Abstract
Background: Transferring a single blastocyst with high implantation potential reduces the likelihood of a multiple pregnancy. However, blastocyst transfer requires long-term culture, increases laboratory work, and increases the risk of having no embryo for transfer. This study aimed to establish a predictive model for developing usable blastocysts based on the morphokinetic and morphologic features of day 3 embryos.
Methods: This retrospective cohort study was performed at IVFAS, An Sinh Hospital, Vietnam. Cycles in which patients ≤ 38 years old were undergoing intracytoplasmic sperm injection treatment and had embryos cultured in a time-lapse monitoring system were included. Patients who had been treated by in vitro maturation, who used surgically retrieved sperm, or who had olycystic ovary yndrome were excluded from the study. The independent t-test was performed to analyze continuous data, while the chi-square test was used to investigate categorical variables. Statistical significance was defined by a p-value < 0.05. Multivariate logistic regression analysis evaluated the relationship between statistically significant variables and blastocyst formation.
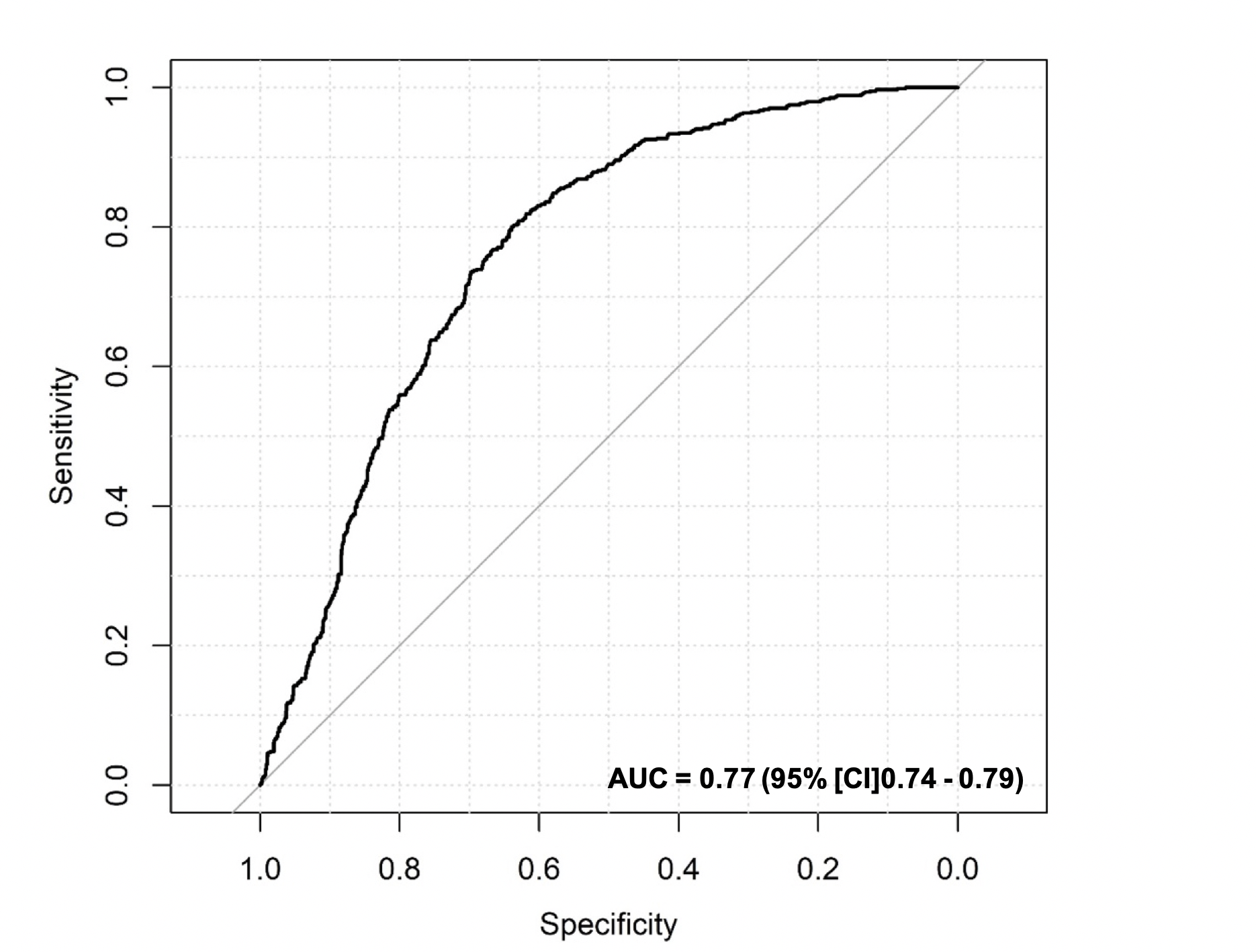
Results: From March 2016 to August 2018, 1,629 embryos extracted from 169 women were collected. The average patient age and anti-Müllerian hormone levels were 32.02 ± 3.77 years and 5.62 ± 3.89 ng/ml, respectively. Using Bayesian model averaging and multivariate logistic regression, the number of blastomeres, fragmentation rate, and time of division to five cells (t5) were identified as the most predictive factors. The final model had an under the receiver operating characteristic curve of 0.77 (95% confidence interval = 0.74 – 0.79), and the predicted and observed probabilities of the usable blastocyst did not significantly differ (Hosmer-Lemeshow test, p > 0.05).
Conclusion: The number of blastomeres, fragmentation rate, and t5 value can be used to predict usable blastocyst formation from the embryo at the cleavage stage.
Introduction
The primary purpose of assisted reproductive technology is to help infertile couples achieve a safe pregnancy and a single healthy baby. Selecting a high-quality embryo for transfer is critical to successfully carrying out a singleton pregnancy. Hence, blastocyst transfer strategies are often used to reduce the number of embryo transfers leading to multiple pregnancies while also achieving a high pregnancy rate. Compared with cleavage embryo transfer, blastocyst transfer is considered to have higher growth potential and synchronization with the endometrium1. However, it requires extended culture times that can compromise embryo quality and alter the spectrum of epigenetic modifications2. Consequently, scientists have focused on selecting a single embryo with high potential at the cleavage stage. Traditional embryo selection is mainly based on morphological observations. Nevertheless, the pregnancy conventional evaluation rate is low, and the embryos need to be removed from the incubator for assessment under a microscope. Therefore, the time-lapse system has been introduced as a new tool for embryo assessment. In addition, there is evidence that having more information about the dynamic division might result in significant pregnancy outcomes than selection based only on morphological assessment3, 4, 5.
Several models for embryo selection based on morphokinetic parameters have been developed to predict blastocyst formation6, 7, 8, 9 or implantation potential10, 11. In 2010, Wong et al. reported that the possibility of blastocyst development depended on the duration of the first cell cycle, the duration of the second cell cycle (cc2), and embryonic synchrony during the second meiosis (s2)6. Meseguer et al. published a hierarchical model that used early embryo morphokinetics to predict embryo implantation10. The findings of Milewski et al. (2015) showed that blastocyst development was related to the timing of embryo division into the 2-cell stage (t2) and the 5-cell stage (t5), as well as the cc212. Several investigations have been conducted in external laboratories to assess the prediction capability of various algorithms13, 14. However, some studies have found that the model has limited predictive power when applied to other clinical centers15, 16. Relatedly, embryos have been found to develop at different speeds in different laboratories for many reasons, such as differing patient populations, ovarian stimulation regimens, and embryo culture conditions, including differing oxygen levels and the use of various commercially produced media, whether sequential or single-step media8, 17. These results indicated that the aforementioned predictive models have several limitations and cannot be easily adjusted to provide a globally accepted model. Therefore, to improve the transferability of the time-lapse algorithm, it is necessary to develop an in-house model specific to a given IVF center, and more data is needed to evaluate the developed method.
Accordingly, this study aimed to establish a model to predict usable blastocyst formation by using the morphological and morphokinetic parameters of an embryo at the cleavage stage.
Methods
Patient population and study design
A retrospective cohort study covering the period from March 2016 to August 2018 was performed at IVFAS (An Sinh Hospital, Viet Nam). The study included only those patients under the age of 38 years who were treated with intracytoplasmic sperm injection (ICSI) using the antagonist protocol and frozen blastocyst cultured in a time-lapse monitoring system. The study did not include patients who underwent in vitro maturation cycles, those with polycystic ovary syndrome, those treated using surgical sperm retrieval (PESA/TESE), or those treated with frozen oocytes/sperm. One hundred sixty-nine patients treated with assisted reproductive technology were ultimately included in this study.
Ovarian stimulation
Ovarian stimulation was performed using follicle-stimulating hormone (FSH) following a gonadotropin-releasing hormone antagonist protocol. The FSH dose was estimated based on patient characteristics such as age, anti-Müllerian hormone level, antral follicle count, and body mass index (BMI) on day 2 of the menstrual cycle. The ovarian response was monitored using ultrasound scan and estradiol and progesterone levels. Recombinant human chorionic gonadotropin (hCG) or diphereline was administered to stimulate oocyte maturation when at least one follicle 17 mm in diameter was present during oocyte stimulation. Then, 36 hours later, the oocytes were retrieved by transvaginal aspiration under ultrasound guidance.
Embryo culture
After retrieval, the oocyte-cumulus-complexes (OCC) were washed and cultured in G-IVF medium (Vitrolife, Sweden) in a benchtop (G815, K-System, Denmark) at 37°C and in 6% CO2 and 5% O2 condition for 2 hours before denudation. Then, to remove the surrounding cumulus cells, oocyte denudation was conducted by mechanical treatment (using Pasteur pipettes) and Hyase enzyme (Vitrolife, Sweden). Then, sperm was injected into all the metaphase II oocytes through ICSI technology in a medium containing HEPES (G-Gamete, Vitrolife). After ICSI, the injected oocytes were transferred into G1-Plus media (Vitrolife, Sweden) at 37°C and 6% CO2, 5% O2 in a benchtop (G210, K-System, Denmark).
Time-lapse recording
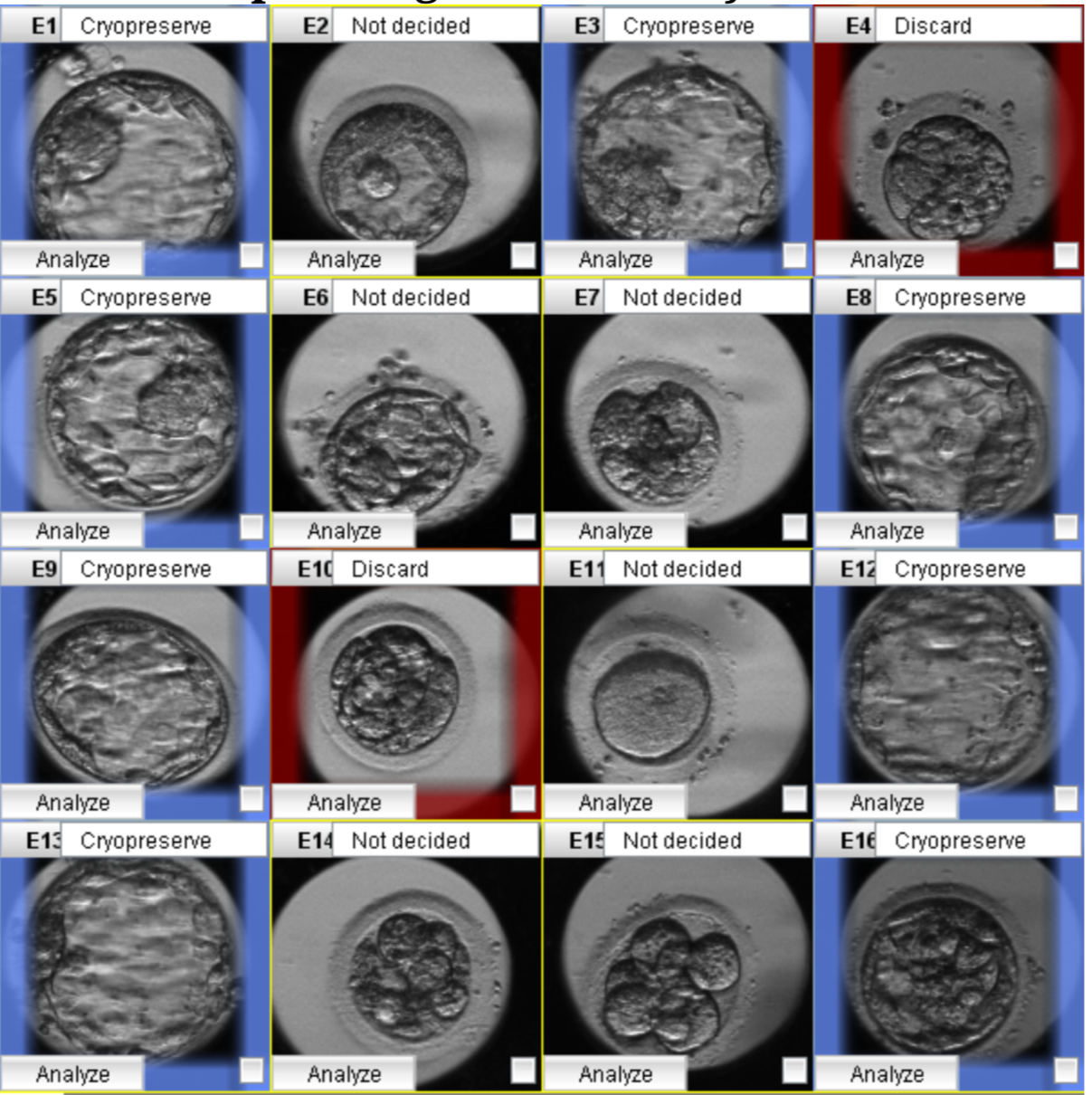
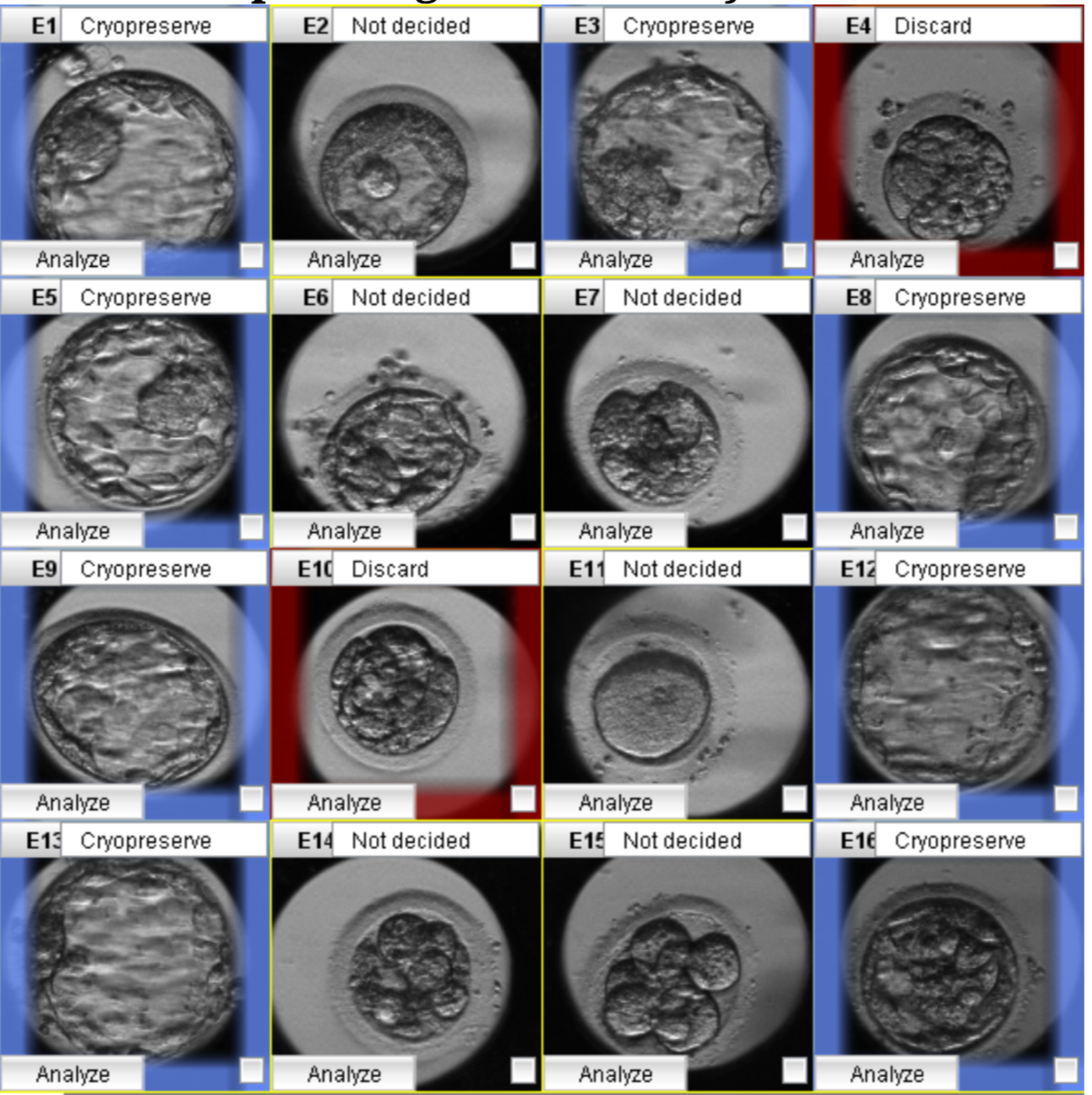
After ICSI, fertilization was assessed at 16-18 hours post-insemination, and the embryos were transferred to time-lapse dishes (Vitrolife, Sweden) containing G-TL medium (Vitrolife, Sweden). Then, each cultured dish was put into a Primo Vision time-lapse system (Vitrolife, Sweden) and placed in a Galaxy 170R incubator at 37°C with 6% CO2 and 5% O2 levels (New Brunswick, Germany). The exposure time for the images was 10 minutes per image. In addition, the entire embryonic development process was monitored using the Primo Vision time-lapse software. Because the time-lapse plate used only has 16 culture wells for 16 embryos, the IVFAS Center used fertilized embryos for time-lapse monitoring to optimize the number of successful ICSI embryos. The timing of embryo division and the presence of any abnormal characteristics were observed. The specific kinetic division was determined by an embryologist using the times of division into the two-cell through eight-cell stage (t2, t3, t4, t5, t8), the time of morula formation (tM), the time of early blastulation, the time of full blastocyst (tB), and the time of hatching. Reverse cleavage (that is, a reduction in the number of cells), direct cleavage (that is, when a single cell divides into more than two cells), and multinucleated blastomeres were among the aberrant cleavages observed during cell division18. Variables associated with cell cycle duration in synchronous cleavage, including synchrony in the division from two to four cells (s2 = t4 − t3), synchrony in the division from five to eight cells (s3 = t8 − t5), the length of the second cell cycle (cc2 = t3 − t2), and the length of the third cell cycle (cc3 = t5 −t3)10, 19, 20, were also monitored. All morphokinetic evaluations were performed with reference to the aforementioned Meseguer et al. study, and the resulting data were inputted into the software10. The morphologic characteristics, the timing of dynamic events, and the presence of abnormal cleavage also were used to help identify embryos for selection (Figure 1)13, 14, 17.
Frozen-thawed blastocyst transfer cycle
The embryos used had been vitrified using the Cryotech method with no more than two embryos per cryotec. Endometrial preparation was then conducted using exogenous estradiol and progesterone until the endometrial thickness reached ≥8 mm. Next, the embryos were thawed and transferred to the uterus under ultrasonographic monitoring five days after progesterone exposure.
Study outcomes
The primary goal of this study was the predictive model for usable blastocyst development, with implantation and continued pregnancy rates as secondary outcomes. The beta-hCG value (≥ 25 mIU/mL) was measured 12 days after embryo transfer. In addition, the presence of a fetal heartbeat confirming clinical pregnancy was determined by ultrasound at seven weeks of gestation.
Model performance
The morphological and morphological parameters were evaluated and analyzed. The present study included two stages: the generation of a predictive model for blastocyst formation and the verification of the value of that predictive model.
In the first stage of this study, multivariate logistic regression analysis was applied to determine the relationship between statistically relevant variables and blastocyst development. Next, Bayesian model averaging (BMA) was performed for pair samples to reduce the number of variables for the final predictive model. This was followed by the use of receiver operating characteristic (ROC) analysis to test the ability of the embryo to achieve usable blastocyst formation. Then, the value of the final predictive model was calibrated in the second stage of the study through internal validation. For this calibration, the predictive values of the final model were compared with actual observed usable blastocyst development (Hosmer-Lemeshow test, p > 0.05). The predictive model was internally validated using 169 KID-transferred blastocysts. Finally, to provide clinicians with a user-friendly interface for the final model, we developed a nomogram that could be used as a graphical tool for generating an individual patient profile.
Statistical analysis
The R statistical software, version 3.3.3 (Vienna, Austria), was used to analyze the data. First, the chi-square test was conducted to analyze categorical data. The analysis included embryos that had not developed to the blastocyst stage. Usable blastocyst can be defined as those that can be used for fresh transfer or frozen transfer. The statistical model chosen was logistic regression and was performed by using defined variables to select the most relevant variables. The independent t-test was performed to investigate continuous variables. The resulting model was also tested for predictive value by ROC curve analysis. Statistical significance was defined by a p-value < 0.05.
Results
The study analyzed 1,629 blastocysts from 169 patients treated from March 2016 to August 2018 with ICSI at IVFAS An Sinh Hospital (Figure 2). In addition, the patient age, BMI, duration of infertility, total FSH dose, and total days of stimulation were all recorded as baseline demographic data (Table 1).
| Characteristics | N = 169 |
|---|---|
| Age - years (mean ± SD) | 32.02 ± 3.77 |
| BMI - kg/m 2 (mean ± SD) | 21.17 ± 2.42 |
| Infertility duration - years (mean ± SD) | 5.14 ± 3.48 |
| Types of Infertility (%) | |
| · Primary infertility | 99/135 (73.3) |
| · Secondary infertility | 36/135 (26.7) |
| Previous treatment cycles (%) | |
| · 1 | 64/151 (42.4) |
| · 2 | 44/151 (29.1) |
| · 3 | 43/151 (28.5) |
| AMH (ng/mL) (mean ± SD) | 5.62 ± 3.89 |
| AFC (mean ± SD) | 16.26 ± 7.04 |
| Characteristics | OR [95% CI] | p-value |
|---|---|---|
| t5 | 1.05 [1.03-1.06] | < 0.001 |
| No. of blastomeres | 1.38 [1.30-1.47] | < 0.001 |
| Fragmentation rate | 0.96 [0.95-0.97] | < 0.001 |
| Parameter | N = 169 |
|---|---|
| Endometrial thickness on FET day (mm) (mean ± SD) | 11.58 ± 2.44 |
| hCG (+) rate (%) | 118 (69.8) |
| Ongoing pregnency rate (%) | 85 (50.3) |
| Clinical pregnancy rate (%) | 89 (52.7) |
Model development
Phase 1: Create a predictive model
In the first stage of this study, BMA and multiple logistic regression were used to analyze morphological and morphokinetic parameters. Applying BMA to all the variables indicated seven essential predictor variables related to usable blastocyst formation: t5, tB, cc3, cell number, fragmentation rate, vacuole appearance, and abnormal division (Figure 3). Next, multiple logistic regression was used to select the most impactful parameters. We used Bayesian information criterion (BIC) approximation and posterior probability to determine the most predictive model. The variable estimations were either negative (red) or positive (blue). The model with the lowest BIC and the highest posterior probability was selected. Finally, the number of blastomeres (as a positive influence) (p < 0.001), the fragmentation rate (as a negative influence) (p < 0.001), and the t5 (as a positive influence) (p < 0.001) were identified as the most predictive factors (Table 2). The prediction model residuals = 1792, and the Akaike information criterion = 1800.
Phase 2: Evaluate and calibrate the value of the predictive model
Next, the value of the predictive model was evaluated and calibrated in the second stage of the research. The prediction accuracy was evaluated through ROC analysis. The area under the curve (AUC) of the predictive model for usable blastocyst development was 0.77 (95% confidence interval (CI): 0.74 – 0.79) (Figure 4). The predictive model's calibration plots showed no significant difference between the predicted and observed probabilities of the blastocyst formation (Hosmer-Lemeshow test: 0.101, p > 0.05). When the prediction model was applied to the internal validation cohort, the AUC was 0.78, with a 95% CI of 0.74 – 0.82, for 512 randomly chosen embryos (31.6%) from the same population (Figure 5). There were 169 single embryos transferred, with an implantation rate of 52.7%, and the ongoing pregnancy rate was 50.3% (Table 3). The nomogram showing the probability of usable blastocyst formation in an individual patient is shown in Figure 6.
Discussion
The time-lapse system is a new tool that could provide morphological and morphokinetic information to help select embryos more effectively than the conventional method. Our research established a predictive model of usable blastocyst formation through two phases. In the first phase, we identified three parameters that significantly affect the probability of usable blastocyst formation. In the second phase, we verified the predictive ability of the predictive model through internal validation and calibration using the Hosmer-Lemeshow test. By predicting the potential development of a usable blastocyst, the method can help achieve a single embryo transfer strategy, reduce the waiting time of patients for pregnancy, and reduce the workload of embryologists. The present study found that the t5 value, the number of blastomeres, and the fragmentation rate could significantly influence the probability of usable blastocyst development.
In conventional assessment, the grading of embryos is based mainly on evaluating the number of blastomeres, the size of blastomeres, and the fragmentation rate. The number of blastomeres was known as the essential morphological predictor that can directly reflect the competence of the embryo. Prior studies had indicated that human embryos with normal behaviors during development should reach the 7- to 8-cell stage on day 3 (D-3)21. In addition, it has been reported that D-3 embryo transfer with two embryos (at the 8-cell stage) can achieve an implantation rate like that of transferring with one blastocyst22. Our research found that the number of blastomeres is one of the major predictive factors in usable blastocyst development, consistent with the results of previous studies. Another essential predictive factor identified in the present study was the fragmentation rate. Fragmentation is described as the presence of a non-nuclear membrane-bound extracellular cytoplasmic structure, including isolated chromosomes developed during the first few cell divisions of the embryo23. It has been shown that embryos with abnormal developmental kinetics, such as irregular cell cycle and cytokinesis durations, are more likely to be fragmented, to express lower numbers of cells, and to not develop into blastocysts6. A high degree of fragmentation might result in a loss of cytoplasmic organelles such as mitochondria24. In addition, it also causes necrosis of the surrounding blastomeres, leading to arrested development and reduced embryonic development space.
Our data demonstrated that cleavage kinetics assessed by time-lapse monitoring can be used to predict blastocyst development and quality23. Some previous studies showed that embryos with earlier cleavage have a significantly better possibility of developing into blastocyst when compared with embryos that have slower development7, 12. For example, Cruz et al. (2012) studied 834 embryos and found that the blastocyst formation capacity and t5 and s2 values were related7. Similarly, a study by Mileskwi et al. (2015) showed that the t2, t5, and cc2 values are related to blastocyst formation12. However, other studies indicated that later embryo cleavage stages are more predictive of blastocyst formation. For example, research by Motate et al. found that late cleavage stages, such as the tM and the third round of cleavage synchrony (s3), are related to blastocyst formation9. Another study reported that s3, t8, and tEB are related to top-quality blastocyst formation25. Our results confirmed that the late cleavage stage, such as the t5 stage, had a good predictive value for usable blastocyst development.
In existing models, morphology assessment has been used as the initial screening for all embryos before assessing the kinetic parameters9, 12, 19. Those evaluations might require more time to finish and reduce the effective role of some morphologic parameters for embryo selection. With abnormal morphological factors, including fragmentation, irregular cleavages, and developmental arrest, the earlier they occur, the more harmful they are to embryo quality12, 26. The ability to predict blastocyst formation can be increased by combining the synchronization in the two-cell stage and the first-cleavage pattern with good standard morphological evaluations7. Furthermore, according to Motato et al. (2016), one of the limitations of their study was that their model did not analyze morphological factors such as the fragmentation rate9. We therefore suggested that morphologic parameters should be incorporated simultaneously with morphokinetic parameters in developing a predictive model. For top-quality blastocyst development prediction, previous research reported that s3, t8, and tEB showed a slightly improved predictive accuracy (AUC: 0.748, 95% CI: 0.697 – 0.79425)25. Another model found that the best predictor for blastocyst development and quality on day 3 was cleavage synchrony from 2 to 8 cells (CS2-8) = ((t3-t2) + (t5-t4))/(t8-t2)) (AUC = 0.786; sensitivity = 83.43; specificity = 62.4630)27.
Yang et al. (2018) demonstrated that both morphological and morphokinetic parameters (fragmentation > 50%, direct cleavage, reverse cleavage, and delayed cleavage; tPNf, t2, and t4) are associated with embryo development potential28. With a similar predictive value, the AUC of our model was 0.77 (95% CI: 0.74 – 0.79, sensitivity: 75%, and specificity: 75%), and the internal validation result was 0.78 (95% CI: 0.74 – 0.82). Our study has expanded on the prior studies in that we incorporated both morphokinetic characteristics, such as t5, and morphologic characteristics, such as the fragmentation rate and cell number, in the scoring system.
If the AUC is between 0.5 and 0.7, a model is considered to have poor performance if it is between 0.7 and 0.8, fair performance and if it is between 0.8 and 0.928, good performance. Embryos are highly certain to expand to the blastocyst stage since they occur late in development. This is why the AUC of the Motato model is high (AUC: 0.849, 95% CI: 0.835 – 0.854) with tM and s39. Furthermore, the existing models for blastocyst development that have been externally validated had reported poor predictive power15, 17. According to Zaninovic et al. (2019), t3, t5, and cc2 were related to blastocyst formation, with the value of AUC being fair (between 0.6 and 0.72)29. Again, our study has expanded on the prior studies because we incorporated both morphokinetic characteristics, such as t5, and morphologic characteristics, such as the fragmentation rate and cell number, in the scoring system. Our result was acceptable, with the AUC being 0.77 (95% CI: 0.74 – 0.79). For predictive IVF models, achieving good discrimination is unfeasible except for models that are developed with artificial intelligence. So, calibration is considered a more meaningful way to measure model performance than discrimination. Our present model had good calibration as measured using the Hosmer-Lemeshow goodness-of-fit statistic, indicating close agreement between the predicted and the observed formation of usable blastocysts.
Furthermore, our developed nomogram has provided new insights into the prediction of blastocyst formation based on the number of blastomeres, fragmentation rate, and t5 for the individual embryos (Figure 6). The main function of our model is to create a patient counseling tool that accounts for all predictors of usable blastocyst formation. With such a tool, embryologists will be able to provide patients with a more accurate prognosis if they analyze morphokinetic and morphologic criteria together, rather than either morphokinetic or morphologic criteria alone.
We acknowledge several limitations in our research. Firstly, despite including almost 1,629 embryos, the sample size of the present study was still not powerful enough. An additional caveat is that we could not exclude biases in this retrospective study. In order to improve the predictive strength of the model, further studies with larger sample numbers and prospective cohorts should be considered. Thirdly, future studies should include external geographic validation and impact analysis to evaluate the benefits of integrating this model into the IVF patient process. Finally, the live birth rate is widely accepted as the optimal endpoint for IVF studies. However, we used clinical pregnancy as a surrogate outcome in our study because of our limited sample size.
Conclusions
Our findings indicate that the rate of fragmentation, the number of blastomeres, and the division time to the five-cell stage can be effectively used to predict the development of the usable blastocysts of the embryo on day 3. This model can provide more personalized and accurate treatment to infertility patients and indicate the probability of usable blastocyst development based on morphologic and morphokinetic value.
Abbreviations
FET: Frozen embryo transfer
FSH: Follicle stimulating hormone
IVM: In-vitro maturation
TLM: Time-lapse monitoring
PCOS: Polycystic ovary syndrome
ICSI: Intracytoplasmic sperm injection
IVF: In-vitro fertilization
Acknowledgments
This study was performed at IVFAS, An Sinh Hospital. The authors would like to acknowledge our colleagues at IVFAS for their assistance in completing the study.
Author’s contributions
The submitted report was read and approved by all contributors. PNDT was the main author and was responsible for the content of the manuscript. NHMT, LNQ, and PTNV completed the IVF and embryo vitrification work. DQV created and designed the experiments. NHMT and PTNV gathered and analyzed the data. DQV supervised the work and finalized the manuscript. The final manuscript was read by all authors and agreed with the publication of the article.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted following the amended Declaration of Helsinki. The IVFAS database contained routinely collected information from all patients, including baseline demographics, cycle data, and outcome data. Patient data was cleansed of personal information. The IVFAS database contained routinely collected information from all patients, including baseline demographics, cycle data, and outcome data. Patient data was cleansed of personal information. Patients’ permission was not required because the data could not be linked back to an individual.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Glujovsky
D.,
Farquhar
C.,
Quinteiro Retamar
A.M.,
Alvarez Sedo
C.R.,
Blake
D.,
Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database of Systematic Reviews.
2016
.
View Article Google Scholar -
Market-Velker
B.A.,
Zhang
L.,
Magri
L.S.,
Bonvissuto
A.C.,
Mann
M.R.,
Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Human Molecular Genetics.
2010;
19
(1)
:
36-51
.
View Article Google Scholar -
Meseguer
M.,
Rubio
I.,
Cruz
M.,
Basile
N.,
Marcos
J.,
Requena
A.,
Embryo incubation and selection in a time-lapse monitoring system improves pregnancy outcome compared with a standard incubator: a retrospective cohort study. Fertility and Sterility.
2012;
98
(6)
.
View Article Google Scholar -
Herrero
J.,
Meseguer
M.,
Selection of high potential embryos using time-lapse imaging: the era of morphokinetics. Fertility and Sterility.
2013;
99
(4)
:
1030-4
.
View Article Google Scholar -
Rubio
I.,
Galán
A.,
Larreategui
Z.,
Ayerdi
F.,
Bellver
J.,
Herrero
J.,
Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertility and Sterility.
2014;
102
(5)
.
View Article Google Scholar -
Wong
C.C.,
Loewke
K.E.,
Bossert
N.L.,
Behr
B.,
De Jonge
C.J.,
Baer
T.M.,
Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nature Biotechnology.
2010;
28
(10)
:
1115-21
.
View Article Google Scholar -
Cruz
M.,
Garrido
N.,
Herrero
J.,
Pérez-Cano
I.,
Muñoz
M.,
Meseguer
M.,
Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reproductive Biomedicine Online.
2012;
25
(4)
:
371-81
.
View Article Google Scholar -
Kirkegaard
K.,
Ahlström
A.,
Ingerslev
H.J.,
Hardarson
T.,
Choosing the best embryo by time lapse versus standard morphology. Fertility and Sterility.
2015;
103
(2)
:
323-32
.
View Article Google Scholar -
Motato
Y.,
de los Santos
M.J.,
Escriba
M.J.,
Ruiz
B.A.,
Remohí
J.,
Meseguer
M.,
Morphokinetic analysis and embryonic prediction for blastocyst formation through an integrated time-lapse system. Fertility and Sterility.
2016;
105
(2)
.
View Article Google Scholar -
Meseguer
M.,
Herrero
J.,
Tejera
A.,
Hilligs∅e
K.M.,
Ramsing
N.B.,
Remohí
J.,
The use of morphokinetics as a predictor of embryo implantation. Human Reproduction (Oxford, England).
2011;
26
(10)
:
2658-71
.
View Article Google Scholar -
Liu
Y.,
Chapple
V.,
Feenan
K.,
Roberts
P.,
Matson
P.,
Time-lapse deselection model for human day 3 in vitro fertilization embryos: the combination of qualitative and quantitative measures of embryo growth. Fertility and Sterility.
2016;
105
(3)
.
View Article Google Scholar -
Milewski
R.,
Kuć
P.,
Kuczyńska
A.,
Stankiewicz
B.,
\Lukaszuk
K.,
Kuczyński
W.,
A predictive model for blastocyst formation based on morphokinetic parameters in time-lapse monitoring of embryo development. Journal of Assisted Reproduction and Genetics.
2015;
32
(4)
:
571-9
.
View Article Google Scholar -
Barrie
A.,
Homburg
R.,
McDowell
G.,
Brown
J.,
Kingsland
C.,
Troup
S.,
Examining the efficacy of six published time-lapse imaging embryo selection algorithms to predict implantation to demonstrate the need for the development of specific, in-house morphokinetic selection algorithms. Fertility and Sterility.
2017;
107
(3)
:
613-21
.
View Article Google Scholar -
Storr
A.,
Venetis
C.,
Cooke
S.,
Kilani
S.,
Ledger
W.,
Time-lapse algorithms and morphological selection of day-5 embryos for transfer: a preclinical validation study. Fertility and Sterility.
2018;
109
(2)
.
View Article Google Scholar -
Fréour
T.,
Le Fleuter
N.,
Lammers
J.,
Splingart
C.,
Reignier
A.,
Barrière
P.,
External validation of a time-lapse prediction model. Fertility and Sterility.
2015;
103
(4)
:
917-22
.
View Article Google Scholar -
Liu
Y.,
Qi
F.,
Matson
P.,
Morbeck
D.E.,
Mol
B.W.,
Zhao
S.,
Between-laboratory reproducibility of time-lapse embryo selection using qualitative and quantitative parameters: a systematic review and meta-analysis. Journal of Assisted Reproduction and Genetics.
2020;
37
(6)
:
1295-302
.
View Article Google Scholar -
Liu
Y.,
Feenan
K.,
Chapple
V.,
Matson
P.,
Assessing efficacy of day 3 embryo time-lapse algorithms retrospectively: impacts of dataset type and confounding factors. Human Fertility (Cambridge, England).
2019;
22
(3)
:
182-90
.
View Article Google Scholar -
Rubio
I.,
Kuhlmann
R.,
Agerholm
I.,
Kirk
J.,
Herrero
J.,
Escribá
M.J.,
Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertility and Sterility.
2012;
98
(6)
:
1458-63
.
View Article Google Scholar -
Chamayou
S.,
Patrizio
P.,
Storaci
G.,
Tomaselli
V.,
Alecci
C.,
Ragolia
C.,
The use of morphokinetic parameters to select all embryos with full capacity to implant. Journal of Assisted Reproduction and Genetics.
2013;
30
(5)
:
703-10
.
View Article Google Scholar -
Campbell
A.,
Fishel
S.,
Bowman
N.,
Duffy
S.,
Sedler
M.,
Thornton
S.,
Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reproductive Biomedicine Online.
2013;
27
(2)
:
140-6
.
View Article Google Scholar -
Kong
X.,
Yang
S.,
Gong
F.,
Lu
C.,
Zhang
S.,
Lu
G.,
The Relationship between Cell Number, Division Behavior and Developmental Potential of Cleavage Stage Human Embryos: A Time-Lapse Study. PLoS One.
2016;
11
(4)
:
e0153697
.
View Article Google Scholar -
Bungum
M.,
Bungum
L.,
Humaidan
P.,
Yding Andersen
C.,
Day 3 versus day 5 embryo transfer: a prospective randomized study. Reproductive Biomedicine Online.
2003;
7
(1)
:
98-104
.
View Article Google Scholar -
Milewski
R.,
Ajduk
A.,
Time-lapse imaging of cleavage divisions in embryo quality assessment. Reproduction (Cambridge, England).
2017;
154
(2)
:
37-53
.
View Article Google Scholar -
Stigliani
S.,
Anserini
P.,
Venturini
P.L.,
Scaruffi
P.,
Mitochondrial DNA content in embryo culture medium is significantly associated with human embryo fragmentation. Human Reproduction (Oxford, England).
2013;
28
(10)
:
2652-60
.
View Article Google Scholar -
Storr
A.,
Venetis
C.A.,
Cooke
S.,
Susetio
D.,
Kilani
S.,
Ledger
W.,
Morphokinetic parameters using time-lapse technology and day 5 embryo quality: a prospective cohort study. Journal of Assisted Reproduction and Genetics.
2015;
32
(7)
:
1151-60
.
View Article Google Scholar -
Yang
S.T.,
Shi
J.X.,
Gong
F.,
Zhang
S.P.,
Lu
C.F.,
Tan
K.,
Cleavage pattern predicts developmental potential of day 3 human embryos produced by IVF. Reproductive Biomedicine Online.
2015;
30
(6)
:
625-34
.
View Article Google Scholar -
Cetinkaya
M.,
Pirkevi
C.,
Yelke
H.,
Colakoglu
Y.K.,
Atayurt
Z.,
Kahraman
S.,
Relative kinetic expressions defining cleavage synchronicity are better predictors of blastocyst formation and quality than absolute time points. Journal of Assisted Reproduction and Genetics.
2015;
32
(1)
:
27-35
.
View Article Google Scholar -
Yang
S.H.,
Wu
C.H.,
Chen
Y.C.,
Yang
C.K.,
Wu
T.H.,
Chen
P.C.,
Effect of morphokinetics and morphological dynamics of cleavage stage on embryo developmental potential: A time-lapse study. Taiwanese Journal of Obstetrics {&}amp; Gynecology.
2018;
57
(1)
:
76-82
.
View Article Google Scholar -
Zaninovic
N.,
Nohales
M.,
Zhan
Q.,
de Los Santos
Z.M.,
Sierra
J.,
Rosenwaks
Z.,
A comparison of morphokinetic markers predicting blastocyst formation and implantation potential from two large clinical data sets. Journal of Assisted Reproduction and Genetics.
2019;
36
(4)
:
637-46
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 4 (2022)
Page No.: 4996-5006
Published on: 2022-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7452 times
- PDF downloaded - 1719 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress