Abstract
Humans harbor various microorganisms, some of which reside naturally in the body, and some of which are transferred from elsewhere. Many of these microbes are considered to be normal flora that do not cause disease, provided that they occur only in their normal anatomical site in the body. The development of malignant lesions requires a long incubation time, even after direct exposure to known carcinogens. Multistep tumorigenesis is required to transform a normal cell into a cancerous one. The role of different microbes in tumorigenesis has expanded to include their potential capacity to form and modulate several cancer hallmarks, including the alteration of the immune response, tumor-promoting inflammation, angiogenesis, tumor growth and proliferation, and pro-carcinogenic metabolite production. Furthermore, microbes may damage the host DNA and induce genomic instability. This review provides a basic overview of the process of tumorigenesis and the role of different microorganisms in cancer accuracy. Then this study discusses the different mechanisms of tumor induction by viruses, bacteria, protozoa, and fungi. Finally, it highlights the necessary health precautions that need to be taken to prevent the development of cancers.
Introduction
Cancer is one of the most common diseases worldwide. It is the result of the uncontrolled growth of abnormal cells due to genetic mutations. Cancer develops when normal cells lose control of their proliferation. They keep growing and dividing rather than dying, and this forms a new, abnormal mass of tissue called a tumor1. Despite the advances made in oncological diagnoses, management, and therapy, there has been a steady increase in the number of cancer patients globally2. Many studies estimate that roughly 20–25% of all human malignancies worldwide are related to microbial infections3, 4. The role of different microbes in tumorigenesis has expanded to include their potential capacity to form and modulate several cancer hallmarks, including the alteration of the immune response5, tumor-promoting inflammation6, 7, angiogenesis8, tumor growth and proliferation9, and pro-carcinogenic metabolite production10. Furthermore, microbes may damage the host DNA and induce genomic instability11, 12. Various oncogenic mechanisms have been suggested for viruses, bacteria, protozoa, and fungi.
Recently, a number of review articles have discussed the mechanism of tumorigenesis for single microbes, including viruses13, bacteria14, protozoa15, and fungi16. Other reviews have comprehensively covered the role of microbes and their relation to cancer17, 18 and their effect on the human immune system19. To the best of our knowledge, no comprehensive review has yet been published that discusses the role of all microorganisms in the incidence of cancer. The current review provides a straightforward overview of oncogenic microorganisms and the process of tumorigenesis. It explores different carcinogenic microorganisms and their mechanisms in cancer induction, and it also highlights the necessary health precautions that can be undertaken to prevent the development of cancer.
Mechanisms of microbial carcinogenesis
Microbial infections have recently been recognized as one of the top causes of many types of cancer, especially in undeveloped and developing counties due to poverty and unhygienic environments20. International cancer research agencies have classified infections due to eleven pathogenic species as Group 1 carcinogens. These include the hepatitis B virus, hepatitis C virus, Helicobacter pylori, Clonorchis sinensis, Opisthorchis viverrini, Schistosoma haematobium, human papillomavirus, human T-cell lymphotropic virus, human immunodeficiency virus, Epstein-Barr virus, and human herpesvirus21, 22. Most of the emphasis has been on viruses due to their direct influence on human genes23 and bacteria, which causes chronic inflammation leading to cancer24. Although fungi and parasitic protozoa have largely been overlooked, they are now being investigated as important factors in microbial carcinogenesis25, 26.
Carcinogenic viruses
The history of tumor virology began in 1911 with the discovery of a filterable agent capable of inducing sarcomas in chickens27. Later on, this filterable agent was shown to be a retrovirus that proved able to transduce a gene, v-src, and induce tumors. The beginning of human tumor virology came in the 1960s following the discovery of the Epstein-Barr virus (EBV)28 that was first observed using electron microscopy in cells cultured from Burkitt's lymphoma29.
International cancer research agencies have classified seven viral pathogens as highly carcinogenic agents. These agencies have also estimated that 1 in 10 cancers is caused by viruses30, 31. Each year, a total of 640,000 cancers are caused by human papillomaviruses (HPVs) alone32. The etiological role of HPV in cervical carcinoma was first proposed in the 1970s by zur Hausen. Recent research indicates that HPV accounts for more than half of infection-linked cancers in females33, 34. von Knebel Doeberitz et al. 35 reported that HPV infects human epithelial cells, integrates into their DNA, and produces oncoproteins including E6 and E7. The oncoproteins are able to disrupt the natural tumor suppressor pathways and inhibit apoptosis. This permits the proliferation of cervical carcinoma cells. HPVs have also been determined to be the main cause of other human cancers including skin cancers in immunosuppressed patients36, head and neck tumors37, and other anogenital cancers38. Rusan et al. (2015)39 described 3 main pathways for HPV integration and tumorigenesis: an increase in oncogene expression, a loss of function of the tumor suppressor genes, and inter- and intra-chromosomal rearrangements. Langsfeld et al. (2016)40 studied the life cycle of HPV and cervical cancer induction and reported that when the viral genomes migrate to the nucleus of the cervical epithelial cells (maintained at ∼100 copies/cell), the virus is continuously amplified in the daughter cells. The expression of oncoproteins (E6 and E7) is increased, leading to a significant enhancement of the cells' proliferation and the accumulation of cellular mutations41. This leads to a loss of cellular differentiation and the cancerous cells invading the dermal layer and neighboring tissues. Figure 1 illustrates the life cycle of HPV during cancer formation as well as the epithelial architecture before and after the virally induced cancer.
Human herpes viruses are a family of oncogenic viruses. This family includes human herpesvirus-8, the main causal agent of Kaposi’s sarcoma and human herpesvirus-6, which has been found to be significantly related to the etiologies of brain cancers and lymphomas43, 44. Although the tumorigenesis mechanisms of these viruses have not yet been firmly established, many studies have suggested that several attributes of these viruses that can promote tumorigenesis45, 46. Choi et al. (2020) 46 investigated the mechanism of tumorigenesis in human herpesvirus and revealed that upon viral infection, the virus increases the metabolites of non-essential amino acids. The K1 oncoprotein of the viruses interacts with and activates the Pyrroline-5-carboxylate reductase enzyme, leading to an increase in the intracellular concentrations of proline. Consequently, the interaction of the viruses' K1 oncoprotein and the reductase enzyme promotes tumorigenesis and tumor cell growth. Kang et al. (2017) 47 reviewed Kaposi’s sarcoma–associated herpesvirus and reported its ability to cause various tumors in humans. The tumors begin with the infection of the endothelial and B- cells by Kaposi's sarcoma–associated herpesvirus, leading to the formation of 1 of 3 malignancies, including Kaposi’s sarcoma and 2 B-cell lymphomas. In the early stages of the endothelial tumor infection (Kaposi's sarcoma), predominant inflammation and aberrant neoangiogenesis have been reported. However, in the later stages due to the proliferation of infected spindle cells, a serious modification of newly formed endothelial cell has been observed, although the origin of the endothelial cells remains elusive48. The increased rate of proliferation of the modified endothelial cells, in addition to its angiogenic and migratory capacities, is the primary mechanism of Kaposi's sarcoma oncogenesis as posited in the previous research.
The Hepatitis C virus belongs to a group of enveloped RNA viruses from the flavivirus family. It is the causative agent for both acute and chronic hepatitis49. In contrast, hepatitis B virus is a DNA virus from a different family, hepadnaviridae50. However, the diseases that result from both hepatitis C and B share many similarities. El-Serag et al. (2012) 51 estimated that roughly 80% of hepatocellular carcinomas worldwide are associated with chronic infections of hepatitis B virus and/or hepatitis C virus. Different studies have found there to be a relationship between intrahepatic cholangiocarcinoma and the hepatitis C or B viruses52. Worldwide, it has been estimated that 2 billion people are infected with hepatitis B virus, and that 1.2 million deaths every year are attributed to subsequent cirrhosis, hepatocellular carcinomas (HCCs), and hepatitis53. A meta-analysis performed in China that included 39 studies from 1954 to 2010 revealed that more than 70% of hepatocellular carcinomas were associated with hepatitis B virus infection, 5% with hepatitis C virus infection, and 6% with hepatitis B and C co-infection, although up to 19% of hepatocellular carcinoma cases showed no relationship with hepatitis B or C54. The majority of patients infected with both viruses developed a chronic hepatitis infection followed by inflammation-induced lesions. This triggered the secretion of various cytokines in the liver55, 56. As a result of these events, cirrhosis and hepatocellular carcinoma were likely to develop. Vescovo et al. (2016) 57 studied the molecular mechanisms of human hepatitis C virus and reported that HCC is a multistep process that leads to malignant transformation. This begins with the viral infection, chronic inflammation, and induction of lesions, followed by fat accumulation (steatohepatitis) in addition to progressive fibrosis. This process occurs over a period of 20 to 40 years, and 10 — 20% of patients went on to develop cirrhosis, whereas 1 — 5% developed typical HCC. The malignant transformation of the liver cells (hepatocytes) occurs due to the increased liver cell turnover resulting from chronic liver injury and subsequent regeneration57. The chronicity of these events, in addition to the oxidative stress, promotes and directly up-regulates the mitogenic pathways that block apoptosis, enhancing cell proliferation and inducing reactive oxygen species (ROS). Hepatitis viruses also triggers the immune response to produce several cytokines such as LTα and LTβ, which have been reported to play a vital role in HCC development58. The prolonged release of ROS is considered to be the main source of genetic mutations and tumorigenesis. Figure 2 presents the role and mechanisms of the hepatitis C virus from infection to HCC.
Epstein-Barr virus is a herpesvirus that has a large genome consisting of double-stranded DNA. This encodes all of the enzymes involved in the replication of its DNA and in nucleotide biosynthesis60. It has been linked to a number of malignancies, such as Hodgkin’s disease61, B- and T-cell lymphomas62, leiomyosarcomas63, post-transplant lymphoproliferative disease64, and nasopharyngeal carcinomas65. Among all of these cancers, the frequency of Burkitt’s lymphoma, leiomyosarcomas and post-transplant lymphoproliferative disease have dramatically increased in the past few years especially in patients who suffer from immunodeficiency, revealing the role of immunosurveillance in the prevention and suppression of malignant transformation66. Naseem et al. (2018) 67 reported multiple factors associated with EBV that contribute to tumorigenesis, including inflammatory changes induced by the viral attack, the hypermethylation of the tumor suppressor genes, the induction of changes in the cell cycle pathways, and host immune evasion by the virus.
Human immunodeficiency virus (HIV) is a carcinogenic virus derived from the lentivirus family. It is responsible for acquired immunodeficiency syndrome (AIDS) and it has resulted in more than 20 million deaths worldwide68. Individuals with HIV have been reported to have a significantly higher incidence of various malignancies compared with the general population due to the progressive immune dysfunction69. Lung cancer is the most common HIV-related cancer, as demonstrated by many studies. However, the underlying mechanism of HIV is still poorly known70, 71. In one study 72, the authors suggested that cancer patients who are infected with HIV have a poorer prognosis compared to non-infected cancer patients. It has been reported that approximately 40% of HIV-associated malignancies were found to be associated with other oncogenic viruses such as EBV, human herpesvirus, HPV, and hepatitis B and C viruses73. Kaposi's sarcoma is an angioproliferative tumor that results from the Human herpesvirus-8 infection of cells of endothelial lineage in HIV patients74. Anampa et al. (2020)75 studied the mechanism of HIV carcinogenesis and reported that the viral infection itself appeared not to be directly involved in carcinogenesis. Instead, it disrupts the immune surveillance of tumors and other carcinogenic pathogens. The same authors reported that HIV induces cytokine dysregulation and genetic alterations, both of which enhance the potential for carcinogenesis. Furthermore, HIV is associated with chronic antigen stimulation which promotes lymphomagenesis75.
Human T lymphotropic virus type I is a type of single-stranded RNA retrovirus characterized by slow transformation and associated with adult T-cell leukemia76. The genome of this virus contains two long-terminal repeats and encodes for several genes, such as flanking gag, env, and pol, in addition to other accessory genes. These genes have been postulated to play a significant role in tumorigenesis77. Several proteins in Human T lymphotropic virus type I have been demonstrated to play key roles in cancer induction through cellular transformation as well as the immortalization of infected T lymphocytes78. It is of note that the Human T lymphotropic virus type I oncoprotein Tax inhibits the innate IFN immune response by mediating an interaction between the mitochondrial antiviral-signaling protein, the stimulator of interferon, and the receptors interacting with protein 1. This interaction causes the suppression of the TANK-binding kinase 1 enzyme–mediated phosphorylation of IFN regulatory factor 3/IFN regulatory factor 779. The accessory protein of Human T lymphotropic virus, the leucine zipper factor, disrupts genomic integrity and inhibits apoptosis as well as the autophagy of the target cells. This leads to the enhancement of cell proliferation and facilitates its evasion from immune surveillance. This mediates oncogenesis78.
Carcinogenic bacteria
Recently, a significant number of studies have implicated that different types of bacteria are involved in the etiology of some cancer types, including Helicobacter pylori in mucosa-associated lymphoid tissue cancer80 as well as gastric cancer81, Salmonella typhi in gallbladder cancer82, Bacteroides fragilis in colon cancer83, and Chlamydia trachomatis in cervical cancer84. This has inspired researchers to further study the mechanisms through which these bacteria promote oncogenesis in order to provide evidence to support such a role. H. pylori is the most abundant bacterial species in the gastric epithelium due to its ability to resist and adapt to gastric acidity. Its presence has been markedly associated with the development of gastric and mucosa-associated lymphoid tissue cancers. A significant number of researchers have linked H. pylori infections with gastric cancer and mucosa-associated lymphoid tissue cancer85, 86, 87, 88, considering it to be among the most important, if not the top, risk factor for gastric cancer in the world. Posselt et al. (2017) 89 studied the mechanism of gastric cancer induction by H. pylori and reported that upon infection, the bacteria actively interferes with the host gastric cells via the secretion of bacterial proteases and the activation of cellular proteases. This may be involved in the induction of cancer. H. pylori regulates and controls the secretion of proteases and thus hosts cytokines in early and late pathogenesis90. It has been reported that H. pylori continuously induces various transcription factors and proteases, including disintegrin and metalloproteinase (ADAM) and various types of matrix metalloproteinases (MMPs). It can highly secrete the host cytokines and interfere with the extracellular matrix proteins or lateral junction complexes91. The chronic ulceration that results from H. pylori pathogenesis will eventually lead to cell proliferation and the formation of tumors89. Figure 3 summarizes the mechanisms of gastric cancer induction by H. pylori.
Alfarouk et al. (2019)92 studied the possible role of H. pylori in gastric cancer and revealed that the carcinogenicity of such bacteria depends on bacteria–host related factors. They reported several genes expressed by the bacteria that accelerate its pathogenicity, in addition to the remodeling of the microenvironment including urease, carbonic anhydrase, Lewis antigen, vacA, cagA, and babA2. The variety of these virulence factors as mucys in H. pylori helps to stabilize its population size in the stomach. This leads to the induction of chronic inflammation93. This creates an unfavorable habitat that alters the pH due to the chronic inflammation around the normal gastric cells. This instigates their malignant transformation and provides an accurate marker of gastric cancer94, 95. The transformation of gastric cells might be our bodies' normal defense against H. pylori as various environmental changes elicit phenotypic plasticity in the gastric cells, enabling them to take on acidophilic phenotypes.
B. fragilis has been reported to be one of the major causes of colorectal cancer96. In one study, Chen et al. (2020)83 reported that B. fragilis accelerates colonization by producing a biofilm in the intestinal tract. This causes a series of inflammatory reactions that result from toxin production. The accumulation of B. fragilis toxin may lead to severe tissue injury and chronic intestinal inflammation which may then develop into colorectal cancer. Snezhkina et al. 97 investigated the mechanisms of colorectal cancer formation by B. fragilis and found that bacterial enterotoxins are able to activate spermine oxidase from the host cells. This produces H2O2 and spermidine as the byproducts of polyamine catabolism. These compounds significantly induce an inflammatory response leading to tissue injury and disturbance. The same authors found two mediators, namely c-Myc and C/EBP𝛽 to be overexpressed in tumors. These mediators play a significant role in cell proliferation, inflammation, and metabolic reprogramming.
S. typhi is another pathogenic bacteria that has been linked to the malignant transformation of the gallbladder98. S. typhi has the potential to promote carcinogenesis due to the production of various secretions such as nitroso compounds, bacterial glucuronidase, and toxic molecules. Cytolethal distending toxins are groups of toxins produced by S. typhi that are able to trigger irreversible cell cycle arrest and apoptosis resulting from DNA damage99. Di Domenico et al. (2017)100 reported that the typhoid toxin produced by S. typhi has strong carcinogenic potential as it induces DNA damage which leads to various alterations in the cell cycle of intoxicated cells101. Furthermore, the biofilm production of S. typhi has been linked to tumorigenesis by promoting a persistent infection in the gallbladder. This leads to a chronic local inflammatory response that exposes the epithelial cells to severe and repeated damage. The presence of chronic infection around gallstones may enhance the formation of an S. typhi biofilm, allowing the bacterial cells to detach from the gallstones and release various carcinogenic molecules. This induce genomic instability in the gallbladder epithelial cells and leads to tumorigenesis100. Figure 4 presents the proposed mechanisms by which S. typhi may induce gallbladder cancer.
C. trachomatis has been reported to be involved in the process of cell proliferation and the inhibition of apoptosis. The induction of chronic inflammation by C. trachomatis and the same as a potential cause of cervical cancer was studied by Zhu et al. (2016)84. The authors concluded that individuals infected with C. trachomatis have a significantly higher risk of cervical cancer. In a different study, Laban et al. (2019)102 evaluated the association of C. trachomatis infection with high-grade serous ovarian cancers and tubal carcinoma. The authors detected bacterial DNA in 84% of high-grade tubal serous cancers which revealed the strong relationship between C. trachomatis and this type of cancer. Although these findings have not yet been supported by a suggested mechanism of carcinogenicity, these findings need to be replicated and further investigated to understand the potential role of C. trachomatis in ovarian and cervical cancers.
Carcinogenic parasites
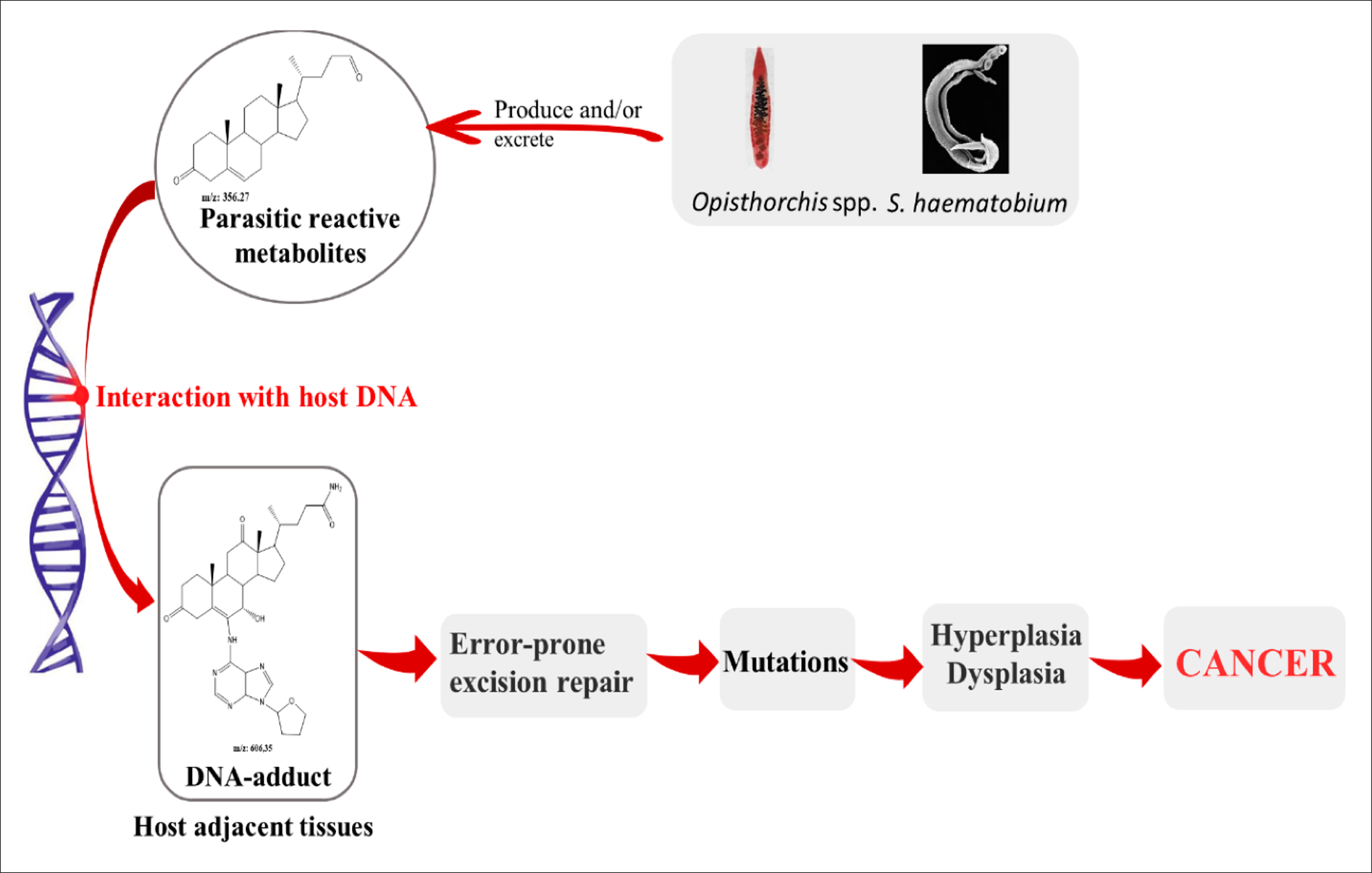
The associations between parasitic infections and cancers have been well established in numerous studies103, 104, 105, 106 (103-106). Schistosoma haematobium, Clonorchis sinensis, and Opistorchis viverrini have been reported to be among the most carcinogenic parasites107, whereas other infectious species have been linked due to being the potential cause of cancers, especially the genera Schistosoma and Opisthorchis 108. Many mechanisms have been suggested for carcinogenic parasites. Among them, 3 have been described for liver flukes, including metabolic oxidative stress, chronic inflammation, and tissue damage due to parasitic attack109. Van Tong et al. (2017)110 studied the relationship between carcinogenesis and human malignancy in different parasites and revealed the high carcinogenicity of 3 helminth diseases including schistosomiasis, clonorchiasis, and opisthorchiasis. The authors illustrated the proposed mechanisms for cancer induction as presented in Figure 5. The chronic inflammation induced by the parasitic infections leads to the enhanced activation of many signaling pathways. This eventually generate somatic mutations that may activate oncogenes111.
The metabolite secretions of Opisthorchis, Clonorchis, and Schistosoma species into the microenvironment of the host may induce metabolic processes such as oxidative stress which facilitates chromosomal DNA damage of the host cells, leading them to becoming cancerous113. The physical damage of host tissues due to parasitic attack, together with the immune response and wound healing process, lead to the secretion of various cytokines to increase cell proliferation and transformation. This also increases the potential for DNA damage and/or mutations114. Combined parasite–host interaction events, namely physical damage, parasite-derived products and chronic inflammation, as well as the combined effects on these processes on the host tissue and their DNA, leads to significant modifications and a higher risk of carcinogenesis due to changes in the cells' growth rate and proliferation, in addition to their survival. This in turn may initiate tumorigenesis and promote malignancy110. Table 1 summarizes the parasites that have been linked to different types of cancers and the proposed mechanism of carcinogenesis for each.
| Parasitic pathogens | Disease | Associated cancer | Proposed mechanism of carcinogenesis | Refs |
|---|---|---|---|---|
| Cryptosporidium parvum | Cryptosporidiosis | Colorectal cancer | Inhibit apoptosis and enhance cells proliferation | 115 |
| Schistosoma mansoni | Schistosomiasis | Colorectal cancer and hepatocellular carcinoma | Chronic inflammation, and oxidative stress | 116 |
| Schistosoma japonicum | Schistosomiasis | Colorectal cancer and squamous cell carcinoma | Chronic inflammation, and oxidative stress | 117 |
| Schistosoma haematobium | Schistosomiasis | Urinary bladder cancer, squamous cell carcinoma | Chronic inflammation, and oxidative stress | 118 |
| Blastocystis hominis | Blastocystis infection | Colorectal cancer | Signaling induction, leading to impaired apoptosis | 119 |
| Toxoplasma gondii | Toxoplasmosis | Brain cancer, meningioma and glioma | Triggering a chronic inflammatory and alteration of cell signaling. | 120 |
| Clonorchis sinensis | Clonorchiasis | Cholangiocarcinoma | Chronic inflammation, cell proliferation & oxidative stress | 121 |
| Trichomonas vaginalis | Vaginitis and urethritis | Prostate, cervical and reproductive tract, cancers | Triggering of proto-oncogenes and altering junctional protein expression | 122 |
| Plasmodium falciparum | Malaria | Burkitt lymphoma | Immune suppression for carcinogenic viruses | 123 |
| Opistorchis viverrini | Opisthorchiasis | Cholangiocarcinoma | Chronic inflammation, cell proliferation & oxidative stress | 124 |
| Taenia solium | Neurocysticercosis | Gliomas | Chronic inflammation and cellular proliferation | 125 |
Carcinogenic fungi
Recent studies have revealed that fungi in the human gut can move into the pancreas under certain circumstances, triggering changes in the pancreatic cells that can lead to tumorigenesis126. Aykut et al. (2019)127 found that the fungal component of the pancreatic microbiome are significantly altered in pancreatic ductal adenocarcinoma. In fact, several fungal genera promote tumor formation. Similarly, Malik et al. (2018)128 found that common resident gut fungi can promote the activation of inflammasome during azoxymethane/dextran sodium sulfate–induced colitis in a mouse model. The authors reported that such fungi were able to alter the cell signaling during inflammasome activation. This resulted in the secretion of various cytokines including interleukin (IL)-18 and interferon-γ, suggesting that during spleen tyrosine kinase-caspase recruitment, domain-9 signaling maintains a microbial—or specifically fungal—ecology that promotes the activation of inflammasome and thus restrains colitis and colon tumorigenesis128. Malassezia species are the most common fungal species in mammalian skin, and they are the best-studied fungal species in skin conditions include atopic dermatitis and dandruff129. Some studies have reported that inflammation caused by the overgrowth of Malassezia may worsen gastric ulcers, weakening the immune system and changing the cell-surface signaling130, 131, 132, 133. Therefore, an abundance of Malassezia species in pancreatic ductal adenocarcinoma tumors may be medically relevant. Aykut et al. (2019)127 found that the administration of antifungal drugs halted pancreatic ductal adenocarcinoma progression in mouse models and significantly improved the ability of chemotherapy, leading to significant shrinking of the tumors. Interestingly, the subsequent repopulation of healed lab animals by a Malassezia fungal species significantly accelerated the growth of pancreatic ductal adenocarcinoma again134. Not much work has considered the relationship between different fungi and cancers but these findings suggests that any microorganism is able to change the normal nature of the human body, making it a potential cause of cancer.
Cancer prevention and health precautions
In the age of personalized medicine and self-treatment, it is extremely important to isolate the causes of health issues to effectively plan personalized therapy. Various epidemiological studies have revealed that leisure time and physical activity can significantly reduce and even prevent at least 13 types of cancer95, 135. Other studies have provided evidence that exercise significantly reduces the risk of developing breast, colon, and prostate cancers136, 137. The nature and duration of exercise training involves whole-body homeostasis. This leads to the widespread adaptation of the body’s cells, tissues, and organs138, 139. Various systemic factors such as the release of catecholamines and myokines during exercise, in addition to sympathetic activation, shear stress, increase blood flow, and an increase body temperature. All immediately exert stress on tumor metabolism and homeostasis140, 141. These acute effects in the long-term will lead to improved blood perfusion, metabolism adjustments, enhanced immunogenicity, and intratumoral adaptations, contributing to slower tumor progression142. Various probiotic strains have been used to treat microbial infections, especially gastrointestinal tract infections, to boost human health as well as to control the biofilm formation that may lead to tumorigenesis143. Jacouton et al. (2017)144 evaluated the role of Lactobacillus casei in colorectal cancer prevention and revealed that it had an immunomodulatory effect mediated by the regulation of different cytokines (particularly IL-22). This was in addition to an antiproliferative effect mediated by Bik, caspase-7 and caspase-9 regulation. The authors suggested using these probiotics in food supplements as a promising strategy for cancer prevention. In a different study, anti-biofilm properties were evaluated in cocktails of probiotic strains against B. fragilis and Escherichia coli strains. They were demonstrated to be highly preventive of tumorigenesis inflammation145, 146. Hindering the biofilm formation of pathogenic gut microbes is said to be an effective method of cancer prevention for which certain strains of probiotic can be utilized147, 148. The production of antagonistic compounds, the modulation of the host immune responses, and competition with pathogens are among the mechanisms that have been suggested due to the beneficial role that probiotics appear to play, as Figure 6 illustrates149. Natural products have been screened for their anticancer properties, and many have been used in the development of cancer preventive and anticancer drugs150. Most anticancer drugs that have been approved by the Food and Drug Administration of the United States are either natural or they mimic natural products151. Song et al. (2016)152 reported that the prevention of any disease requires either the avoidance or reduction of risk factors (i.e., carcinogenic materials or microorganisms) or the early detection of and intervention in disease evolution. In this regard, following a natural diet regime and avoiding oxidants and synthetic materials are major factors that may limit tumorigenesis in addition to boosting the immune system to help combat carcinogenic microorganisms. Figure 6 presents the mechanisms by which probiotics target tumorigenic gut microbial biofilms.
Conclusion
Recent research has uncovered a great deal of information regarding the mechanisms used by different microorganisms to cause, colonize, or cure cancer. However, many questions remain. It has long been known that many microorganisms can trigger tumorigenesis in humans but to date, the exact molecular mechanisms of many of these microbes have remained unclear. The continued exploration of these questions will bring research ever closer to the prevention, early diagnosis, and truly effective treatment of this scourge of mankind. Here we have discussed the role of viruses, bacteria, protozoa, and fungi in tumorigenesis and elucidated the possible molecular events that may be involved in the carcinogenic properties of each type of pathogen. We have also explored the structural basis for how the host cells interact with these microorganisms to produce the hallmarks of cancer. Microbial secretions, as well as immune-regulating cytokines, may play an essential role as mutagenic factors.
Abbreviations
AIDS: Acquired immunodeficiency syndrome
DNA: Deoxyribonucleic acid
EBV: Epstein-Barr virus
HCC: Hepatocellular carcinoma
HIV: Human immunodeficiency virus
HPV: Human papilloma virus
IL: Interleukin
ROS: Reactive oxygen species
Acknowledgments
The authors would like to thank the collaboration between Sabratha University, Sabratha, Libya, University Teknologi MARA, Shah Alam, Malaysia and the Universiti Sains Malaysia, Penang, Malaysia that made this work possible.
Author’s contributions
All authors contributed equally to this work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Jaiswal
R.,
Sedger
L.M.,
Intercellular vesicular transfer by exosomes, microparticles and oncosomes-implications for cancer biology and treatments. Front Oncol.
2019;
9
:
125
.
View Article PubMed Google Scholar -
Banu
A.,
Gousuddin
M.,
Yahya
E.B.,
Green synthesized monodispersed silver nanoparticles' characterization and their efficacy against cancer cells. Biomed Res Ther.
2021;
8
(8)
:
4476-82
.
View Article Google Scholar -
Araldi
R.P.,
Sant'Ana
T.A.,
Módolo
D.G.,
de Melo
T.C.,
Spadacci-Morena
D.D.,
de Cassia Stocco
R.,
The human papillomavirus (HPV)-related cancer biology: an overview. Biomed Pharmacother.
2018;
106
:
1537-56
.
View Article PubMed Google Scholar -
Wong
S.H.,
Kwong
T.N.Y.,
Wu
C-Y.,
Yu
J.,
Clinical applications of gut microbiota in cancer biology. Semin Cancer Biol.
2019;
55
:
28-36
.
View Article PubMed Google Scholar -
Russo
E.,
Taddei
A.,
Ringressi
M.N.,
Ricci
F.,
Amedei
A.,
The interplay between the microbiome and the adaptive immune response in cancer development. Therap Adv Gastroenterol.
2016;
9
(4)
:
594-605
.
View Article PubMed Google Scholar -
Sethi
V.,
Kurtom
S.,
Tarique
M.,
Lavania
S. ,
Malchiodi
Z.,
Hellmund
L.,
Zhang
L.,
Sharma
U.,
Giri
B.,
Garg
B.,
Ferrantella
A.,
Vickers
S. M.,
Banerjee
S.,
Dawra
R.,
Roy
S.,
Ramakrishnan
S.,
Saluja
A.,
Dudeja
V.,
Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology.
2018;
155
(1)
:
33-37.e6
.
View Article PubMed Google Scholar -
O'Keefe
S.J.,
Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol.
2016;
13
(12)
:
691-706
.
View Article PubMed Google Scholar -
Reinhardt
C.,
Bergentall
M.,
Greiner
T.U.,
Schaffner
F.,
Ostergren-Lundén
G.,
Petersen
L.C.,
Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature.
2012;
483
(7391)
:
627-31
.
View Article PubMed Google Scholar -
Wu
S.,
Rhee
K.J.,
Albesiano
E.,
Rabizadeh
S.,
Wu
X.,
Yen
H.R.,
A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med.
2009;
15
(9)
:
1016-22
.
View Article PubMed Google Scholar -
Louis
P.,
Hold
G.L.,
Flint
H.J.,
The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol.
2014;
12
(10)
:
661-72
.
View Article PubMed Google Scholar -
Nougayrède
J.P.,
Homburg
S.,
Taieb
F.,
Boury
M.,
Brzuszkiewicz
E.,
Gottschalk
G.,
Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science.
2006;
313
(5788)
:
848-51
.
View Article PubMed Google Scholar -
Yahya
E.B.,
Alqadhi
A.M.,
Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci.
2021;
269
:
119087
.
View Article PubMed Google Scholar -
Swanson
M.S.,
Kokot
N.,
Sinha
U.K.,
The role of HPV in head and neck cancer stem cell formation and tumorigenesis. Cancers (Basel).
2016;
8
(2)
:
24
.
View Article PubMed Google Scholar -
Kalisperati
P.,
Spanou
E.,
Pateras
I.S.,
Korkolopoulou
P.,
Varvarigou
A.,
Karavokyros
I.,
Inflammation, DNA damage, Helicobacter pylori and gastric tumorigenesis. Front Genet.
2017;
8
:
20
.
View Article PubMed Google Scholar -
Callejas
B.E.,
Martínez-Saucedo
D.,
Terrazas
L.I.,
Parasites as negative regulators of cancer. Biosci Rep.
2018;
38
(5)
.
View Article PubMed Google Scholar -
Wang
Y.,
Zhang
D.,
Hou
Y.,
Shen
S.,
Wang
T.,
The adaptor protein CARD9, from fungal immunity to tumorigenesis. Am J Cancer Res.
2020;
10
(8)
:
2203-25
.
PubMed Google Scholar -
Dzutsev
A.,
Goldszmid
R.S.,
Viaud
S.,
Zitvogel
L.,
Trinchieri
G.,
The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol.
2015;
45
(1)
:
17-31
.
View Article PubMed Google Scholar -
Vinasco
K.,
Microbial carcinogenesis: lactic acid bacteria in gastric cancer. Biochimica et Biophysica Acta (BBA)-. Rev Can.
2019;
1872
(2)
:
188309
.
-
Elinav
E.,
Nowarski
R.,
Thaiss
C.A.,
Hu
B.,
Jin
C.,
Flavell
R.A.,
Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer.
2013;
13
(11)
:
759-71
.
View Article PubMed Google Scholar -
Goodman
B.,
Gardner
H.,
The microbiome and cancer. J Pathol.
2018;
244
(5)
:
667-76
.
View Article PubMed Google Scholar -
de Martel
C.,
Ferlay
J.,
Franceschi
S.,
Vignat
J.,
Bray
F.,
Forman
D.,
Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol.
2012;
13
(6)
:
607-15
.
View Article PubMed Google Scholar -
Cancer
I.A.,
Working Group on the Evaluation of Carcinogenic Risks to Humans
IARC,
Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum.
2012;
100
:
1-441
.
PubMed Google Scholar -
Saleh
A.,
Halawa
A.,
Is BK virus carcinogenic?. Nephrology Dialysis Transplantation.
2020;
35
(Supplement_3)
:
gfaa142.P1649
.
View Article Google Scholar -
Yaghoubi
A.,
Khazaei
M.,
Jalili
S.,
Hasanian
S.M.,
Avan
A.,
Soleimanpour
S.,
Cho
W.C.,
Bacteria as a double-action sword in cancer. Biochim Biophys Acta Rev Cancer.
2020;
1874
(1)
:
188388
.
View Article Google Scholar -
Klimesova
K.,
Jiraskova Zakostelska
Z.,
Tlaskalova-Hogenova
H.,
Oral bacterial and fungal microbiome impacts colorectal carcinogenesis. Front Microbiol.
2018;
9
:
774
.
View Article PubMed Google Scholar -
Mukherjee
A.,
A Systematic Review on Parasite Induced Carcinogenesis. International Journal of Innovative Science and Research Technology.
2020;
5
(6)
:
1095-1099
.
-
Rous
P.,
A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med.
1911;
13
(4)
:
397-411
.
View Article PubMed Google Scholar -
Epstein
M.A.,
Achong
B.G.,
Barr
Y.M.,
Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet.
1964;
1
(7335)
:
702-3
.
View Article PubMed Google Scholar -
Pagano
J.S.,
Epstein-Barr virus: the first human tumor virus and its role in cancer. Proc Assoc Am Physicians.
1999;
111
(6)
:
573-80
.
View Article PubMed Google Scholar -
Rodriguez-Acevedo
A.J.,
Green
A.C.,
Sinclair
C.,
van Deventer
E.,
Gordon
L.G.,
Indoor tanning prevalence after the International Agency for Research on Cancer statement on carcinogenicity of artificial tanning devices: systematic review and meta-analysis. Br J Dermatol.
2020;
182
(4)
:
849-59
.
View Article PubMed Google Scholar -
Yahya
E.B.,
A. M. Alqadhi,
Abdulsamad
M. A.,
Allaq
A. A.,
Asian Nipah virus and the potential of new pandemic. Pakistan Journal of Biotechnology.
2021;
18
(1-2)
:
17-22
.
View Article Google Scholar -
Zapatka
M.,
Borozan
I.,
Brewer
D.S.,
Iskar
M.,
Grundhoff
A.,
Alawi
M.,
Pathogens
PCAWG,
Consortium
PCAWG,
The landscape of viral associations in human cancers. Nat Genet.
2020;
52
(3)
:
320-30
.
View Article PubMed Google Scholar -
Hausen
H. Zur,
Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol.
1977;
78
:
1-30
.
View Article PubMed Google Scholar -
Bukhari
N.,
Joseph
J.P.,
Hussain
S.S.,
Khan
M.A.,
Wakim
M.J.,
Yahya
E.B.,
Prevalence of Human Papilloma Virus Sub Genotypes following Head and Neck Squamous Cell Carcinomas in Asian Continent, A Systematic Review Article. Asian Pac J Cancer Prev.
2019;
20
(11)
:
3269-77
.
View Article PubMed Google Scholar -
von Knebel Doeberitz
M.,
Oltersdorf
T.,
Schwarz
E.,
Gissmann
L.,
Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res.
1988;
48
(13)
:
3780-6
.
PubMed Google Scholar -
Baez
C.F.,
Gonçalves
M.T.,
da Rocha
W.M.,
Magalhães de Souza
L.,
Savassi-Ribas
F.,
de Oliveira Almeida
N.K.,
Investigation of three oncogenic epitheliotropic viruses shows human papillomavirus in association with non-melanoma skin cancer. Eur J Clin Microbiol Infect Dis.
2019;
38
(6)
:
1129-33
.
View Article PubMed Google Scholar -
Wood
O.,
Woo
J.,
Seumois
G.,
Savelyeva
N.,
McCann
K.J.,
Singh
D.,
Consortium
SPARC,
Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget.
2016;
7
(35)
:
56781-97
.
View Article PubMed Google Scholar -
Thida
M.,
Okada
S.,
Shwe
M.M.,
Thu
H.M.,
Aye
K.S.,
Myint
A.A.,
Mar
K.S.,
Oo
K.K.,
Aye
K.S.,
Thant
K.Z.,
Determination of oncogenic human papillomavirus (HPV) genotypes in Anogenital cancers in Myanmar. Acta Med Okayama.
2016;
70
(2)
:
103-10
.
View Article PubMed Google Scholar -
Rusan
M.,
Li
Y.Y.,
Hammerman
P.S.,
Genomic landscape of human papillomavirus-associated cancers. Clin Cancer Res.
2015;
21
(9)
:
2009-19
.
View Article PubMed Google Scholar -
Langsfeld
E.,
Laimins
L.A.,
Human papillomaviruses: research priorities for the next decade. Trends Cancer.
2016;
2
(5)
:
234-40
.
View Article PubMed Google Scholar -
Lambert
P.F.,
Münger
K.,
Rösl
F.,
Hasche
D.,
Tommasino
M.,
Beta human papillomaviruses and skin cancer. Nature.
2020;
588
(7838)
:
20-1
.
View Article PubMed Google Scholar -
Morgan
E.L.,
Macdonald
A.,
Manipulation of JAK/STAT signalling by high-risk HPVs: potential therapeutic targets for HPV-associated malignancies. Viruses.
2020;
12
(9)
:
977
.
View Article PubMed Google Scholar -
Amirian
E.S.,
Scheurer
M.E.,
Chromosomally-integrated human herpesvirus 6 in familial glioma etiology. Med Hypotheses.
2012;
79
(2)
:
193-6
.
View Article PubMed Google Scholar -
Ganem
D.,
KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J Clin Invest.
2010;
120
(4)
:
939-49
.
View Article PubMed Google Scholar -
Li
T.,
Ju
E.,
Gao
S.J.,
Kaposi sarcoma-associated herpesvirus miRNAs suppress CASTOR1-mediated mTORC1 inhibition to promote tumorigenesis. J Clin Invest.
2019;
129
(8)
:
3310-23
.
View Article PubMed Google Scholar -
Choi
U.Y.,
Lee
J.J.,
Park
A.,
Zhu
W.,
Lee
H.R.,
Choi
Y.J.,
Oncogenic human herpesvirus hijacks proline metabolism for tumorigenesis. Proc Natl Acad Sci USA.
2020;
117
(14)
:
8083-93
.
View Article PubMed Google Scholar -
Kang
S.,
Myoung
J.,
Primary lymphocyte infection models for KSHV and its putative tumorigenesis mechanisms in B cell lymphomas. J Microbiol.
2017;
55
(5)
:
319-29
.
View Article PubMed Google Scholar -
Gramolelli
S.,
Ojala
P.M.,
Kaposi's sarcoma herpesvirus-induced endothelial cell reprogramming supports viral persistence and contributes to Kaposi's sarcoma tumorigenesis. Curr Opin Virol.
2017;
26
:
156-62
.
View Article PubMed Google Scholar -
Rossi
C.,
Butt
Z.A.,
Wong
S.,
Buxton
J.A.,
Islam
N.,
Yu
A.,
Hepatitis Testers Cohort Team
BC,
Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J Hepatol.
2018;
69
(5)
:
1007-14
.
View Article PubMed Google Scholar -
Ramsey
S.D.,
Unger
J.M.,
Baker
L.H.,
Little
R.F.,
Loomba
R.,
Hwang
J.P.,
Prevalence of hepatitis B virus, hepatitis C virus, and HIV infection among patients with newly diagnosed cancer from academic and community oncology practices. JAMA Oncol.
2019;
5
(4)
:
497-505
.
View Article PubMed Google Scholar -
El-Serag
H.B.,
Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology.
2012;
142
(6)
:
1264-1273.e1
.
View Article PubMed Google Scholar -
Ralphs
S.,
Khan
S.A.,
The role of the hepatitis viruses in cholangiocarcinoma. J Viral Hepat.
2013;
20
(5)
:
297-305
.
View Article PubMed Google Scholar -
Lavanchy
D.,
Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat.
2004;
11
(2)
:
97-107
.
View Article PubMed Google Scholar -
de Martel
C.,
Maucort-Boulch
D.,
Plummer
M.,
Franceschi
S.,
World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology.
2015;
62
(4)
:
1190-200
.
View Article PubMed Google Scholar -
Heim
M.H.,
Thimme
R.,
Innate and adaptive immune responses in HCV infections. J Hepatol.
2014;
61
(1)
:
14-25
.
View Article PubMed Google Scholar -
Westbrook
R.H.,
Dusheiko
G.,
Natural history of hepatitis C. J Hepatol.
2014;
61
(1)
:
58-68
.
View Article PubMed Google Scholar -
Vescovo
T.,
Refolo
G.,
Vitagliano
G.,
Fimia
G.M.,
Piacentini
M.,
Molecular mechanisms of hepatitis C virus-induced hepatocellular carcinoma. Clin Microbiol Infect.
2016;
22
(10)
:
853-61
.
View Article PubMed Google Scholar -
Sung
W.K.,
Zheng
H.,
Li
S.,
Chen
R.,
Liu
X.,
Li
Y.,
Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet.
2012;
44
(7)
:
765-9
.
View Article PubMed Google Scholar -
Hayes
C.N.,
Zhang
P.,
Zhang
Y.,
Chayama
K.,
Molecular mechanisms of hepatocarcinogenesis following sustained virological response in patients with chronic hepatitis C virus infection. Viruses.
2018;
10
(10)
:
531
.
View Article PubMed Google Scholar -
Young
L.S.,
Yap
L.F.,
Murray
P.G.,
Epstein-Barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer.
2016;
16
(12)
:
789-802
.
View Article PubMed Google Scholar -
Murray
P.G.,
Young
L.S.,
An etiological role for the Epstein-Barr virus in the pathogenesis of classical Hodgkin lymphoma. Blood.
2019;
134
(7)
:
591-6
.
View Article PubMed Google Scholar -
Crombie
J.L.,
LaCasce
A.S.,
Epstein Barr virus associated B-cell lymphomas and iatrogenic lymphoproliferative disorders. Front Oncol.
2019;
9
:
109
.
View Article PubMed Google Scholar -
Aida
N.,
Ito
T.,
Maruyama
M.,
Saigo
K.,
Akutsu
N.,
Aoyama
H.,
A Case of Epstein-Barr Virus-Associated Leiomyosarcoma Concurrently With Posttransplant Lymphoproliferative Disorders After Renal Transplantation. Clin Med Insights Case Rep.
2019;
12
:
1179547619867330
.
View Article PubMed Google Scholar -
Ru
Y.,
Chen
J.,
Wu
D.,
Epstein-Barr virus post-transplant lymphoproliferative disease (PTLD) after hematopoietic stem cell transplantation. Eur J Haematol.
2018;
101
(3)
:
283-90
.
View Article PubMed Google Scholar -
Chang
A.M.,
Chiosea
S.I.,
Altman
A.,
Pagdanganan
H.A.,
Ma
C.,
Programmed death-ligand 1 expression, microsatellite instability, Epstein-Barr virus, and human papillomavirus in nasopharyngeal carcinomas of patients from the philippines. Head Neck Pathol.
2017;
11
(2)
:
203-11
.
View Article PubMed Google Scholar -
Li
Z.,
Tsai
M.H.,
Shumilov
A.,
Baccianti
F.,
Tsao
S.W.,
Poirey
R.,
Epstein-Barr virus ncRNA from a nasopharyngeal carcinoma induces an inflammatory response that promotes virus production. Nat Microbiol.
2019;
4
(12)
:
2475-86
.
View Article PubMed Google Scholar -
Naseem
M.,
Barzi
A.,
Brezden-Masley
C.,
Puccini
A.,
Berger
M.D.,
Tokunaga
R.,
Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat Rev.
2018;
66
:
15-22
.
View Article PubMed Google Scholar -
Tota
J.E.,
Engels
E.A.,
Madeleine
M.M.,
Clarke
C.A.,
Lynch
C.F.,
Ortiz
A.P.,
Risk of oral tongue cancer among immunocompromised transplant recipients and human immunodeficiency virus-infected individuals in the United States. Cancer.
2018;
124
(12)
:
2515-22
.
View Article PubMed Google Scholar -
Patel
P.,
Hanson
D.L.,
Sullivan
P.S.,
Novak
R.M.,
Moorman
A.C.,
Tong
T.C.,
Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med.
2008;
148
(10)
:
728-36
.
View Article PubMed Google Scholar -
Chinula
L.,
Moses
A.,
Gopal
S.,
HIV-associated malignancies in sub-Saharan Africa: progress, challenges, and opportunities. Curr Opin HIV AIDS.
2017;
12
(1)
:
89-95
.
View Article PubMed Google Scholar -
Sigel
K.,
Makinson
A.,
Thaler
J.,
Lung cancer in persons with HIV. Curr Opin HIV AIDS.
2017;
12
(1)
:
31-8
.
View Article PubMed Google Scholar -
Yanik
E.L.,
Kaunitz
G.J.,
Cottrell
T.R.,
Succaria
F.,
McMiller
T.L.,
Ascierto
M.L.,
Association of HIV status with local immune response to anal squamous cell carcinoma: implications for immunotherapy. JAMA Oncol.
2017;
3
(7)
:
974-8
.
View Article PubMed Google Scholar -
Hayakawa
Y.,
Kobayashi
K.,
Sakamoto
N.,
Matsuoka
M.,
Nozaka
T.,
Misumi
Y.,
A case of esophageal cancer with human immunodeficiency virus infection that progressed rapidly after neoadjuvant chemoradiotherapy. Clin J Gastroenterol.
2020;
13
(1)
:
17-21
.
View Article PubMed Google Scholar -
Galanina
N.,
Goodman
A.M.,
Cohen
P.R.,
Frampton
G.M.,
Kurzrock
R.,
Successful treatment of HIV-associated Kaposi sarcoma with immune checkpoint blockade. Cancer Immunol Res.
2018;
6
(10)
:
1129-35
.
View Article PubMed Google Scholar -
Anampa
J.,
Barta
S. K.,
Haigentz
M.,
Sparano
J. A.,
Human Immunodeficiency Virus (HIV) Infection and Cancer, in Abeloff's Clinical Oncology. 2020, Elsevier. p. 894-903. e4.
.
-
Jalaeikhoo
H.,
Soleymani
M.,
Rajaeinejad
M.,
Keyhani
M.,
Prevalence of Human T-lymphotropic virus type 1 (HTLV-1) Infection in Patients with Hematologic Disorders and Non-Hematologic Malignancies in a Tertiary Referral Hospital. Archives of Iranian medicine.
2017;
20
(4)
:
0-0
.
-
Sharma
V.K.,
Raimondi
V.,
Ruggero
K.,
Pise-Masison
C.A.,
Cavallari
I.,
Silic-Benussi
M.,
Expression of miR-34a in T-cells infected by human T-lymphotropic virus 1. Front Microbiol.
2018;
9
:
832
.
View Article PubMed Google Scholar -
Zhang
L.L.,
Wei
J.Y.,
Wang
L.,
Huang
S.L.,
Chen
J.L.,
Human T-cell lymphotropic virus type 1 and its oncogenesis. Acta Pharmacol Sin.
2017;
38
(8)
:
1093-103
.
View Article PubMed Google Scholar -
Hirata
M.,
Shinden
Y.,
Nagata
A.,
Nomoto
Y.,
Saho
H.,
Nakajo
A.,
Clinical Features of Breast Cancer Patients with Human T-Cell Lymphotropic Virus Type-1 Infection. Asian Pac J Cancer Prev.
2019;
20
(6)
:
1909-12
.
View Article PubMed Google Scholar -
Choi
J.,
Successful Endoscopic Resection of Residual Colonic Mucosa-Associated Lymphoid Tissue Lymphoma after Polypectomy. Clin Endosc.
2020
.
-
Moss
S.F.,
The clinical evidence linking Helicobacter pylori to gastric cancer. Cell Mol Gastroenterol Hepatol.
2016;
3
(2)
:
183-91
.
View Article PubMed Google Scholar -
Koshiol
J.,
Wozniak
A.,
Cook
P.,
Adaniel
C.,
Acevedo
J.,
Azócar
L.,
Gallbladder Cancer Chile Working Group
Salmonella enterica serovar Typhi and gallbladder cancer: a case-control study and meta-analysis. Cancer Med.
2016;
5
(11)
:
3310-3235
.
View Article PubMed Google Scholar -
Cheng
W.T.,
Kantilal
H.K.,
Davamani
F.,
The Mechanism of Bacteroides fragilis Toxin Contributes to Colon Cancer Formation. Malays J Med Sci.
2020;
27
(4)
:
9-21
.
View Article PubMed Google Scholar -
Zhu
H.,
Shen
Z.,
Luo
H.,
Zhang
W.,
Zhu
X.,
Chlamydia trachomatis infection-associated risk of cervical cancer: a meta-analysis. Medicine (Baltimore).
2016;
95
(13)
:
e3077
.
View Article PubMed Google Scholar -
Maeda
M.,
Moro
H.,
Ushijima
T.,
Mechanisms for the induction of gastric cancer by Helicobacter pylori infection: aberrant DNA methylation pathway. Gastric Cancer.
2017;
20
(1)
:
8-15
.
View Article PubMed Google Scholar -
Rokkas
T.,
Rokka
A.,
Portincasa
P.,
A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann Gastroenterol.
2017;
30
(4)
:
414-23
.
View Article PubMed Google Scholar -
Zhang
X.Y.,
Zhang
P.Y.,
Aboul-Soud
M.A.,
From inflammation to gastric cancer: role of Helicobacter pylori. Oncol Lett.
2017;
13
(2)
:
543-8
.
View Article PubMed Google Scholar -
Maleki Kakelar
H.,
Barzegari
A.,
Dehghani
J.,
Hanifian
S.,
Saeedi
N.,
Barar
J.,
Pathogenicity of Helicobacter pylori in cancer development and impacts of vaccination. Gastric Cancer.
2019;
22
(1)
:
23-36
.
View Article PubMed Google Scholar -
Posselt
G.,
Crabtree
J.E.,
Wessler
S.,
Proteolysis in Helicobacter pylori-induced gastric cancer. Toxins (Basel).
2017;
9
(4)
:
134
.
View Article PubMed Google Scholar -
Kusano
C.,
Gotoda
T.,
Ishikawa
H.,
Moriyama
M.,
The administrative project of Helicobacter pylori infection screening among junior high school students in an area of Japan with a high incidence of gastric cancer. Gastric Cancer.
2017;
20
(1)
:
16-9
.
View Article PubMed Google Scholar -
Kakiuchi
T.,
Matsuo
M.,
Endo
H.,
Nakayama
A.,
Sato
K.,
Takamori
A.,
A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in Saga Prefecture: a preliminary report. J Gastroenterol.
2019;
54
(8)
:
699-707
.
View Article PubMed Google Scholar -
Alfarouk
K.O.,
Bashir
A.H.,
Aljarbou
A.N.,
Ramadan
A.M.,
Muddathir
A.K.,
AlHoufie
S.T.,
The possible role of Helicobacter pylori in gastric cancer and its management. Front Oncol.
2019;
9
:
75
.
View Article PubMed Google Scholar -
Colotta
F.,
Allavena
P.,
Sica
A.,
Garlanda
C.,
Mantovani
A.,
Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis.
2009;
30
(7)
:
1073-81
.
View Article PubMed Google Scholar -
Gatenby
R.A.,
Gillies
R.J.,
Brown
J.S.,
Of cancer and cave fish. Nat Rev Cancer.
2011;
11
(4)
:
237-8
.
View Article PubMed Google Scholar -
Iqbal
M.O.,
Yahya
E.B.,
Andleeb
S.,
Ahmed
M.M.,
Javaid
M.U.,
Shakeel
W.,
Iqbal
I.,
In vivo Assessment of Reversing Cisplatin-Induced Nephrotoxicity Using Jatropha mollissima crude extract and its potential cytotoxicity. Saudi J Biol Sci.
2021;
:
In press
.
View Article Google Scholar -
Haghi
F.,
Goli
E.,
Mirzaei
B.,
Zeighami
H.,
The association between fecal enterotoxigenic B. fragilis with colorectal cancer. BMC Cancer.
2019;
19
(1)
:
879
.
View Article PubMed Google Scholar -
Snezhkina
A.V.,
Krasnov
G. S.,
Lipatova
A. V.,
Sadritdinova
A. F.,
Kardymon
O. L.,
Fedorova
M. S. ,
The Dysregulation of Polyamine Metabolism in Colorectal Cancer Is Associated with Overexpression of c-Myc and C/EBPβ rather than Enterotoxigenic Bacteroides fragilis Infection. Oxid Med Cell Longev.
2016;
2016
:
2353560
.
View Article Google Scholar -
Tsuchiya
Y.,
Loza
E.,
Villa-Gomez
G.,
Trujillo
C.C.,
Baez
S.,
Asai
T.,
Metagenomics of microbial communities in gallbladder bile from patients with gallbladder cancer or cholelithiasis. Asian Pacific journal of cancer prevention. Asian Pac J Cancer Prev.
2018;
19
(4)
:
961-7
.
PubMed Google Scholar -
Fowler
C.C.,
Chang
S.J.,
Gao
X.,
Geiger
T.,
Stack
G.,
Galán
J.E.,
Emerging insights into the biology of typhoid toxin. Curr Opin Microbiol.
2017;
35
:
70-7
.
View Article PubMed Google Scholar -
Di Domenico
E.G.,
Cavallo
I.,
Pontone
M.,
Toma
L.,
Ensoli
F.,
Biofilm producing Salmonella typhi: chronic colonization and development of gallbladder cancer. Int J Mol Sci.
2017;
18
(9)
:
1887
.
View Article PubMed Google Scholar -
Vagholkar
K.,
Pawanarkar
A.,
Iyengar
M.,
Vagholkar
S.,
Chronic Salmonella typhi carrier state: a precursor to gall bladder cancer. International Surgery Journal.
2016;
3
(2)
:
464-7
.
View Article Google Scholar -
Laban
M.,
Ibrahim
E.A.,
Hassanin
A.S.,
Nasreldin
M.A.,
Mansour
A.,
Khalaf
W.M.,
Chlamydia trachomatis infection in primary fallopian tube and high-grade serous ovarian cancers: a pilot study. Int J Womens Health.
2019;
11
:
199-205
.
View Article PubMed Google Scholar -
Salim
O.E.H.,
Hamid
H.K.,
Mekki
S.O.,
Suleiman
S.H.,
Ibrahim
S.Z.,
Colorectal carcinoma associated with schistosomiasis: a possible causal relationship. World J Surg Oncol.
2010;
8
(1)
:
68
.
View Article PubMed Google Scholar -
Han
I.H.,
Kim
J.H.,
Jang
K.S.,
Ryu
J.S.,
Inflammatory mediators of prostate epithelial cells stimulated with Trichomonas vaginalis promote proliferative and invasive properties of prostate cancer cells. Prostate.
2019;
79
(10)
:
1133-46
.
View Article PubMed Google Scholar -
Molan
A.L.,
Rasheed
E.H.,
Study the possible link between toxoplasmosis and different kinds of cancer in Iraq. American Journal of Life Science Researches.
2016;
4
(3)
:
83-8
.
View Article Google Scholar -
Berahmat
R.,
Mahami-Oskouei
M.,
Rezamand
A.,
Spotin
A.,
Aminisani
N.,
Ghoyounchi
R.,
Cryptosporidium infection in children with cancer undergoing chemotherapy: how important is the prevention of opportunistic parasitic infections in patients with malignancies?. Parasitol Res.
2017;
116
(9)
:
2507-15
.
View Article PubMed Google Scholar -
Benamrouz
S.,
Conseil
V.,
Creusy
C.,
Calderon
E.,
Dei-Cas
E.,
Certad
G.,
Parasites and malignancies, a review, with emphasis on digestive cancer induced by Cryptosporidium parvum (Alveolata: Apicomplexa) . Parasite.
2012;
19
(2)
:
101-15
.
View Article PubMed Google Scholar -
Machicado
C.,
Marcos
L.A.,
Carcinogenesis associated with parasites other than Schistosoma, Opisthorchis and Clonorchis: A systematic review. Int J Cancer.
2016;
138
(12)
:
2915-21
.
View Article PubMed Google Scholar -
Plieskatt
J.L.,
Deenonpoe
R.,
Mulvenna
J.P.,
Krause
L.,
Sripa
B.,
Bethony
J.M.,
Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB J.
2013;
27
(11)
:
4572-84
.
View Article PubMed Google Scholar -
van Tong
H.,
Brindley
P.J.,
Meyer
C.G.,
Velavan
T.P.,
Parasite infection, carcinogenesis and human malignancy. EBioMedicine.
2017;
15
:
12-23
.
View Article PubMed Google Scholar -
Grivennikov
S.I.,
Greten
F.R.,
Karin
M.,
Immunity, inflammation, and cancer. Cell.
2010;
140
(6)
:
883-99
.
View Article PubMed Google Scholar -
Vale
N.,
Gouveia
M.J.,
Gärtner
F.,
Current and Novel Therapies Against Helminthic Infections: The Potential of Antioxidants Combined with Drugs. Biomolecules.
2020;
10
(3)
:
350
.
View Article PubMed Google Scholar -
Saha
S.K.,
Lee
S.B.,
Won
J.,
Choi
H.Y.,
Kim
K.,
Yang
G.M.,
Correlation between oxidative stress, nutrition, and cancer initiation. Int J Mol Sci.
2017;
18
(7)
:
1544
.
View Article PubMed Google Scholar -
Patergnani
S.,
Danese
A.,
Bouhamida
E.,
Aguiari
G.,
Previati
M.,
Pinton
P.,
Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int J Mol Sci.
2020;
21
(21)
:
8323
.
View Article PubMed Google Scholar -
Sulżyc-Bielicka
V.,
Ko\lodziejczyk
L.,
Jaczewska
S.,
Bielicki
D.,
Safranow
K.,
Bielicki
P.,
Colorectal cancer and Cryptosporidium spp. infection. PLoS One.
2018;
13
(4)
:
e0195834
.
View Article PubMed Google Scholar -
Calvisi
D.F.,
Schistoma mansoni and Hepatocellular Carcinoma: is it all about c-Jun and STAT3?. Hepatology.
2020
.
View Article Google Scholar -
Hamid
H.K.,
Schistosoma japonicum-Associated Colorectal Cancer: A Review. Am J Trop Med Hyg.
2019;
100
(3)
:
501-5
.
View Article PubMed Google Scholar -
Cacho-Díaz
B.,
Neurocysticercosis in cancer patients. Neurociencia.
2019;
20
(6)
:
262-5
.
-
Dematei
A.,
Fernandes
R.,
Soares
R.,
Alves
H.,
Richter
J.,
Botelho
M.C.,
Angiogenesis in Schistosoma haematobium-associated urinary bladder cancer. APMIS.
2017;
125
(12)
:
1056-62
.
View Article PubMed Google Scholar -
Lovy
A.,
Knowles
B.,
Labbe
R.,
Nolan
L.,
Activity of edible mushrooms against the growth of human T4 leukemic cancer cells, HeLa cervical cancer cells, and Plasmodium falciparum. J Herbs Spices Med Plants.
2000;
6
(4)
:
49-57
.
View Article Google Scholar -
Zhu
Z.,
Davidson
K.T.,
Brittingham
A.,
Wakefield
M.R.,
Bai
Q.,
Xiao
H.,
Trichomonas vaginalis: a possible foe to prostate cancer. Med Oncol.
2016;
33
(10)
:
115
.
View Article PubMed Google Scholar -
Schwartz
D.A.,
Helminths in the induction of cancer: opisthorchis viverrini, Clonorchis sinensis and cholangiocarcinoma. Trop Geogr Med.
1980;
32
(2)
:
95-100
.
PubMed Google Scholar -
Imam
A.,
Al-Anzi
F.G.,
Al-Ghasham
M.A.,
Al-Suraikh
M.A.,
Al-Yahya
A.O.,
Rasheed
Z.,
Serologic evidence of Toxoplasma gondii infection among cancer patients. A prospective study from Qassim region, Saudi Arabia. Saudi Med J.
2017;
38
(3)
:
319-21
.
View Article PubMed Google Scholar -
Mohamed
A.M.,
Ahmed
M.A.,
Ahmed
S.A.,
Al-Semany
S.A.,
Alghamdi
S.S.,
Zaglool
D.A.,
Predominance and association risk of Blastocystis hominis subtype I in colorectal cancer: a case control study. Infect Agent Cancer.
2017;
12
(1)
:
21
.
View Article PubMed Google Scholar -
Sripa
B.,
Brindley
P.J.,
Mulvenna
J.,
Laha
T.,
Smout
M.J.,
Mairiang
E.,
The tumorigenic liver fluke Opisthorchis viverrini multiple pathways to cancer. Trends Parasitol.
2012;
28
(10)
:
395-407
.
View Article PubMed Google Scholar -
Dambuza
I.M.,
Brown
G.D.,
Fungi accelerate pancreatic cancerNature Publishing Group 2019.
View Article Google Scholar -
Aykut
B.,
Pushalkar
S.,
Chen
R.,
Li
Q.,
Abengozar
R.,
Kim
J.I.,
The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature.
2019;
574
(7777)
:
264-7
.
View Article PubMed Google Scholar -
Malik
A.,
Sharma
D.,
Malireddi
R. K. S.,
Guy
C. S.,
Chang
T-C.,
Olsen
S. R.,
SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity.
2018;
49
(3)
:
515-530.e5
.
View Article PubMed Google Scholar -
Krüger
W.,
Vielreicher
S.,
Kapitan
M.,
Jacobsen
I.D.,
Niemiec
M.J.,
Fungal-bacterial interactions in health and disease. Pathogens.
2019;
8
(2)
:
70
.
View Article PubMed Google Scholar -
Tragiannidis
A.,
Bisping
G.,
Koehler
G.,
Groll
A.H.,
Minireview: malassezia infections in immunocompromised patients. Mycoses.
2010;
53
(3)
:
187-95
.
View Article PubMed Google Scholar -
Guého
E.,
Boekhout
T.,
Ashbee
H.R.,
Guillot
J.,
Van Belkum
A.,
Faergemann
J.,
The role of Malassezia species in the ecology of human skin and as pathogens. Med Mycol.
1998;
36
(1)
:
220-9
.
PubMed Google Scholar -
Abogmaza
A.F.,
Keer
K. F.,
Takrizzah
A. A.,
Yahya
E. B.,
A Review on the Medicinal and Aromatic Plants Growing in Libya and Their Therapeutic Properties. International Research Journal of Science and Technology.
2020;
2
(1)
:
327-334
.
View Article Google Scholar -
Iqbal
M.O.,
Yahya
E.B.,
In vivo assessment of reversing aminoglycoside antibiotics nephrotoxicity using Jatropha mollissima crude extract. Tissue Cell.
2021;
72
:
101525
.
View Article PubMed Google Scholar -
Daley
D.,
Mani
V.R.,
Mohan
N.,
Akkad
N.,
Ochi
A.,
Heindel
D.W.,
Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med.
2017;
23
(5)
:
556-67
.
View Article PubMed Google Scholar -
Moore
S.C.,
Lee
I.M.,
Weiderpass
E.,
Campbell
P.T.,
Sampson
J.N.,
Kitahara
C.M.,
Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med.
2016;
176
(6)
:
816-25
.
View Article PubMed Google Scholar -
Holmes
M.D.,
Chen
W.Y.,
Feskanich
D.,
Kroenke
C.H.,
Colditz
G.A.,
Physical activity and survival after breast cancer diagnosis. JAMA.
2005;
293
(20)
:
2479-86
.
View Article PubMed Google Scholar -
Kenfield
S.A.,
Stampfer
M.J.,
Giovannucci
E.,
Chan
J.M.,
Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol.
2011;
29
(6)
:
726-32
.
View Article PubMed Google Scholar -
Gabriel
B.M.,
Zierath
J.R.,
The limits of exercise physiology: from performance to health. Cell Metab.
2017;
25
(5)
:
1000-11
.
View Article PubMed Google Scholar -
Yahya
E.B.,
Alzalouk
M.M.,
Alfallous
K.A.,
Abogmaza
A.F.,
Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed Res Ther.
2020;
7
(10)
:
4032-40
.
View Article Google Scholar -
Allaq
A.A.,
Sidik
N.J.,
Abdul-Aziz
A.,
Ahmed
I.A.,
Cumin (Cuminum cyminum L.): A review of its ethnopharmacology, phytochemistry. Biomed Res Ther.
2020;
7
(9)
:
4016-21
.
View Article Google Scholar -
Yahya
E.B.,
Amirul
A.A.,
H P S
A.K.,
Olaiya
N.G.,
Iqbal
M.O.,
Jummaat
F.,
Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers (Basel).
2021;
13
(10)
:
1612
.
View Article PubMed Google Scholar -
Yahya
E.B.,
Jummaat
F.,
Amirul
A.A.,
Adnan
A.S.,
Olaiya
N.G.,
Abdullah
C.K.,
A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics (Basel).
2020;
9
(10)
:
648
.
View Article PubMed Google Scholar -
Kich
D.M.,
Vincenzi
A.,
Majolo
F.,
Volken de Souza
C.F.,
Goettert
M.I.,
Probiotic: effectiveness nutrition in cancer treatment and prevention. Nutr Hosp.
2016;
33
(6)
:
1430-7
.
View Article PubMed Google Scholar -
Jacouton
E.,
Chain
F.,
Sokol
H.,
Langella
P.,
Bermúdez-Humarán
L.G.,
Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front Immunol.
2017;
8
:
1553
.
View Article PubMed Google Scholar -
Shin
D.S.,
Rhee
K.J.,
Eom
Y.B.,
Effect of probiotic Clostridium butyricum NCTC 7423 supernatant on biofilm formation and gene expression of Bacteroides fragilis. J Microbiol Biotechnol.
2020;
30
(3)
:
368-77
.
View Article PubMed Google Scholar -
Abdelhamid
A.G.,
Esaam
A.,
Hazaa
M.M.,
Cell free preparations of probiotics exerted antibacterial and antibiofilm activities against multidrug resistant E. coli. Saudi Pharm J.
2018;
26
(5)
:
603-7
.
View Article PubMed Google Scholar -
Almashgab
A.M.,
Yahya
E.B.,
Banu
A.,
The Cytotoxicity Effects of Outer Membrane Vesicles Isolated from Hospital and Laboratory Strains of Pseudomonas Aeruginosa on Human Keratinocyte Cell Line. Malays J Sci.
2020;
39
(3)
:
45-53
.
View Article Google Scholar -
Rizal
S.,
Lai
T.K.,
Muksin
U.,
Olaiya
N.G.,
Abdullah
C.K.,
Ikramullah
Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre. Polymers (Basel).
2020;
12
(11)
:
2554
.
View Article PubMed Google Scholar -
Barzegari
A.,
Kheyrolahzadeh
K.,
Hosseiniyan Khatibi
S.M.,
Sharifi
S.,
Memar
M.Y.,
Zununi Vahed
S.,
The battle of probiotics and their derivatives against biofilms. Infect Drug Resist.
2020;
13
:
659-72
.
View Article PubMed Google Scholar -
Abdul Khalil
H.P.,
Adnan
A.S.,
Yahya
E.B.,
Olaiya
N.G.,
Safrida
S.,
Hossain
M.S.,
A Review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers (Basel).
2020;
12
(8)
:
1759
.
View Article PubMed Google Scholar -
Bishayee
A.,
Sethi
G.,
Bioactive natural products in cancer prevention and therapy: Progress and promise. in Seminars in cancer biology. 2016. Elsevier. 10.1016/j.semcancer.2016.08.006.
.
-
Song
M.,
Vogelstein
B.,
Giovannucci
E.L.,
Willett
W.C.,
Tomasetti
C.,
Cancer prevention: molecular and epidemiologic consensus. Science.
2018;
361
(6409)
:
1317-8
.
View Article PubMed Google Scholar -
Chew
S.S.,
Tan
L.T.,
Law
J.W.,
Pusparajah
P.,
Goh
B.H.,
Ab Mutalib
N.S.,
Targeting Gut Microbial Biofilms-A Key to Hinder Colon Carcinogenesis?. Cancers (Basel).
2020;
12
(8)
:
2272
.
View Article PubMed Google Scholar
Comments
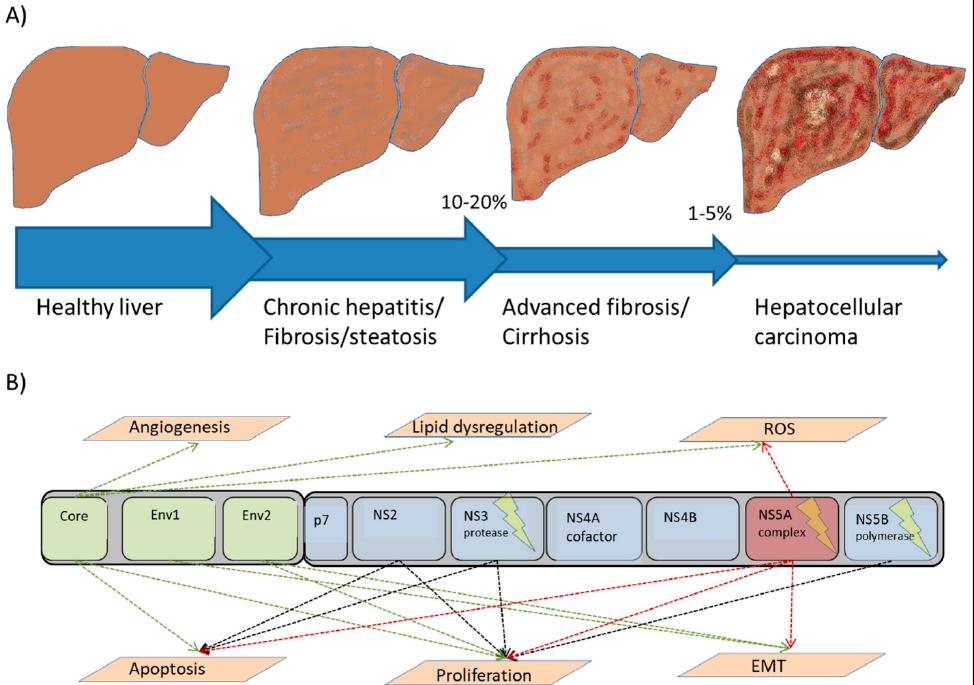
Article Details
Volume & Issue : Vol 8 No 9 (2021)
Page No.: 4525-4539
Published on: 2021-09-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8609 times
- PDF downloaded - 1566 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress