Abstract
Dermatomyositis is an inflammatory autoimmune disease that causes significant muscle damage. The diagnosis and treatment of this disease commonly requires both therapeutic and dental care. This article describes a clinical case of dermatomyositis where the patient has been receiving therapy since 2018. Stable disease remission was achieved with therapy, with dual-energy X-ray absorptiometry (DXA) indicating an improvement in bone tissue parameters. However, despite this remission, the patient experienced progressive dental manifestations (characteristic complaints, presence of oral mucosal lesions, destruction of jawbone structures). The primary cause of these dental manifestations was not found to be local oral cavity factors, suggesting they were the consequence of both disease pathology and steroid treatment that targeted the oral cavity tissues. The collaboration between an internist and dentist contributed to the early diagnosis and prevention of possible complications associated with this disease.
Introduction
Dermatomyositis is a rare, acquired inflammatory autoimmune disease that causes significant muscle damage. According to Gokhale Y. et al., the incidence of this disease is 9.3 cases per 1 million people1. The disease is associated with a high rate of premature mortality. D’Silva K.M. et al. reported on patient mortality across two different time periods (early versus late cohort). In the early cohort, mortality was 71.5 deaths per 1000 person-years, while in the late cohort, it was 49.1 deaths per 1000 person-years, suggesting little improvement in the rate of premature mortality across the two time periods2. Moreover, Marie I. (2012) found that over 80% of treated patients developed long-term disabilities or malignancies3. Dermatomyositis is characterized by a wide range of clinical manifestations, as its pathological process involves various systems. In the initial stages of the disease, autoimmune inflammation develops in the striated muscles and skin; however, the lungs may also be involved. Much of the previous literature focuses on the internal organ lesions associated with the disease. However, the consequences of the disease are broad, with pathological manifestations observed both in facial skeletal bone structures and in the oral cavity. While some previous studies have investigated the dental manifestations of dermatomyositis4, 5, 6, 7, 8. The primary aim of this study was to determine the dental status changes in a patient with dermatomyositis.
Case presentation
In 2018, a patient (patient record No. 7463) sought medical attention, complaining of breath shortness following minimal physical activity, heart palpitations, a dry, nonproductive cough, muscle weakness and pain, severe general weakness, and a body temperature of 38°C. The patient’s history showed that in 2006, they were diagnosed with erythema nodosum. In 2016, during pregnancy, the patient was diagnosed with Raynaud’s syndrome. Following breastfeeding termination, edema and cyanosis developed around the eyes. Over time, wrist joint pain and femoral muscle weakness also developed, with an increase in muscle weakness being noted since 2017. X-ray examination of the thoracic organs revealed bilateral pneumonia, which was unsuccessfully treated with antibiotics. Additionally, the patient’s Raynaud’s syndrome worsened during this treatment. In April 2017, the patient was examined by a rheumatologist, who identified positive antinuclear antibodies (ANAs) and prescribed 32 mg/day of Medrol as treatment, which improved the condition. The patient was diagnosed with a subacute course of active phase primary dermatomyositis (activity III) and antisynthetase syndrome, involving skin lesions (paraorbital edema, heliotropic erythema, and erythematous rash), décolleté symptoms, joint symptoms (non-erosive arthritis, polyarthralgia, and Ro-changes І FN II stage), severe Raynaud’s syndrome, lung damage (interstitial lung disease, DN II stage), heart damage (myopericarditis, myocardiofibrosis, HF II A stage, ІІІ), kidney damage (XXI, grade I), immunological phenomena (ANAs, IgG antibodies to Jo-l, IgG antibodies to RNP), Itsenko-Cushing disease (drug origin), systemic osteoporosis, and compression fractures of the first vertebra of the lumbar spine (L1). Pulse therapy was conducted using Medrol and Endoxan therapy. Due to the severity of the Raynaud’s syndrome, on September 24, 2018, the distal phalanges of right-hand fingers II and III left-hand finger II were surgically resected. Following this, courses of Octagam therapy were prescribed. Once remission was achieved, Medrol therapy continued at a decreased dosage of 8 mg/day. In December 2018, the patient was determined to be pregnant; an induced abortion (vacuum-exoculation) was performed. Following this, the patient’s condition significantly worsened; their shortness of breath worsened, while muscle weakness, fever, and joint pain developed. To combat this, 500 mg intravenous rituximab was administered on day 1 and day 14 of each two-week cycle. After 6 months, 32 mg/day of methylprednisolone was prescribed in addition to the rituximab therapy, which was decreased to a single 500 mg every 6 months. Once clinical and laboratory remission was confirmed, the methylprednisolone dose was reduced to 8 mg/day. Since 2018, the patient has been prescribed 2000 IU/day of vitamin D and 1000 mg/day of oral denosumab, as well as a subcutaneous injection of 60 mg of denosumab every 6 months.
In 2021, the patient noticed the development of severe mouth and nose dryness, halitosis, gum bleeding when brushing their teeth or eating, changes in tooth mobility and position, and changes in gum shape. They were referred to a dentist for a consultation.
The consultation involved the examination of the perioral region, lips, and oral mucosa, assessment of hygiene and periodontal indices, and measurement of periodontal pocket depth, epithelial attachment loss, and salivation rate changes. In addition, X-ray examination (panoramic radiography) and cone-beam computed tomography (CBCT) were performed. The examination found that the patient had no difficulty opening their mouth, and their temporomandibular joint movements were full, symmetrical, and painless. However, the skin of the perioral region was noted to be dry and scaly with pronounced maceration, while the lip’s border was dry, covered with scaly crusts, and tight in the center. Color changes in the mucous membrane of the upper lip were observed, caused by capillary pattern changes from dilated blood vessels (telangiectasia). No color changes were seen on the lower lip.
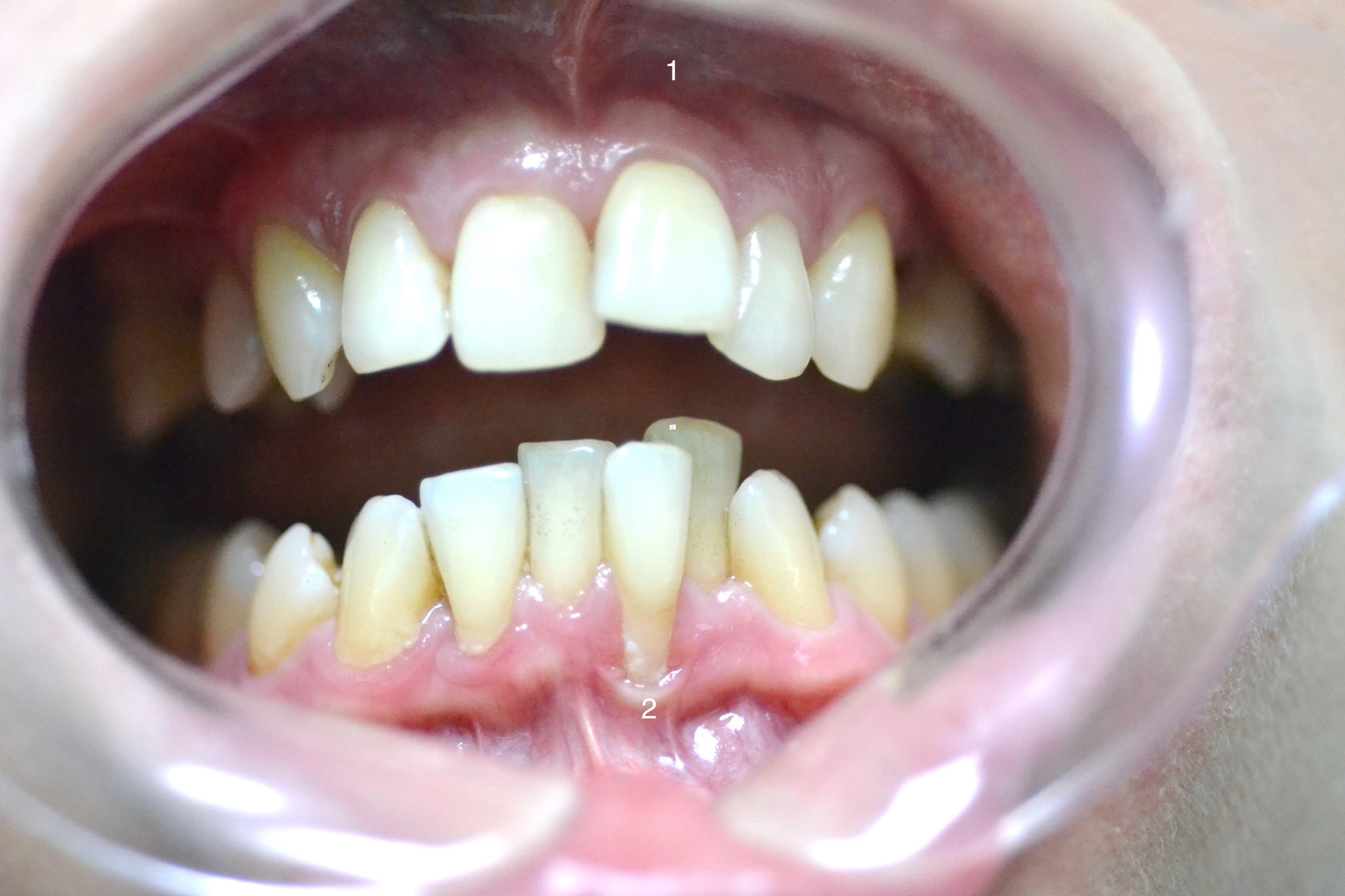
The gums of the frontal upper and lower jaw were dry with a matte sheen, with pale coloration along the papillary-marginal edge and hyperemia of the alveolar ridge (Figure 1). The gum biotype was thin, with gum palpation around the 23rd tooth causing pain. Around the lower anterior teeth, in particular, the 31st tooth, local, roller-shaped gum edge recession and thickening (McCall’s festoon) was observed, caused by badly adhered frenum and mucous membrane strands. Based on the clinical picture, it was determined that the patient was in remission from grade 1–2 generalized periodontitis.
The saliva was viscous, thick, foamy, and formed threads when stretched. The rate of unstimulated salivation on an empty stomach was significantly lower than normal, at 0.1 mL/minute.
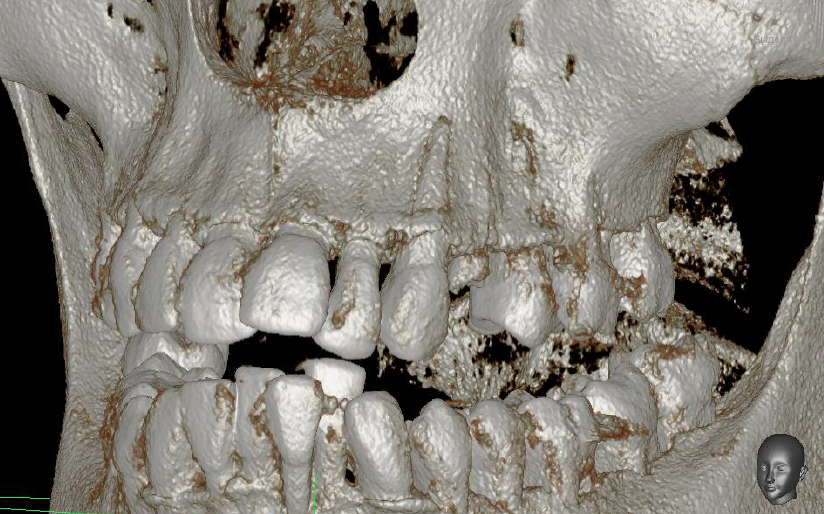
Analysis of the CBCT data revealed nonconformity between the clinical and radiological pictures of the periodontium. The CBCT data demonstrated destruction along the length of the alveolar wall, with signs of chronic inflammatory process exacerbation (Figure 2), as well as scalloped bone edges with altered resorption levels on both the upper and lower jaw. Furthermore, on the 23rd tooth, the middle and lower third of the root was exposed due to fenestration of the vestibular cortical plate of the alveolar ridge.
In the lower jaw, rarefaction of bony septa, resorption in the alveolar septa apexes, bony pockets, and the absence of the cortical vestibular plate around the 31st tooth were observed. The bone tissue pattern indicated progressive heterogeneous osteoporosis in alveolar ridges of the upper and lower jaws (Figure 3).
Throughout the entire observation period, the patient’s bone tissue was assessed using DXA. The densitometry data is shown in Table 1.
| T-zone | Z-zone | |||||||
| 2017 | 2018 | 2019 | 2021 | 2017 | 2018 | 2019 | 2021 | |
| Femora | ||||||||
| Neck | -0.6 | -0.6 | -0.5 | -0.4 | -0.5 | -0.6 | -0.5 | -0.4 |
| Troch | 0.0 | -0.8 | -0.6 | -0.6 | -0.1 | -0.8 | -0.6 | -0.6 |
| Inter | 0.1 | 0.2 | 0.0 | 0.0 | 0.1 | 0.2 | 0. | 0.0 |
| Total | -0.2 | -0.3 | -0.4 | -0.3 | -0.2 | -0.4 | -0.3 | -0.3 |
| Ward’s | -0.3 | -0.4 | -0.3 | -0.2 | 0.0 | -0.2 | 0.0 | 0.1 |
| Lumbar spine | ||||||||
| L1 | -2.5 | -1.1 | -0.7 | 0.0 | -2.4 | -1.0 | -0.7 | 0.1 |
| L2 | -0.7 | -2.1 | 0.0 | 0.2 | -0.7 | -2.1 | 0.0 | 0.2 |
| L3 | -1.6 | -1.6 | -0.1 | -0.1 | -1.6 | -1.6 | 0.0 | 0.0 |
| L4 | -0.6 | -2.1 | -1.1 | -0.8 | -0.6 | -2.1 | -1.0 | -0.7 |
| Total | -1.2 | -1.8 | -0.5 | -0.3 | -1.2 | -1.8 | -0.5 | -0.2 |
Discussion
Osteoporosis in dermatomyositis patients can be caused by several isolated factors; however, most clinicians associate the occurrence of osteoporosis with steroid hormone therapy. Lee C.W. et al. found that dermatomyositis patients were 2.99 times more likely to develop osteoporosis than those without dermatomyositis, regardless of whether they received corticosteroid and immunosuppressant treatment9. Similarly, Vincze A. et al. found that 13.5% of study participants with dermatomyositis had osteoporosis10, while Gupta L. et al. reported that over half of examined inflammatory myositis patients had asymptomatic vertebral body fractures11. One of the most likely causes of bone mineral density reduction in such cases is muscle weakness and patient immobilization. Therefore, the symptoms of dermatomyositis can be managed through steroid therapy. However, the presence of xerostomia, changes to the lip border and oral cavity mucous membrane, periodontal pocket development, and jaw bone osteoporosis in patients suggest that the salivary glands and periodontium tissues and structures are targets for both dermatomyositis and the drugs administered for its treatment.
Conclusion
Dermatomyositis is a unique inflammatory disease. Its treatment requires collaboration between an internist and dentist in order to achieve early diagnosis and prevent possible complications. Once diagnosed, patients should have frequent dental follow-ups, even without visible evidence of intraoral disease. To prevent dental lesions, personalized oral hygiene algorithms should be developed that consider oral cavity risk factors by creating individual risk webs. These algorithms should specify the frequency of dental visits and include the monitoring and removal of dental plaque and relief of xerostomia signs. Furthermore, education should be given regarding at-home oral hygiene (item selection, means of hygiene, method of tooth brushing), salivation control, and how to stabilize teeth using removable and nonremovable splinting.
Abbreviations
None.
Acknowledgments
None.
Author’s contributions
N.E.: Conceived of the study, carried out the clinical studies, drafted the manuscript, data acquisition.
A.I.: Data analysis Guarantor, Literature search, Clinical studies, participated in the design of the study, Data analysis.
All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The procedures have been reviewed and approved by the Ethics Committee of Government Institution “L.T. Malaya Therapy National Institute of the National Academy of Medical Sciences of Ukraine” All procedures were in accordance with the ethical standards of the institutional and/or national research committee, as well as the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards. “Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent may be made available for review by the editor-in-chief of this journal."
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Gokhale
Y.,
Patankar
A.,
Holla
U.,
Shilke
M.,
Kalekar
L.,
Karnik
N.D.,
Dermatomyositis during COVID-19 Pandemic (A Case Series): Is there a Cause Effect Relationship?. The Journal of the Association of Physicians of India.
2020;
68
(11)
:
20-4
.
PubMed Google Scholar -
D'Silva
K.M.,
Li
L.,
Lu
N.,
Ogdie
A.,
Avina-Zubieta
J.A.,
Choi
H.K.,
Persistent premature mortality gap in dermatomyositis and polymyositis: a United Kingdom general population-based cohort study. Rheumatology (Oxford, England).
2020;
11
.
View Article PubMed Google Scholar -
Marie
I.,
Morbidity and mortality in adult polymyositis and dermatomyositis. Current Rheumatology Reports.
2012;
14
(3)
:
275-85
.
View Article PubMed Google Scholar -
Gonçalves
L.M.,
Bezerra-Júnior
J.R.,
Gordón-Núñez
M.A.,
Libério
S.A.,
de Fátima Vasconcelos Pereira
A.,
da Cruz
M.C.,
Oral manifestations as important symptoms for juvenile dermatomyositis early diagnosis: a case report. International Journal of Paediatric Dentistry.
2011;
21
(1)
:
77-80
.
View Article PubMed Google Scholar -
Dourmishev
L.A.,
Dourmishev
A.L.,
Oral Manifestations of Dermatomyositis. Dermatomyositis: advances in Recognition. Understanding and Management.
2009;
61-3
:
61-3
.
View Article Google Scholar -
Geist
S.M.,
Tanaka
T.I.,
Oral lichen planus in a dermatomyositis patient that resolved after intravenous immunoglobulin therapy. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.
2014;
118
(4)
:
e111-4
.
View Article PubMed Google Scholar -
Wojcik
M.,
López-Torres
A.,
Neely
A.,
Haddow
M.,
Kinaia
B.,
Management of a Periodontal Patient With Dermatomyositis: A Case Report. Clinical Advances in Periodontics.
2021;
11
(1)
:
39-42
.
View Article PubMed Google Scholar -
Deepa
D.,
Arun Kumar
K.V.,
Chawla
A.,
Patil
P.,
Den- tal management of a patient associated with Dermatomyositis. Univ Re! s. Journal of Dentistry.
2015;
5
(3)
:
207-10
.
View Article Google Scholar -
Lee
C.W.,
Muo
C.H.,
Liang
J.A.,
Sung
F.C.,
Hsu
C.Y.,
Kao
C.H.,
Increased osteoporosis risk in dermatomyositis or polymyositis independent of the treatments: a population-based cohort study with propensity score. Endocrine.
2016;
52
(1)
:
86-92
.
View Article PubMed Google Scholar -
Vincze
A.,
Bodoki
L.,
Szabó
K.,
Nagy-Vincze
M.,
Szalmás
O.,
Varga
J.,
The risk of fracture and prevalence of osteoporosis is elevated in patients with idiopathic inflammatory myopathies: cross-sectional study from a single Hungarian center. BMC Musculoskeletal Disorders.
2020;
21
(1)
:
426
.
View Article PubMed Google Scholar -
Gupta
L.,
Lawrence
A.,
Edavalath
S.,
Misra
R.,
Prevalence and predictors of asymptomatic vertebral fractures in inflammatory myositis. International Journal of Rheumatic Diseases.
2018;
21
(3)
:
725-31
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 6 (2022)
Page No.: 5084-5088
Published on: 2022-06-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 5026 times
- PDF downloaded - 1551 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress