
68-72
.
View Article PubMed Google Scholar -
Rahman
K.,
Studies on free radicals, antioxidants, and co-factors. Clinical Interventions in Aging.
2007;
2
(2)
:
219-36
.
PubMed Google Scholar -
Pejić
S.,
Kasapović
J.,
Todorović
A.,
Stojiljković
V.,
Pajović
S.B.,
Lipid peroxidation and antioxidant status in blood of patients with uterine myoma, endometrial polypus, hyperplastic and malignant endometrium. Biological Research.
2006;
39
(4)
:
619-29
.
View Article PubMed Google Scholar -
Mohammad
T.U.,
Abass
E.A.,
Salman
M.A.,
Estimation Arginase Activity in the Serum of Uterine Fibroid Females and its Relationship with Other Parameters. Journal of Natural Sciences Research..
2014;
4
(24)
:
1-5
.
-
Oyeyemi
A.O.,
Oyeyemi
R.B.,
Molehin
O.R.,
Evaluation of Some Mineral Elements and Antioxidant Status in Fibroid Patients in Ado Ekiti, Nigeria. Journal of Research in Pharmaceutical Science.
2016;
3
(2)
:
1-3
.
Comments
Article Details
Volume & Issue : Vol 9 No 7 (2022)
Page No.: 5140-5148
Published on: 2022-07-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 5984 times
- PDF downloaded - 1554 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress